Abstract
Amphibians across the planet face the threat of population decline and extirpation caused by the disease chytridiomycosis. Despite consensus that the fungal pathogens responsible for the disease are conservation issues, strategies to mitigate their impacts in the natural world are, at best, nascent. Reducing risk associated with the movement of amphibians, non-amphibian vectors and other sources of infection remains the first line of defence and a primary objective when mitigating the threat of disease in wildlife. Amphibian-associated chytridiomycete fungi and chytridiomycosis are already widespread, though, and we therefore focus on discussing options for mitigating the threats once disease emergence has occurred in wild amphibian populations. All strategies have shortcomings that need to be overcome before implementation, including stronger efforts towards understanding and addressing ethical and legal considerations. Even if these issues can be dealt with, all currently available approaches, or those under discussion, are unlikely to yield the desired conservation outcome of disease mitigation. The decision process for establishing mitigation strategies requires integrated thinking that assesses disease mitigation options critically and embeds them within more comprehensive strategies for the conservation of amphibian populations, communities and ecosystems.
This article is part of the themed issue ‘Tackling emerging fungal threats to animal health, food security and ecosystem resilience’.
Keywords: chytridiomycosis, mitigation, conservation strategy
1. Introduction
We are confronting an expanding array of pathogenic fungi that cause extensive mortality, demographic decline and extirpations in livestock, crop and wildlife hosts [1]. Developing strategies to limit the spread and impact of these pathogens is a priority that crosses the boundaries of politics, economics, science and health, and falls within the remit of the medical, veterinary, agricultural and conservation sciences. Despite the increasing range of animal and plant taxa threatened by fungal pathogens, conservation science has not advanced disease mitigation in nature as a priority. This shortcoming has no better example than research on amphibian-associated chytridiomycete fungi. Our recognition of the threat posed by the global and regional emergences of the chytrid Batrachochytrium dendrobatidis (hereafter, Bd), has spurred significant advances in understanding the biology of the fungus and the dynamics of chytridiomycosis since the disease was first identified nearly 20 years ago [2]. Similarly, we have gained important insights into the European emergence of another chytrid fungus, Batrachochytrium salamandrivorans (Bsal) [3]. Unfortunately, the development of field interventions for disease management has lagged far behind and managing amphibian health in nature remains a largely unexplored topic [4–6]. Because applied conservation always operates under enormous financial constraints, it is important to critically assess the viability of conservation strategies before significant investment, which has rarely been done for strategies for controlling chytridiomycosis in wild amphibians [6–8]. Here, we assess some of the commonly proposed approaches to control the spread and impact of amphibian chytridiomycosis in the field. We assume that an ideal strategy will be: (i) safe, legal and ethical, (ii) effective and reliable, (iii) transferrable across host species, communities and environments, (iv) relatively simple to implement, and (v) cost-effective.
Countering disease-driven amphibian declines should consist of a multifaceted approach adapted to the stages of pathogen emergence (pre-arrival, invasion front, epidemic and established) [9]. Current approaches include prevention and short-term solutions (e.g. ex situ breeding programmes, cryopreservation) but long-term, in situ, sustainable solutions are required if the goal of amphibian conservation is to be attained. This implies neutralizing the disease threat in wild populations. Although we do not discuss the prevention of pathogen introduction here in any detail, attempts to do this (e.g. via trade regulations, such as the recent establishment of restrictions on caudate amphibian trade in the USA in response to the emergence of B. salamandrivorans, https://federalregister.gov/a/2016-00452) are probably the most effective disease mitigation measure available [9,10]. The international movement of amphibians plays a continuing role in establishing and extending the distribution of amphibian-associated chytrids and other pathogens, but the control of chytridiomycosis and other purely wildlife diseases is largely overlooked in commercial trade [3,11–13]. The World Organisation for Animal Health (OIE) is the international body that can regulate this, but even though its remit includes wildlife conservation it has a poor track record in doing so. Batrachochytrium dendrobatidis has been listed by the OIE but enforcement of chytridiomycosis control in the amphibian trade has not been implemented by OIE member states [14].
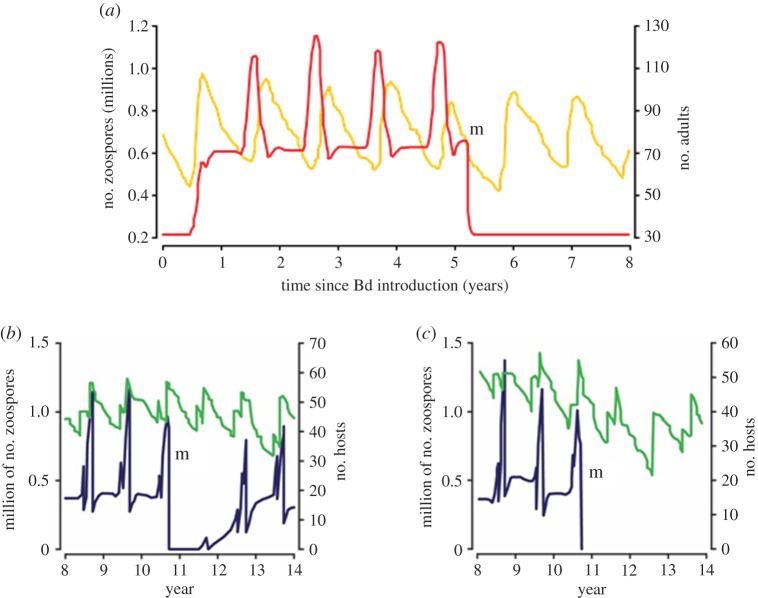
Here, we review strategies for mitigating amphibian disease following pathogen emergence. These range from minimizing effects on host populations to pathogen eradication. Short-term solutions have been discussed in detail or summarized elsewhere and these are considered vital in temporarily preserving amphibian populations at risk [4,6,15,16]. For example, interventions with antifungals during an epidemic can alter infection dynamics and alleviate disease, but in the absence of long-term disease management in situ, any short-term measure is unlikely to result in significant conservation success [17]. We focus on measures that offer the potential for long-term chytridiomycosis management in situ. Bd currently infects hundreds of amphibian species on all continents where amphibians occur (figure 1) [18]. Amphibian infections with Bd predate the late twentieth century identification of lethal chytridiomycosis, and global emergence of the lethal form of the disease at this time was widespread [19,20]. Chytridiomycosis continues to emerge across four continents, precluding its elimination from widespread and complex infected host communities [18]. Instead of focusing on short-term solutions, we examine a more pragmatic approach that strives for long-term, host–pathogen coexistence. An ambitious aim would be to preserve a maximum proportion and diversity of amphibian species across as many of their distributions as possible. This implies that conservation triage will be necessary, accepting the loss of individual populations and even species [21,22]. Indeed, culling of reservoir and superspreader hosts requires consideration (figure 2). Irrespective, aims and methods will depend on local conservation priorities and should be defined by local conservation managers [24].
Figure 1.
Examples of lethal chytridiomycosis from Latin America (a) and Europe (b). (a) A Craugastor underwoodi dead and in situ killed by lethal chytridiomycosis in Monte Verde, Costa Rica. The isolate derived from this animal in 2008 has served as the source of DNA for real-time polymerase chain reaction (qPCR)–positive controls for two of the authors to this day. (b) An Alytes muletensis, again dead and in situ, found on Mallorca, Spain.
Figure 2.
The relative impact of culling and antifungal treatment in a simple, single-species population paramaterised using data on the Mallorcan midwife toad. Mitigation is undertaken at point m. See [23] for explanation of model structure and parameter estimation. (a) Culling of tadpoles, undertaken at point m, results in pathogen elimination. The yellow line is adult population size and the red line is free-swimming zoospore density. (b–c) Comparison between culling and tadpole antifungal treatment and release, assuming maintenance of infection in the adult population and keeping model parameters identical across models. Here, green lines are adult population size and blue lines are free-swimming zoospore density. In (b), mitigation is unsuccessful due to increased host density after treated tadpoles are returned to the pond. In (c), pathogen elimination is attributable to more persistent reduction in host density.
Amphibian chytridiomycosis treatments have been developed for captive populations, but translating these to managing infections in wild amphibian populations and communities is not straightforward. This is because amphibians affected by chytridiomycosis occupy terrestrial, arboreal, aquatic and subterranean habitats that can overlap in a single landscape. Host population sizes fluctuate enormously, often exhibit highly dynamic spatial dispersion, and are frequently undetectable for much of the year. Therefore, it is not surprising that the number of studies of infection and disease in the wild, and those exploring management of infection in captivity, far outstrip those on in situ intervention. We know of few published studies describing the outcomes of attempted mitigation, and only two describing success. Four different strategies to mitigate the impacts of chytridiomycosis in nature have been attempted and published: translocation/reintroduction, augmentation of the host microbiome with probiotics, treatment of individuals with antifungals and a combination of antifungal treatment with chemical disinfection of the environment [16,17,25–27].
2. Trialled and tested
Translocations/reintroductions often have strong appeal because they can promote the idea that ‘something is being done’. They are erroneously perceived to be cost-effective, simple to implement and transferrable. However, without a solid understanding of host–pathogen dynamics and the biology of the host and pathogen in the landscape, translocations/reintroductions have little probability of success. Several attempts have been made to repatriate amphibians affected by chytridiomycosis in Europe, North America, the Caribbean and Africa but none have led to successful, long-term amphibian re-establishment [4,26,28,29] (but see [30] for evidence of short-term post-release survival). Although the majority of failures have been associated with the re-emergence of lethal chytridiomycosis in the translocated/reintroduced species, the cause behind failure to re-establish in almost every case could not be attributed clearly [26,28] (but see [11]). This is important because lethal chytridiomycosis can be a secondary consequence of other threatening processes, which would mean conservation efforts focused on the fungus could be misdirected [28,29,31]. The inability to unambiguously identify cause demonstrates the relative immaturity of the science of amphibian reintroduction as a means of mitigating chytridiomycosis, falsifies the assumptions of simplicity and transferability, and violates the requirement of threat mitigation before reintroduction [32]. It also calls for greater investment in pathological investigations in concert with post-release field monitoring. Given our incomplete understanding of Bd dynamics and the potential for development of resistance to Bd in wild populations, the use of translocations/reintroductions as a research tool is perhaps more appropriate than as a mitigation strategy against Bd.
A decade ago, Harris and co-workers discovered that a subset of bacteria isolated from the skin of living amphibians has the ability to inhibit Bd growth in vitro [33]. Since then bacteria that inhibit Bd have been isolated from amphibians from across the Americas, Africa, Europe and Australia. Field studies of amphibian microbiomes indicate that the bacterial community on amphibian skin changes with amphibian life-history stage, with fewer Bd-inhibitory species in later life stages, suggesting that targets for field intervention may be age-specific [34]. An expanding research programme is underway to ascertain if resistance to or limitation of infection can be enhanced by augmenting amphibian skin microbiomes with inhibitory bacteria. Encouragingly, a limited, but successful, field trial has been published along with a strategy for the isolation and potential application of probiotics to augment skin microbiomes [25,35]. This strategy outlines the advantages of bioaugmentation, including the use of local bacterial isolates, and describes the potential for environmental application of bacteria that will interact with an entire amphibian community [35].
Several general issues need to be overcome before probiotics can be considered a viable mitigation strategy. First, the potential risk probiotics pose to ecosystem and public health requires assessment and the practicalities of probiotic development are also largely unassessed [36]. For example, there is little available information regarding the relationship between chytrid growth inhibition in vitro and effective inhibition of fungal growth or the development of disease in vivo. Experimental efforts using probiotics to control Campylobacter in poultry show that the relationship will probably not be straightforward and that some bacteria that are inhibitory are ineffective against pre-existing infections [37,38]. Efficient and persistent host and environmental colonization needs to be established: amphibian skin microbiomes are dynamic and can be unstable and unpredictable, and bacterial community composition changes over the animal's lifetime [32]. Bioaugmentation requires a deeper understanding of bacterial community assembly, stability and permeability, couched in the context of amphibian host community, the skin secretions produced by species members of the community and how these are in turn influenced by environmental heterogeneity [39,40]. Probiotics should also exert their beneficial effect across Bd genotypes. It has already been documented that the ability to inhibit one isolate of Bd does not translate across different isolates of the globally pandemic lineage [41]. Finally, a probiotic should show characteristics that render it suitable for mass production, including prolonged shelf life. As it stands, we have an unclear understanding of how interactions among all these factors will influence the development of effective probiotic therapies against chytridiomycosis. The research required to gain this understanding will probably be less cost-effective, implementable and transferrable than that for chemical treatments (see below), and, if animal experiment requirements are extensive and not well-justified, ethically questionable. However, if candidate bacteria can be characterized that meet the required criteria, their application could be far more cost-effective, ethical and less controversial than chemical treatment.
Antifungals applied directly to susceptible hosts have proved ineffective as a long-term strategy for in situ chytrid mitigation, as they afford no persistent benefits after treatment is stopped [17,27]. However, in an isolated and structurally simple ecosystem containing a single amphibian host species, antifungal treatments of individuals combined with chemical treatment of the environment did eliminate Bd and clearance persisted across years [27]. These findings suggest that the environmental application of fungicides may be a viable, cost-effective, simple to implement and broadly transferrable strategy for controlling infection in some wild amphibian populations. Environmental treatment might not be applicable to many amphibian communities and species, however, and the environmental application of chemical pesticides has significant ecological, legal and ethical ramifications. To be effective in the long term, fungicides may have to be applied on a regular basis, much as they are in agricultural systems. Although any strategy that requires ongoing maintenance and has the potential for collateral impacts might seem untenable, decades of fungicide applications to food crops have had a significant and positive effect on global food yields [42]. The parallel suggests that in the face of the chytridiomycosis crisis environmental treatment with fungicides should be considered as a viable, long-term management strategy for wild amphibians threatened by the disease. Very little effort has been expended in investigating existing chemical compounds that are effective against amphibian-associated chytrids, or the development of chemical agents that specifically target chytrids, despite the evidence that some chemical pesticides mitigate infection in the aquatic environment without compromising amphibian development and larval survival [43] (but see [44]). Although the use of agricultural pesticides is greatly debated, the focal, short-term application of antifungals targeted at a reduction of infection prevalence and infection load in specific cases of acute chytridiomycosis-driven amphibian die-offs is worth exploring [45]. The application of any such measure should be weighed against its potential negative impacts on biodiversity, ecosystem function, human health and the potential for amphibian-associated chytrids to develop resistance to these treatments [46]. Advances in our understanding of the virulence factors and cellular components key for chytrid reproduction, growth and infectivity should inform the selection of compounds that exhibit multi-modal antifungal action, and also guide the development of application strategies [47,48].
3. Horizon-scanning or wishful thinking?
Several mitigation strategies are gaining traction in the literature although they remain untested in real-world settings. Evidence is accumulating that at least some species are responding to the emergence of chytridiomycosis through natural selection on immunity [49,50]. As a result, two arguments that incorporate selection into mitigation strategies are being promoted [51]. The first is based on the idea that, given time, natural selection will operate on immunogenetic variation in amphibian populations. To enable this, amphibians need to persist in the face of the pathogen, and translocation/repatriation has been proposed as a method to facilitate population persistence during the process of selection. The second strategy is to breed selectively for resistant or tolerant genotypes for release into the wild [52]. Both strategies seek to establish resistant or tolerant populations and are based on the assumptions that amphibian host immune responses to chytrids can be selected for and that immune function will be protective in a wild setting.
We can apply the points for and against translocations/reintroductions that we outlined above to the strategy of translocation/repatriation, compounded with the need to understand resistance and tolerance in captive populations before any release could be ethically undertaken. But what about selective breeding? We are aware of a single example where captive selection and subsequent breeding created defined lines that exhibit variation in immunity in an amphibian: the genus Xenopus [53,54]. The knowledge base on Xenopus captive breeding, cell biology, genetics and immunity took decades to develop. Advances are being made in comparative immunogenetics that could conceivably guide breeding designs, but this is still a long way from understanding host species immune responses to chytrids and exploring heritable variation of amphibian immunity with the goal of selective breeding [55]. The elucidation of mechanisms underpinning resistance against Bd would greatly facilitate the development of resistance markers that could be used in marker-assisted selective breeding programmes. The chances of finding any such marker, or a set of markers, are hampered by the context-dependent interaction of Bd with the amphibian host [56]. Establishing captive colonies upon which selection can be imposed is a non-trivial task and requires extensive investment and resources. Even if assisted selection does produce genotypes that have the ability to resist or tolerate infection with chytrids, there is no guarantee that these abilities will function when transferred to a natural setting. Research has repeatedly shown how environmental variation can dictate the outcome of the amphibian host/chytrid pathogen interaction and the ability to mount innate immune responses to Bd can be significantly impaired simply by modifying ambient temperature [31,56–59]. We do not dismiss the possibility that assisted selection might provide conservation benefits, only caution that the current knowledge base indicates significant research is still required before natural and assisted selection can be applied widely to chytrid mitigation. If genetic determinants of host resistance are identified in multiple amphibian species and new technologies for genetic manipulation prove amenable to immunogenetic modification of susceptible amphibian species, the situation might change, but it will also open up new ethical issues for conservationists [60–62]. Clearly, it is imperative to continue investigating the genetic basis of amphibian resistance and novel means by which it can be augmented.
At least three published studies have investigated whether frogs could be immunized against Bd. Systemic injections of killed Bd were ineffective at reducing the probability of infection or death [63,64]. By contrast, increasing numbers of exposures to killed Bd or live Bd culture followed by clearance with antifungals was negatively correlated with strength of infection and positively correlated with survival following subsequent exposure to Bd [65]. The authors themselves questioned how their findings might be applied in a conservation setting but noted the potential for priming hosts against infection prior to release to the wild. These findings are contradicted by Hudson et al., where the repeated use of antifungals on naturally infected frogs generated no long-term benefits once antifungal treatments ceased [17]. Perhaps more importantly, every immunization study to date has focused on post-metamorphic animals, and immunization of pre-metamorphic stages might not be possible as adaptive immunity is not available to pre-metamorphic stages [66] (but see [54]). Amplification of infection is commonly associated with larval stages, with high rates of mortality occurring at metamorphic climax. Controlling infection in amphibian larvae will be a key factor in mitigating impacts of chytridiomycosis because amphibian population growth rates are highly sensitive to survival rates of post-metamorphic juveniles [67–70].
The ideal vaccine for in situ use should elicit a strong protective response across life stages and across species against a broad spectrum of relevant and virulent chytrid genotypes, be safe, and have both its production and administration feasible. Indeed, the research process should engage with the relevant authorities from the outset, as policy applicable to vaccinating free-living wildlife populations also requires development. So far, immunization experiments have been conducted with fairly straightforward and crude fungal preparations. Designing effective vaccines is a time- and money-consuming undertaking, and for diseases in a range of species, fungal vaccines have proved far more difficult to develop than their bacterial and viral counterparts. To date, with few exceptions, potential vaccines against human fungal pathogens are still in preclinical stages of development and very few effective veterinary vaccines are available [71–73]. Although vaccinations currently afford no clear contribution to chytridiomycosis mitigation in wild populations, continued research on vaccines will undoubtedly aid in our understanding of amphibian immunity and host–pathogen interactions, both topics essential for a variety of mitigation strategies including immunization, selection and bioaugmentation.
Manipulating environments to reduce infectivity or virulence of Bd is another strategy that may hold promise. The principle behind this ecological, rather than evolutionary, approach underlies environmental treatments (e.g. see [27]), but in practice is accomplished by exploiting environmental variations that reduce chytrid growth and zoospore density and does not require elimination of the pathogen from the environment. The concept follows the recognition that environmental variability can inhibit, as well as exacerbate, the impacts of chytridiomycosis, with evidence of reduced virulence even in highly susceptible host species [6,74–77]. Refuges from disease, but not necessarily infection, could be created by altering habitats to reinforce environmental factors not conducive to Bd growth within the host or zoospore survival outside of it. Habitat management is already integral to most amphibian conservation programmes and often involves repeated efforts to maintain useful habitats (e.g. [78]), suggesting that environmental manipulations for the purposes of disease control could have quick uptake by the conservation community, with both concepts and strategies readily transferrable. Interventions could be chemical (e.g. altering salinity), physical (e.g. altering temperatures to not favour chytrid growth and reproduction) or biotic (e.g. promoting the abundance of organisms that consume environmental zoospores) [76,79–81]. These strategies will probably focus, at least initially, on manipulating the aquatic environment, as environmental persistence of Bd in water is deemed essential for amphibian decline and extinction scenarios [82,83]. Theory and empirical evidence shows that conservation efforts targeting aquatic life stages that reduced disease-driven losses of newly metamorphosed juveniles should improve recruitment and reduce or reverse the effects of disease-driven decline; additional population models addressing this topic are clearly needed [15,82,84].
Although environmental manipulations may create pockets of tolerance or resistance, they offer limited opportunities for amphibians with broad geographical ranges and/or disproportionately affected complex communities and habitats. As with environmental disinfection, even in simple settings environmental manipulations must be assessed for their impacts on biodiversity and other ecosystem functions. As with translocations/reintroductions, host ecology must be well understood before changes to the habitat are undertaken. For now, environmental manipulation might provide long-term refuges for focal species of high conservation concern, but offers no broad scope for chytridiomycosis mitigation.
A focus on disease mitigation may not always be the best way forward because simpler actions might achieve the required results: improving habitat quality might enable losses from disease at one stage of the amphibian life cycle to be compensated for in gains at other life stages. For example, one might use pond draining to cull predators of amphibian larvae. As a consequence, tadpole survival might increase, leading to increased juvenile recruitment. Even if many juveniles continue to die of chytridiomycosis, this action might still facilitate population persistence. There is some empirical evidence that this might work, and existing theory of harvested and exploited populations might guide such a strategy [5,85].
4. Single strategies or a marriage of methods?
Clearly, we do not know how to manage amphibian diseases in the wild and yet conservation managers have to make decisions and manage populations. They cannot wait until we understand amphibian-chytrid host–pathogen biology in great detail; a lack of action because of imperfect information is a management decision [86]. From our review, it is clear that a single strategy is unlikely to achieve the conservation outcome of disease mitigation. Each strategy has pros and cons but by combining methods strategically in situ mitigation is likely to have a greater probability of success. There are a number of tools to decide which management actions are best or most likely to succeed in the presence of uncertainty. Structured decision-making and value of information analysis can be used to find the best management option and to define the direction of research most likely to illuminate critical uncertainties [87–89]. For example, structured decision-making might identify important gaps in our understanding of chytrid epidemiology. These approaches have only recently been used in the context of chytrid mitigation [7,24]. Converse et al. used such an approach to study the effects of translocations in a toad metapopulation and found that efforts to reduce disease spread had weak effects, selection for resistance would increase the number of sites occupied by toads and translocations would speed up species recovery [7].
Shortcomings of individual strategies outlined above may be compensated for by combining two or more strategies. In that sense, our outline of the major alternatives for Bd mitigation and the applicability and challenges of each forms a starting template that can inform decision-making processes. The science of decision-making links management options to measurable objectives (e.g. population persistence). Post-management monitoring then determines the outcome of management actions against the objectives and is used to update models for the next round of decision-making. This approach allows real-time assessment of the impact of management alternatives so that management can be rapidly modified to improve outputs [8,90].
For these approaches to work, researchers investigating mitigation strategies have to engage in the conservation management process and be willing to alter research programmes based on the outputs of structured decision-making and adaptive management exercises. Precedence for this can be found in the literature on chytridiomycosis ecology, evolution and epidemiology and is exemplified by the initial effort to identify chytridiomycosis as the cause of amphibian mass mortality [2]. Coordinating research and management efforts have already been proposed for Australian amphibian species at risk from chytridiomycosis [6]. Joined-up efforts will require field trials across a more extensive range of settings and amphibian communities than are currently being attempted. It remains to be decided, the authors of this review disagree on this point, at which stage of methods development sufficient knowledge has accumulated to justify field trials.
What must be considered at all stages of the conservation management process, however, are the ethical and legal issues associated with whatever strategies are proposed or adopted. Strategies that are illegal or unethical are inapplicable irrespective of their cost- and field-effectiveness, reliability, transferability or simplicity. Ethical issues may be identified at any scale. Our example of conservation triage is a knotty ethical question: what is an acceptable format for deciding which species to conserve and which to cull or allow to go extinct? Expending effort on the mitigation of chytridiomycosis should also be subject to ethical consideration, as should any decision to expend highly limited resources available for biodiversity conservation [91]. Disease as a conservation issue remains a novel concept for most policy-makers and conservation practitioners, so legal frameworks may have to be challenged and modified to account for responses to this new and growing threat to amphibian biodiversity. Ethical issues may be difficult to address, but failing to mitigate chytridiomycosis, a disease widely accepted as predominantly driven by human activities, is the least ethical option of all.
5. Conclusion
Despite decades of research into amphibian-chytrid host–pathogen biology, no effective method to reduce the impact of chytridiomycosis has emerged and been tested broadly in the field. A few case and proof-of-concept studies have produced mixed or limited success at best. A more collaborative approach to chytrid mitigation research is necessary, one that should start with an approach from the family of tools from decision sciences to define the most important research questions. Such exercises to identify those questions should be conducted by interdisciplinary research teams that are working with conservation managers and that can put research outputs into the context of the overall conservation objectives. It is always uncertain how the findings of research undertaken away from the field setting will transfer to the real world, but it is clear from our review that significant ex situ research efforts are required for all mitigation methods to ensure that the results of field trials can be fully explained. A lack of in situ evidence from chytridiomycosis mitigation efforts, however, indicates that field trials are not yet an objective in many research programmes, despite invoking amphibian conservation as a potential consequence of research discoveries. Clearly, if we are to mitigate chytridiomycosis, research must be focused on delivering outputs that can be rapidly and critically assessed and, when warranted, implemented in field trials as soon as possible.
Acknowledgements
This is contribution number 547 of the USGS Amphibian Research and Monitoring Initiative (ARMI).
Authors' contributions
All authors reviewed and approved the final manuscript. Contributions were made by all authors to all components of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
T.W.J.G. acknowledges generous funding provided by NERC (NE/K012509/1 and NE/N009967/1) and the Morris Animal Foundation (D12ZO-002) and thanks the Royal Society for hosting for the presentation that this manuscript was preliminarily based on. J.B. acknowledges generous funding from the BBVA Foundation.
Disclaimer
Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
- 1.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. ( 10.1038/nature10947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger L, et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl Acad. Sci. USA 95, 9031–9036. ( 10.1073/pnas.95.15.9031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martel A, et al. 2014. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346, 630–631. ( 10.1126/science.1258268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodhams DC, et al. 2011. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front. Zool. 8, 8 ( 10.1186/1742-9994-8-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheele BC, Hunter DA, Skerratt LF, Brannelly LA, Driscoll DA. 2015. Low impact of chytridiomycosis on frog recruitment enables persistence in refuges despite high adult mortality. Biol. Conserv. 182, 36–43. ( 10.1016/j.biocon.2014.11.032) [DOI] [Google Scholar]
- 6.Skerratt LF, et al. 2016. Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildl. Res. 43, 105–120. ( 10.1071/WR15071) [DOI] [Google Scholar]
- 7.Converse SJ, Bailey LL, Mosher BA, Funk WC, Gerner BD, Muths E. 2016. A model to inform management actions as a response to chytridiomycosis-associated decline. Ecohealth ( 10.1007/s10393-016-1117-9) [DOI] [PubMed]
- 8.Grant EHC, Zipkin EF, Nichols JD, Campbell JP. 2013. A strategy for monitoring and managing declines in an amphibian community. Conserv. Biol. 27, 1245–1253. ( 10.1111/cobi.12137) [DOI] [PubMed] [Google Scholar]
- 9.Langwig KE, et al. 2015. Context-dependent conservation responses to emerging wildlife diseases. Front. Ecol. Environ. 13, 195–202. ( 10.1890/140241) [DOI] [Google Scholar]
- 10.Gray MJ, et al. 2015. Batrachochytrium salamandrivorans: the North American response and a call for action. PLoS Pathog. 11, e1005251 ( 10.1371/journal.ppat.1005251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker SF, et al. 2008. Invasive pathogens threaten species recovery programs. Curr. Biol. 18, R853–R854. ( 10.1016/j.cub.2008.07.033) [DOI] [PubMed] [Google Scholar]
- 12.Weldon C, Fisher MC. 2011. The effect of trade-mediated spread of amphibian chytrid on amphibian conservation. In Fungal diseases: an emerging challenge to human, animal, and plant health (ed. IOM (Institute of Medicine)), pp. 355–367. Washington, DC: The National Academies Press. [Google Scholar]
- 13.Wombwell E, Garner TWJ, Cunningham AA, Quest R, Pritchard S, Rowcliffe JM, Griffiths R. 2016. Detection of Batrachochytrium dendrobatidis in amphibians imported into the UK for the pet trade. Ecohealth. ( 10.1007/s10393-016-1138-4) [DOI] [PubMed] [Google Scholar]
- 14.Schloegel LM, Daszak P, Cunningham AA, Speare R, Hill B. 2010. Two amphibian diseases, chytridiomycosis and ranaviral disease, are now globally notifiable to the World Organization for Animal Health (OIE): an assessment. Dis. Aquat. Organ. 92, 101–108. ( 10.3354/dao02140) [DOI] [PubMed] [Google Scholar]
- 15.Scheele BC, Hunter DA, Grogan LF, Berger L, Kolby JE, McFadden MS, Marantelli G, Skerratt LF, Driscoll DA. 2014. Interventions for reducing extinction risk in chytridiomycosis-threatened amphibians. Conserv. Biol. 28, 1195–1205. ( 10.1111/cobi.12322) [DOI] [PubMed] [Google Scholar]
- 16.Smith RK, Sutherland WJ. 2014. Amphibian conservation: global evidence for the effects of interventions. Exeter, UK: Pelagic Publishing. [Google Scholar]
- 17.Hudson MA, et al. 2016. In-situ itraconazole treatment improves survival rate during an amphibian chytridiomycosis epidemic. Biol. Conserv. 195, 37–45. ( 10.1016/j.biocon.2015.12.041) [DOI] [Google Scholar]
- 18.Olson DH, et al. 2013. Mapping the Global Emergence of Batrachochytrium dendrobatidis, the Amphibian Chytrid Fungus. PLoS ONE 8, e56802 ( 10.1371/journal.pone.0056802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weldon C, du Preez LH, Hyat AD, Muller R, Speare R. 2004. Origin of the amphibian chytrid fungus. Emerg. Infect. Dis. 10, 2100–2105. ( 10.3201/eid1012.030804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrer RA, et al. 2011. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl Acad. Sci. USA 108, 18 732–18 736. ( 10.1073/pnas.1111915108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottrill MC, et al. 2008. Is conservation triage just smart decision making? Trends Ecol. Evol. 23, 649–654. ( 10.1016/j.tree.2008.07.007) [DOI] [PubMed] [Google Scholar]
- 22.Muths E, Fisher RN. 2016. An alternative framework for responding to the amphibian crisis. Oryx. ( 10.1017/S0030605315001131) [DOI] [Google Scholar]
- 23.Doddington BJ, Bosch J, Oliver JA, Grassly NC, Garcia G, Schmidt BR, Garner TWJ, Fisher MC. 2013. Context-dependent amphibian host population response to an invading pathogen. Ecology 94, 1795–1804. ( 10.1890/12-1270.1) [DOI] [PubMed] [Google Scholar]
- 24.Grant EHC, et al. 2015. Salamander chytrid fungus ( Batrachochytrium salamandrivorans ) in the United States—developing research, monitoring, and management strategies. U.S. Geological Survey Open-File Report 2015–1233, p. 16. See 10.3133/ofr20151233. [DOI]
- 25.Vredenburg VT, Briggs CJ, Harris RN. 2011. Host pathogen dynamics of amphibian chytridiomycosis: the role of the skin microbiome in health and disease. In Fungal diseases: an emerging threat to human, animal, and plant health (eds Olson L, Choffnes E, Relman D, Pray L), pp. 342–355. Washington, DC: National Academy Press. [Google Scholar]
- 26.Muths E, Bailey LL, Watry MK. 2014. Animal reintroductions: an innovative assessment of survival. Biol. Conserv. 172, 200–208. ( 10.1016/j.biocon.2014.02.034) [DOI] [Google Scholar]
- 27.Bosch J, Sanchez-Tomé, Fernández-Loras A, Oliver JA, Fisher MC, Garner TWJ. 2015. Successful elimination of a lethal wildlife infectious disease in nature. Biol. Lett. 11, 20150874 ( 10.1098/rsbl.2015.0874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fellers GM, Bradford DF, Pratt D, Wood LL. 2007. Demise of repatriated populations of mountain yellow-legged frogs (Rana muscosa) in the Sierra Nevada of California. Herpetol. Conserv. Biol. 2, 5–21. [Google Scholar]
- 29.Rija AA, Khatibu FH, Kohi EM, Muheto R. 2011. Status and reintroduction of the Kihansi spray toad Nectophrynoides asperginis in Kihansi gorge: challenges and opportunities. In Proc. of the 7th TAWIRI Scientific Conference, pp. 11–20.
- 30.Brannelly LA, Hunter DA, Skerratt LF, Scheele BC, Lenger D, McFadden MS, Harlow PS, Berger L. 2016. Chytrid infection and post-release fitness in the reintroduction of an endangered alpine tree frog. Anim. Conserv. 19, 153–162. ( 10.1111/acv.12230) [DOI] [Google Scholar]
- 31.Bosch J, Carrascal LM, Durán L, Walker S, Fisher MC. 2007. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc. R. Soc. B 274, 253–260. ( 10.1098/rspb.2006.3713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IUCN/SSC. 2013. Guidelines for reintroductions and other conservation translocations. Version 1.0. Gland, Switzerland: IUCN Species Survival Commission, viiii+57 pp. [Google Scholar]
- 33.Harris RN, James TY, Lauer A, Simon MA, Patel A. 2006. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. Ecohealth 3, 53–56. ( 10.1007/s10393-005-0009-1) [DOI] [Google Scholar]
- 34.Kueneman JG, Woodhams DC, Van Treuren W, Archer HM, Knight R, McKenzie VJ. 2016. Inhibitory bacteria reduce fungi on early life stages of endangered Colorado boreal toads (Anaxyrus boreas). ISME J. 10, 934–944. ( 10.1038/ismej.2015.168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KPC, Harris RN. 2013. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol. Lett. 16, 807–820. ( 10.1111/ele.12099) [DOI] [PubMed] [Google Scholar]
- 36.Woodhams DC, Bletz M, Kueneman J, McKenzie V. 2016. Managing amphibian disease with skin microbiota. Trends Microbiol. 24, 161–164. ( 10.1016/j.tim.2015.12.010) [DOI] [PubMed] [Google Scholar]
- 37.Robyn J, Rasschaert G, Messens W, Pasmans F, Heyndrickx M. 2012. Screening for lactic acid bacteria capable of inhibiting Campylobacter jejuni in in vitro simulations of the broiler chicken caecal environment. Beneficial Microbes 3, 299–308. ( 10.3920/BM2012.0021) [DOI] [PubMed] [Google Scholar]
- 38.Robyn J, Rasschaert G, Hermans D, Pasmans F, Heyndrickx M. 2013. In vivo broiler experiments to assess anti-Campylobacter jejuni activity of a live Enterococcus faecalis strain. Poultry Sci. 92, 265–271. ( 10.3382/ps.2012-02712) [DOI] [PubMed] [Google Scholar]
- 39.Daskin JH, Alford RA. 2012. Context-dependent symbioses and their potential roles in wildlife diseases. Proc. R. Soc. B 279, 1457–1465. ( 10.1098/rspb.2011.2276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodhams DC, et al. 2014. Interacting symbionts and immunity in the Amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS ONE 9, e96375 ( 10.1371/journal.pone.0096375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antwis RE, Preziosi RF, Harrison XA, Garner TWJ. 2015. Amphibian symbiotic bacteria do not show a universal ability to inhibit growth of the global panzootic lineage of Batrachochytrium dendrobatidis. Appl. Environ. Microbiol. 81, 3706–3711. ( 10.1128/AEM.00010-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. 2002. Agricultural sustainability and intensive production practices. Nature 418, 671–677. ( 10.1038/nature01014) [DOI] [PubMed] [Google Scholar]
- 43.Hanlon SM, Parris MJ. 2014. The interactive effects of chytrid fungus, pesticides, and exposure timing on gray treefrog (Hyla versicolor) larvae. Environ. Toxicol. Chem. 33, 216–222. ( 10.1002/etc.2419) [DOI] [PubMed] [Google Scholar]
- 44.Hanlon SM, Lynch KJ, Kerby JL, Parris MJ. 2015. The effects of a fungicide and chytrid fungus on anuran larvae in aquatic mesocosms. Environ. Sci. Pollut. Res. 22, 12929–12940. ( 10.1007/s11356-015-4566-8) [DOI] [PubMed] [Google Scholar]
- 45.Dayan FE, Cantrell CL, Duke SO. 2009. Natural products in crop protection. Bioorg. Med. Chem. 17, 4022–4034. ( 10.1016/j.bmc.2009.01.046) [DOI] [PubMed] [Google Scholar]
- 46.Stearns SC. 2012. Evolutionary medicine: its scope, interest and potential. Proc. R. Soc. B 279, 4305–4321. ( 10.1098/rspb.2012.1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farrer RA, Henk DA, Garner TWJ, Balloux F, Woodhams DC, Fisher MC. 2013. Chromosomal copy number variation, selection and uneven rates of recombination reveal cryptic genome diversity linked to pathogenicity. PLoS Genet. 9, e1003703 ( 10.1371/journal.pgen.1003703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holloman DW. 2015. Fungicide resistance: 40 years on and still a major problem. In Fungicide resistance in plant pathogens (eds Ishii H, Holloman DW), pp. 3–11. New York, NY: Springer. [Google Scholar]
- 49.Savage AE, Zamudio KR. 2016. Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc. R. Soc. B 283, 20153115 ( 10.1098/rspb.2015.3115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brannelly LA, Hunter DA, Skerratt LF, Scheele BC, Lenger D, McFadden MS, Harlow PS, Berger L. 2016. Chytrid infection and post-release fitness in the reintroduction of an endangered alpine tree frog. Anim. Conserv. 19, 153–162. ( 10.1111/acv.12230) [DOI] [Google Scholar]
- 51.Kilpatrick AM. 2006. Facilitating the evolution of resistance to avian malaria in Hawaiian birds. Biol. Conserv. 128, 475–485. ( 10.1016/j.biocon.2005.10.014) [DOI] [Google Scholar]
- 52.Venesky MD, Mendelson JR III, Sears BF, Stiling P, Rohr JR. 2012. Selecting for tolerance against pathogens and herbivores to enhance success of reintroduction and translocation. Conserv. Biol. 26, 586–592. ( 10.1111/j.1523-1739.2012.01854.x) [DOI] [PubMed] [Google Scholar]
- 53.Edholm E-S, Goyos A, Taran J, Andino FDJ, Ohta Y, Robert J. 2014. Unusual evolutionary conservation and further species-specific adaptations of a large family of nonclassical MHC class Ib genes across different degrees of genome ploidy in the amphibian subfamily Xenopodinae. Immunogenetics 66, 411–426. ( 10.1007/s00251-014-0774-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robert J, Edholm ES. 2014. A prominent role for invariant T cells in the amphibian Xenopus laevis tadpoles. Immunogenetics 66, 513–523. ( 10.1007/s00251-014-0781-6) [DOI] [PubMed] [Google Scholar]
- 55.Savage AE, Kiemnec-Tyburczy KM, Ellison AR, Fliescher RC, Zamudio KR. 2014. Conservation and divergence in the frog immunome: pyrosequencing and de novo assembly of immune tissue transcriptomes. Gene 542, 98–108. ( 10.1016/j.gene.2014.03.051) [DOI] [PubMed] [Google Scholar]
- 56.Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. 2012. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 3, 146–151. ( 10.1038/nclimate1659) [DOI] [Google Scholar]
- 57.Ribas L, et al. 2009. Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis. PLoS ONE, 4, e8408 ( 10.1371/journal.pone.0008408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garner TWJ, Rowcliffe JM, Fisher MC. 2011. Climate change, chytridiomycosis or condition: an experimental test of amphibian survival. Glob. Change Biol. 17, 667–675. ( 10.1111/j.1365-2486.2010.02272.x) [DOI] [Google Scholar]
- 59.Clare FC, et al. 2016. Climate forcing of an emerging pathogenic fungus across a montane multi-host community. Phil. Trans. R. Soc. B 371, 20150454 ( 10.1098/rstb.2015.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho SW, Kim S, Kim JM, Kim JS. 2013. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232. ( 10.1038/nbt.2507) [DOI] [PubMed] [Google Scholar]
- 61.Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. 2013. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239. ( 10.1038/nbt.2508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson JA, Altwegg R, Evans DM, Ewen JG, Gordon IJ, Pettorelli N, Young JK. 2016. Is there a future for genome-editing technologies in conservation? Anim. Conserv. 19, 97–101. ( 10.1111/acv.12273) [DOI] [Google Scholar]
- 63.Rollins-Smith LA, Ramsey JP, Reinert LK, Woodhams DC, Livo LJ, Carey C. 2009. Immune defenses of Xenopus laevis against Batrachochytrium dendrobatidis. Front. Biosci. S1, 68–91. ( 10.2741/s8) [DOI] [PubMed] [Google Scholar]
- 64.Stice MJ, Briggs CJ. 2010. Immunization is ineffective at preventing infection and mortality due to the amphibian chytrid fungus Batrachochytrium dendrobatidis. J. Wildl. Dis. 46, 70–77. ( 10.7589/0090-3558-46.1.70) [DOI] [PubMed] [Google Scholar]
- 65.McMahon TA, et al. 2014. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511, 224–227. ( 10.1038/nature13491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andino FDJ, Chen G, Li Z, Grayfer L, Robert J. 2012. Susceptibility of Xenopus laevis tadpoles to infection by the ranavirus Frog-Virus 3 correlates with a reduced and delayed innate immune response in comparison with adult frogs. Virology 432, 435–443. ( 10.1016/j.virol.2012.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geiger CC, Schmidt BR. 2013. Laboratory tests of antifungal agents to treat tadpoles against the pathogen Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 103, 191–197. ( 10.3354/dao02576) [DOI] [PubMed] [Google Scholar]
- 68.Hels T, Nachman G. 2002. Simulating viability of a spadefoot toad Pelobates fuscus metapopulation in a landscape fragmented by a road. Ecography 25, 730–744. ( 10.1034/j.1600-0587.2002.250609.x) [DOI] [Google Scholar]
- 69.Conroy SDS, Brook BW. 2003. Demographic sensitivity and persistence of the threatened white- and orange-bellied frogs of Western Australia. Popul. Ecol. 45, 105–114. ( 10.1007/s10144-003-0145-9) [DOI] [Google Scholar]
- 70.Di Minin E, Griffiths RA. 2011. Viability analysis of a threatened amphibian population: modelling the past, present and future. Ecography 34, 162–169. ( 10.1111/j.1600-0587.2010.06263.x) [DOI] [Google Scholar]
- 71.Spellberg B. 2011. Vaccines for invasive fungal infections. F1000 Med Rep 3, 13 ( 10.3410/M3-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cassone A, Casadevall A. 2012. Recent progress in vaccines against fungal diseases. Curr. Opin. Microbiol. 15, 427–433. ( 10.1016/j.mib.2012.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mignon B, Tabart J, Baldo A, Mathy A, Losson B, Vermout S. 2008. Immunization and dermatophytes. Curr. Opin. Infect. Dis. 21, 134–140. ( 10.1097/QCO.0b013e3282f55de6) [DOI] [PubMed] [Google Scholar]
- 74.Blooi M, Martel A, Vercammen F, Haesebrouck F, Pasmans F. 2015. Treatment of urodelans based on temperature dependent infection dynamics of Batrachochytrium salamandrivorans. Sci. Rep. 5, 8037 ( 10.1038/srep08037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spitzen-van der Sluijs A, Martel A, Hallmann C, Bosman W, Garner TWJ, Van Rooij P, Jooris R, Haesebrouck F, Pasmans F. 2014. Environmental determinants of recent endemism of Batrachochytrium dendrobatidis infections in amphibian assemblages in the absence of disease outbreaks. Conserv. Biol. 28, 1302–1311. ( 10.1111/cobi.12281) [DOI] [PubMed] [Google Scholar]
- 76.Puschendorf R, Hodgson L, Alford RA, Skerratt LF, VanDerWal J. 2013. Underestimated ranges and overlooked refuges from amphibian chytridiomycosis. Diver. Distrib. 19, 1313–1321. ( 10.1111/ddi.12091) [DOI] [Google Scholar]
- 77.Walker SF, et al. 2010. Factors driving pathogenicity versus prevalence of the amphibian pathogen Batrachochytrium dendrobatidis and chytridiomycosis in Iberia. Ecol. Lett. 13, 372–382. ( 10.1111/j.1461-0248.2009.01434.x) [DOI] [PubMed] [Google Scholar]
- 78.Denton JS, Hitchings SP, Beebee TJC, Gent A. 1997. A recovery program for the natterjack toad (Bufo calamita) in Britain. Conserv. Biol. 11, 1329–1338. ( 10.1046/j.1523-1739.1997.96318.x) [DOI] [Google Scholar]
- 79.Schmeller DS, et al. 2014. Microscopic aquatic predators strongly affect infection dynamics of a globally emerged pathogen. Curr. Biol. 24, 176–180. ( 10.1016/j.cub.2013.11.032) [DOI] [PubMed] [Google Scholar]
- 80.Roznik EA, Sapsford DA, Pike L, Schwarzkopf L, Alford RA. 2015. Natural disturbance reduces disease risk in endangered rainforest frog populations. Sci. Rep. 5, 13472 ( 10.1038/srep13472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stockwell MP, Storrie LJ, Pollard CJ, Clulow J, Mahoney MJ. 2015. Effects of pond salinization on survival rate of amphibian hosts infected with the chytrid fungus. Conserv. Biol. 29, 391–399. ( 10.1111/cobi.12402) [DOI] [PubMed] [Google Scholar]
- 82.Mitchell KM, Churcher TS, Garner TWJ, Fisher MC. 2008. Persistence of the emerging pathogen Batrachochytrium dendrobatidis outside the amphibian host greatly increases the probability of host extinction. Proc. R. Soc. B 275, 329–334. ( 10.1098/rspb.2007.1356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Briggs CJ, Knapp RA, Vredenburg VT. 2010. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc. Natl Acad. Sci. USA 107, 9695–9700. ( 10.1073/pnas.0912886107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muths E, Scherer RD, Pilliod DS. 2011. Compensatory effects of recruitment and survival when amphibian populations are perturbed by disease. J. Appl. Ecol. 48, 873–879. ( 10.1111/j.1365-2664.2011.02005.x) [DOI] [Google Scholar]
- 85.Lebreton J-D. 2005. Dynamical and statistical models for exploited populations. Aust. N. Z. J. Stat. 47, 49–63. ( 10.1111/j.1467-842X.2005.00371.x) [DOI] [Google Scholar]
- 86.Martin TG, et al. 2012. Acting fast helps avoid extinction. Conserv. Lett. 5, 274–280. ( 10.1111/j.1755-263X.2012.00239.x) [DOI] [Google Scholar]
- 87.Kendall WL. 2001. Using models to facilitate complex decisions. In Modeling in natural resource management: valid development, interpretation and application (eds Shenk TM, Franklin AB), pp. 147–170. Washington, DC: Island Press. [Google Scholar]
- 88.Martin J, Runge MC, Nichols JD, Lubow BC, Kendall WL. 2009. Structured decision making as a conceptual framework to identify thresholds for conservation and management. Ecol. Appl. 19, 1079–1090. ( 10.1890/08-0255.1) [DOI] [PubMed] [Google Scholar]
- 89.Canessa S, Guillera-Arroita G, Lahoz-Monfort JJ, Southwell DM, Armstrong DP, Chadès I, Lacy RC, Converse SJ. 2015. When do we need more data? A primer on calculating the value of information for applied ecologists. Methods Ecol. Evol. 6, 1219–1228. ( 10.1111/2041-210X.12423) [DOI] [Google Scholar]
- 90.Nichols JD, Johnson FA, Williams BK, Boomer SG. 2015. On formally integrating science and policy: walking the walk. J. Appl. Ecol. 52, 539–543. ( 10.1111/1365-2664.12406) [DOI] [Google Scholar]
- 91.Sarkar S, David FM. 2012. Conservation biology: ethical foundations. Nat. Educ. Knowledge 3, 3. [Google Scholar]