Abstract
Streptococcus mutans is a major pathogen of human dental caries. Strains harbouring the cnm gene, which encodes Cnm, a collagen-binding protein, contribute to the development of several systemic diseases. In this study, we analysed S. mutans strains isolated from the oral cavity of immunoglobulin (Ig)A nephropathy (IgAN) patients to determine potential relationships between cnm and caries status as well as IgAN conditions. Saliva specimens were collected from 109 IgAN patients and the cnm status of isolated S. mutans strains was determined using PCR. In addition, the dental caries status (decayed, missing or filled teeth [DMFT] index) in patients who agreed to dental consultation (n = 49) was evaluated. The DMFT index and urinary protein levels in the cnm-positive group were significantly higher than those in the cnm-negative group (p < 0.05). Moreover, the urinary protein levels in the high DMFT (≥15) group were significantly higher than those in the low DMFT (<15) group (p < 0.05). Our results show that isolation of cnm-positive S. mutans strains from the oral cavity may be associated with urinary protein levels in IgAN patients, especially those with a high dental caries status.
Immunoglobulin (Ig)A nephropathy (IgAN) is the most frequent chronic glomerulonephritis in the world1,2. IgAN occurs at any age, most commonly with clinical onset in the second and third decades of life3. Approximately 30–40% of IgAN patients progress to end-stage kidney disease within 20 years1,2. However, there is no disease-targeted treatment for IgAN4 since pathogenesis of the disease remains unknown. IgAN patients often manifest deterioration of macroscopic haematuria in upper respiratory infections such as tonsillitis3. Some bacteria have been reported as the source of initiating antigens or involved in the pathogenesis of IgAN5,6,7,8,9, and Haemophilus parainfluenzae antigen and Staphylococcus aureus cell envelope antigens have been detected in renal tissue of IgAN patients5,8. In addition, periodontitis-related bacteria were also suggested to be associated with IgAN10. These reports suggest that infection may trigger IgAN.
Streptococcus mutans, a Gram-positive oral streptococcal species known to be a major pathogen of human dental caries11, is occasionally isolated from the blood of patients with infective endocarditis12. S. mutans strains harbouring the cnm gene, which encodes Cnm, a cell surface collagen-binding protein, exhibit binding ability to the extracellular matrix, which may be a possible virulence factor in infective endocarditis13. Furthermore, our recent studies suggest that S. mutans strains expressing Cnm on the cell surface may be associated with aggravated cerebral haemorrhaging14,15, non-alcoholic steatohepatitis16 and inflammatory bowel disease11. We occasionally encountered IgAN patients with inflammatory bowel disease in the clinical setting and an association between inflammatory bowel disease and IgAN has also been noted17. Thus, we hypothesized that the presence of cnm-positive S. mutans strains may affect IgAN. We previously showed that the rate of cnm-positive S. mutans strains isolated from the oral cavity was found to be significantly higher in IgAN patients than in non-diseased controls, suggesting that the presence of cnm-positive S. mutans strains in the oral cavity may influence IgAN severity18. Based upon the present study, we hypothesized that cnm status and dental caries status exacerbate urinary protein levels in IgAN patients.
Results
Characteristics of IgAN and non-diseased control groups
At presentation, IgAN patients and non-diseased control subjects exhibited similar age and sex distributions (Table 1). The rates of S. mutans isolation in the IgAN and control groups were similar, whereas cnm-positive strains were significantly more prevalent in the IgAN group than in the control group (p = 0.0465).
Table 1. Characteristics of IgAN and non-diseased control groups.
| Characteristics | Control subjects (n = 61) | IgAN patients (n = 109) | p-value |
|---|---|---|---|
| Age (yr; mean ± SD) | 42.1 ± 10.6 | 43.8 ± 13.6 | 0.4073 |
| Gender (M/F) | 33/28 | 62/47 | 0.7279 |
| S. mutans isolated/total subjects (%) | 80.3 | 79.8 | 0.9367 |
| cnm-positiveS. mutans/total subjects (%) | 11.5 | 27.5 | 0.0465 |
| cnm-positiveS. mutans/total S. mutansisolated subjects (%) | 14.2 | 34.4 | 0.0486 |
Bold values indicate statistical significance at p < 0.05.
Dental caries status between IgAN patients and non-diseased control subjects
Forty-nine of the 109 IgAN patients and 49 of the 61 non-diseased control subjects were evaluated for dental caries status. No significant differences were found between IgAN and control groups regarding missing teeth (MT) and filled teeth (FT) index values (Table 2). However, the IgAN group was significantly associated with higher values for decayed teeth (DT) index (p = 0.0058) and decayed, missing or filled teeth (DMFT) index values (p = 0.0074).
Table 2. Dental caries status between IgAN patients and control subjects.
| Characteristics | Control subjects (n = 49) | IgAN patients (n = 49) | p-value |
|---|---|---|---|
| DT index | 0.3 ± 0.5 | 1.2 ± 2.3 | 0.0058 |
| MT index | 0.7 ± 3.4 | 1.8 ± 4.8 | 0.2616 |
| FT index | 8.6 ± 5.4 | 10.1 ± 6.2 | 0.1849 |
| DMFT index | 9.6 ± 5.9 | 13.1 ± 6.9 | 0.0074 |
Bold values indicate statistical significance at p < 0.05.
Background differences between S. mutans-negative groups and cnm-positive and cnm-negative S. mutans groups
IgAN patients and non-diseased control subjects were divided into three groups each, an S. mutans-negative group and cnm-positive and cnm-negative S. mutans groups. No significant differences in gender were found between the cnm-positive S. mutans in IgAN patients group and other groups, whereas the cnm-positive S. mutans in IgAN patients group was significantly associated with % urinary protein 1+ or higher (Table 3). In addition, the association between % urinary protein 1+ or higher and cnm-positive S. mutans in IgAN patients remained significantly different in subsequent logistic regression analysis adjusted for age and gender (p = 0.0011) (Supplementary Table S1). Furthermore, the cnm-positive S. mutans in the IgAN patients group was also significantly associated with higher age, higher serum creatinine, lower estimated glomerular filtration rates (eGFR), % urinary occult blood 1+ or higher and higher DMFT index. However, these data did not remain significantly different in subsequent logistic regression analyses adjusted for age and gender (data not shown).
Table 3. Background differences between S. mutans negative groups and cnm-positive and cnm-negative S. mutans groups.
| Characteristics | Control subjects | IgAN patients | ||||
|---|---|---|---|---|---|---|
| S. mutans negative (n = 12) | S. mutans positive | S. mutans negative (n = 22) | S. mutans positive | |||
| cnm-negative (n = 42) | cnm-positive (n = 7) | cnm-negative (n = 57) | cnm-positive (n = 30) | |||
| Age (yr; mean ± SD) | 41.2 ± 8.8 p = 0.0357 | 41.8 ± 11.2 p = 0.0057 | 45.6 ± 10.3 p = 0.3836 | 41.3 ± 14.7 p = 0.0122 | 41.5 ± 12.6 p = 0.0023 | 50.1 ± 13.1 |
| Gender (M/F) | 7/5 p = 0.7701 | 24/18 p = 0.7504 | 2/5 p = 0.2398 | 15/7 p = 0.2917 | 31/26 p = 0.9258 | 16/14 |
| Serum creatinine (mg/dl; mean ± SD) | 0.8 ± 0.2 p = 0.1247 | 0.7 ± 0.2 p = 0.0088 | 0.7 ± 0.2 p = 0.1410 | 1.2 ± 1.2 p = 0.6026 | 0.9 ± 0.4 p = 0.1953 | 1.1 ± 0.6 |
| eGFR (ml/min; mean ± SD) | 82.4 ± 15.6 p = 0.0032 | 89.4 ± 13.2 p < 0.0001 | 77.5 ± 7.0 p = 0.0532 | 70.6 ± 27.7 p = 0.0845 | 73.6 ± 22.8 p = 0.0057 | 60.5 ± 22.5 |
| % Urinary Protein 1 + or higher | 0 p < 0.0001 | 0 p < 0.0001 | 0 p < 0.0001 | 18.2 p < 0.0001 | 47.4 p = 0.0271 | 66.7 |
| % Urinary occult blood 1 + or higher | 0 p = 0.0004 | 0 p < 0.0001 | 0 p = 0.0036 | 22.7 p = 0.0248 | 29.8 p = 0.0491 | 46.7 |
| DMFT index (mean ± SD) | 3.8 ± 3.9 p = 0.0002 (n = 5) | 10.1 ± 6.1 p = 0.0020 (n = 38) | 11.2 ± 3.3 p = 0.0942 (n = 6) | 10.5 ± 8.2 p = 0.0239 (n = 11) | 12.5 ± 6.8 p = 0.0698 (n = 24) | 16.3 ± 4.8 (n = 14) |
The p-values in each column indicate comparison with the cnm-positive S. mutans in IgAN patients group.
Bold values indicate statistical significance at p < 0.05.
Background differences in IgAN patients harbouring cnm-positive and cnm-negative S. mutans strains
A total of 109 IgAN patients (mean age 43.8 ± 13.6 years, 62 males and 47 females) were analysed. S. mutans strains were isolated from 87 subjects with (n = 30) or without (n = 57) cnm-positive S. mutans strains in the oral cavity. Patients without S. mutans isolated strains (n = 22) were included in the cnm-negative S. mutans group (Fig. 1A). No significant differences were found between the cnm-positive S. mutans and cnm-negative S. mutans groups regarding sex, height, body weight, body mass index (BMI), serum creatinine, serum IgA, urinary red blood cell, anamnesis of tonsillectomy rate, anamnesis of steroid therapy rate or duration from kidney biopsy (Table 4).
Figure 1. Patient enrolment.
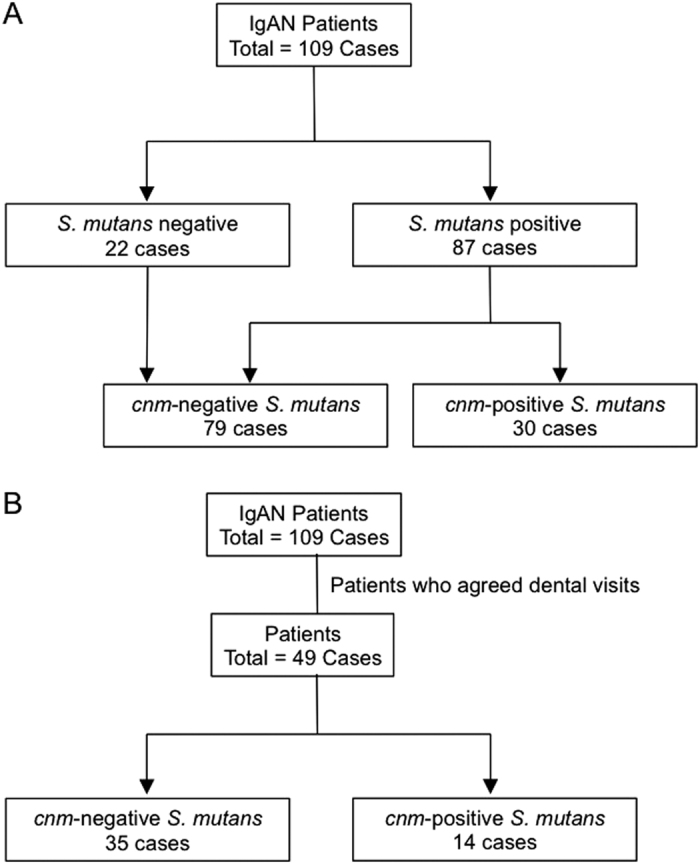
A total of 109 patients with IgAN were enrolled, among whom 30 showed positivity for the cnm gene (A). The dental caries status was evaluated in 49 patients, among whom 14 showed positivity for the cnm gene (B). IgAN: immunoglobulin (Ig)A nephropathy.
Table 4. Background differences between cnm-positive and cnm-negative S. mutans groups in IgAN patients.
| Characteristics | Total (n = 109) | cnm-negative S. mutans (n = 79) | cnm-positive S. mutans (n = 30) | p-value |
|---|---|---|---|---|
| Age (yr; mean ± SD) | 43.8 ± 13.6 | 41.4 ± 13.1 | 50.1 ± 13.1 | 0.0026 |
| Gender (M/F) | 62/47 | 46/33 | 16/14 | 0.6486 |
| Height (cm; mean ± SD) | 164.1 ± 8.5 | 165.0 ± 8.8 | 161.8 ± 7.4 | 0.0867 |
| Body weight (kg; mean ± SD) | 60.6 ± 12.1 | 60.9 ± 10.7 | 59.9 ± 15.4 | 0.6976 |
| BMI (kg/m2;mean ± SD) | 22.3 ± 3.6 | 22.2 ± 3.2 | 22.7 ± 4.6 | 0.5834 |
| Systolic blood pressure (mmHg; mean ± SD) | 123.8 ± 16.1 | 120.8 ± 14.4 | 131.8 ± 17.8 | 0.0011 |
| Diastolic blood pressure (mmHg; mean ± SD) | 78.2 ± 11.9 | 76.3 ± 11.5 | 83.4 ± 11.4 | 0.0047 |
| Serum albumin (g/dl; mean ± SD) | 4.2 ± 0.5 | 4.2 ± 0.4 | 4.0 ± 0.5 | 0.0026 |
| Serum total cholesterol (mg/dl; mean ± SD) | 193.3 ± 36.6 | 188.4 ± 31.3 | 205.9 ± 45.8 | 0.0258 |
| Serum creatinine (mg/dl; mean ± SD) | 1.0 ± 0.7 | 1.0 ± 0.7 | 1.1 ± 0.6 | 0.5161 |
| eGFR (ml/min/1.73 m2; mean ± SD) | 69.4 ± 24.2 | 72.8 ± 24.1 | 60.5 ± 22.5 | 0.0173 |
| Serum IgA (mg/dl; mean ± SD) | 291.3 ± 112.6 | 282.8 ± 113.3 | 312.9 ± 109.9 | 0.2242 |
| Urinary protein (g/gCr; mean ± SD) | 0.7 ± 1.7 | 0.4 ± 0.9 | 1.5 ± 2.8 | 0.0021 |
| Urinary red blood cell (/HPF; mean ± SD) | 5.3 ± 14.7 | 4.0 ± 12.1 | 8.7 ± 19.9 | 0.1319 |
| RAS-I medication rate (%) | 76.1 | 70.9 | 90 | 0.0444 |
| Anamnesis of tonsillectomy rate (%) | 40.4 | 39.2 | 43.3 | 0.7005 |
| Anamnesis of steroid therapy rate (%) | 77.1 | 72.2 | 90 | 0.0723 |
| Duration from kidney biopsy (month; mean ± SD) | 92.5 ± 68.5 | 91.7 ± 68.3 | 94.7 ± 70.3 | 0.8398 |
BMI: body mass index, eGFR: estimated glomerular filtration rate and RAS-I: renin-angiotensin system inhibitor. Bold values indicate statistical significance at p < 0.05.
The cnm-positive S. mutans group was significantly associated with higher age (p = 0.0026), higher systolic blood pressure (p = 0.0011), higher diastolic blood pressure (p = 0.0047), lower serum albumin (p = 0.0026), higher serum total cholesterol (p = 0.0258) and higher urinary protein levels (p = 0.0021) (Table 4). In addition, the association between urinary protein levels >0.5 g/gCr and cnm-positive S. mutans remained significantly different in subsequent logistic regression analysis adjusted for age and sex (p = 0.0147) (Supplementary Table S2). The cnm-positive S. mutans group was also significantly associated with lower eGFR (p = 0.0173) and higher renin-angiotensin system inhibitor (RAS-I) medication rates (p = 0.0444), although these data did not remain significantly different in subsequent logistic regression analysis adjusted for age and sex (data not shown).
Dental caries status in the cnm-positive S. mutans and cnm-negative S. mutans groups in IgAN patients
Forty-nine of the 109 IgAN patients were evaluated for dental caries status (Fig. 1B), among whom 14 subjects showed positivity for the cnm gene. No significant differences were found between cnm-positive S. mutans and cnm-negative groups regarding the DT and MT indices. However, the cnm-positive S. mutans group was significantly associated with higher values for the FT (p = 0.0017) and DMFT indices (p = 0.0400) (Table 5). In addition, the association between cnm-positive S. mutans and higher DMFT index values remained significantly different in subsequent logistic regression analysis adjusted for age and sex (p = 0.0468) (Supplementary Table S3).
Table 5. Dental caries status between cnm-positive and cnm-negative S. mutans groups in IgAN patients.
| Characteristics | Total (n = 49) | cnm-negative S. mutans (n = 35) | cnm-positive S. mutans (n = 14) | p-value |
|---|---|---|---|---|
| DT index (mean ± SD) | 1.2 ± 2.3 | 0.6 ± 1.3 | 1.5 ± 2.6 | 0.2735 |
| MT index (mean ± SD) | 1.8 ± 4.8 | 2.2 ± 5.5 | 0.9 ± 1.7 | 0.3848 |
| FT index (mean ± SD) | 10.1 ± 6.2 | 8.4 ± 6.0 | 14.4 ± 4.5 | 0.0017 |
| DMFT index (mean ± SD) | 13.1 ± 6.9 | 11.9 ± 7.2 | 16.3 ± 4.8 | 0.0400 |
DT: decayed teeth, MT: missing teeth, FT: filled teeth and DMFT: decayed, missing or filled teeth. Bold values indicate statistical significance at p < 0.05.
Clinical differences between high and low DMFT index groups in IgAN patients
A total of 49 IgAN patients (mean age 44.4 ± 13.9 years, 27 males and 22 females) were analysed (Fig. 2), among whom 20 subjects showed DMFT index values of 15 or greater (designated as the ‘high DMFT group’). The remaining 29 subjects with DMFT index values lower than 15 were designated as the ‘low DMFT group’. There were no significant differences found between the high and low DMFT groups regarding sex, height, body weight, BMI, diastolic blood pressure, serum albumin, serum IgA, urinary red blood cell, RAS-I medication rate, anamnesis of tonsillectomy rate, anamnesis of steroid therapy rate or duration from kidney biopsy. However, the high DMFT group was significantly associated with higher age (p = 0.0108), higher urinary protein levels (p = 0.0246), higher serum creatinine (p = 0.0026) and lower eGFR (p = 0.0012) (Table 6). In addition, the association between urinary protein levels >0.5 g/gCr and higher DMFT index values remained significantly different in subsequent logistic regression analysis adjusted for age and sex (p = 0.0076) (Supplementary Table S4). Furthermore, the high DMFT group was also significantly associated with higher systolic blood pressure (p = 0.0432), although these data did not remain significantly different in subsequent logistic regression analysis adjusted for age and gender (data not shown).
Figure 2. Dental caries status of IgAN patients.

Among 49 subjects, 20 had DMFT values of 15 or greater and were designated as the ‘high DMFT group’. IgAN: immunoglobulin (Ig)A nephropathy, DMFT: decayed, missing or filled teeth.
Table 6. Clinical data between the high and low DMFT index groups in IgAN patients.
| Characteristics | Total (n = 49) | Low DMFT (less than 15) (n = 29) | High DMFT (15 and more) (n = 20) | p-value |
|---|---|---|---|---|
| Age (yr; mean ± SD) | 44.4 ± 13.9 | 40.3 ± 12.4 | 50.4 ± 14.1 | 0.0108 |
| Gender (M/F) | 27/22 | 16/13 | 11/9 | 0.9907 |
| Height (cm; mean ± SD) | 163.3 ± 7.9 | 162.9 ± 8.1 | 163.9 ± 7.8 | 0.7027 |
| Body weight (kg; mean ± SD) | 62.1 ± 13.4 | 61.7 ± 12.2 | 62.7 ± 15.2 | 0.7946 |
| BMI (kg/m2; mean ± SD) | 23.0 ± 4.4 | 22.8 ± 3.6 | 23.3 ± 5.3 | 0.6846 |
| Systolic blood pressure (mmHg; mean ± SD) | 126.0 ± 16.3 | 122.1 ± 16.6 | 131.7 ± 14.6 | 0.0432 |
| Diastolic blood pressure (mmHg; mean ± SD) | 78.8 ± 11.5 | 76.6 ± 12.9 | 82.0 ± 8.5 | 0.1065 |
| Serum albumin (g/dl; mean ± SD) | 4.2 ± 0.4 | 4.3 ± 0.4 | 4.1 ± 0.4 | 0.1139 |
| Serum creatinine (mg/dl; mean ± SD) | 0.9 ± 0.3 | 0.8 ± 0.2 | 1.1 ± 0.4 | 0.0026 |
| eGFR (ml/min/1.73 m2; mean ± SD) | 70.7 ± 22.3 | 78.9 ± 18.5 | 58.7 ± 22.4 | 0.0012 |
| IgA (mg/dl; mean ± SD) | 306.9 ± 115.3 | 309.6 ± 125.6 | 302.9 ± 102.0 | 0.8479 |
| Urinary protein (g/gCr; mean ± SD) | 0.5 ± 1.0 | 0.3 ± 0.3 | 0.9 ± 1.4 | 0.0246 |
| Urinary red blood cell (/HPF; mean ± SD) | 7.0 ± 16.4 | 7.9 ± 19.2 | 5.6 ± 11.6 | 0.6305 |
| RAS-I medication rate (%) | 79.6 | 75.9 | 85 | 0.4459 |
| Anamnesis of tonsillectomy rate (%) | 44.9 | 44.8 | 45.0 | 0.9907 |
| Anamnesis of steroid therapy rate (%) | 75.5 | 75.9 | 75.0 | 0.9464 |
| Duration from kidney biopsy (month;mean ± SD) | 73.4 ± 55.1 | 65.9 ± 46.9 | 84.3 ± 64.8 | 0.2554 |
BMI: body mass index, eGFR: estimated glomerular filtration rate, RAS-I: renin-angiotensin system inhibitor. Bold values indicate statistical significance at p < 0.05.
cnm-positivity and the DMFT index are important factors for proteinuria in IgAN patients
A total of 49 IgAN patients who underwent evaluation of dental caries status were divided into four groups according to cnm positivity and DMFT index value as follows: A: cnm-negative S. mutans and low DMFT group (n = 23), B: cnm-negative S. mutans and high DMFT group (n = 12), C: cnm-positive S. mutans and low DMFT group (n = 6) and D: cnm-positive S. mutans and high DMFT group (n = 8). Group D (1.7 ± 2.0 g/g creatinine) was significantly associated with higher urinary protein levels compared with all other groups (Group A: 0.3 ± 0.3 g/g creatinine, p = 0.0001; Group B: 0.4 ± 0.5 g/g creatinine, p = 0.0013; and Group C: 0.4 ± 0.2 g/g creatinine, p = 0.0047) (Fig. 3A), although Group D was not significantly associated with higher age compared with all other groups (Fig. 3B). In addition, Group B was significantly associated with higher age compared with Group A (p = 0.0059).
Figure 3. Relationship between proteinuria and cnm positivity and DMFT status.

(A) Group D was significantly associated with higher urinary protein levels than all other groups (*p < 0.05). (B) Group D was not significantly associated with higher age than all other groups. A: cnm-negative S. mutans and low DMFT group (n = 23), B: cnm-negative S. mutans and high DMFT group (n = 12), (C) cnm-positive S. mutans and low DMFT group (n = 6) and (D) cnm-positive S. mutans and high DMFT group (n = 8). DMFT: decayed, missing or filled teeth. The high DMFT group was designated as patients having a DMFT index of 15 or greater. The low DMFT group was designated as patients having a DMFT index of lower than 15.
Discussion
To our knowledge, this is the first study demonstrating that cnm positivity and dental caries status are associated with urinary protein levels in IgAN patients. Although several studies using in vivo approaches in the 1980s and 1990s hypothesized a correlation between S. mutans and nephritis19,20, there have been no reports regarding comparable human data. There are currently no established disease-targeted treatments for IgAN because the precise pathogenic mechanism involved remains unclear4. Therefore, we believe that the strong correlation between S. mutans and IgAN presented in this study could provide important information for future research regarding IgAN therapy.
The present study clearly demonstrated that IgAN patients harbouring cnm-positive S. mutans strains in the oral cavity showed significantly higher DMFT index values and urinary protein levels compared with the cnm-negative S. mutans group. In addition, the cnm-positive S. mutans group showed significantly lower serum albumin levels, higher serum total cholesterol levels and higher blood pressure than the cnm-negative group. In general, proteinuria causes hypoalbuminemia and hypercholesterolemia, both of which are sometimes seen in IgAN21. Hypertension in IgAN is also associated with renal damage22. Therefore, it is reasonable to consider that these clinical data also strengthen our hypothesis, in which cnm-positive S. mutans strains present in the oral cavity aggravate IgAN symptoms. However, the precise mechanism by which the presence of cnm-positive S. mutans strains in the oral cavity influences serum albumin, cholesterol and blood pressure in IgAN patients remains to be elucidated. The present study also demonstrated that cnm-positive strains were significantly more prevalent in the IgAN group than in the non-diseased control group, and the prevalence of dental caries was significantly higher in the IgAN group than in the non-diseased control group. These data suggest that IgAN patients may have more dental caries than non-diseased controls, which may exacerbate IgAN conditions. However, other factors such as socioeconomic status may be involved and should be analysed in future studies.
The concept of the ‘kidney-gut axis’ has recently gained attention23,24 and there is increasing clinical evidence that patients with chronic kidney disease have a distinctly dysbiotic intestinal bacterial community (termed ‘gut microbiota’). This, in turn, drives a cascade of metabolic abnormalities, including uremic toxin production, inflammation and immunosuppression, which ultimately promote progressive kidney failure and cardiovascular disease23. Marked differences in gut microbiota composition were found between healthy controls and patients with end-stage renal disease using phylogenetic microarrays25. Some researchers have also suggested a novel hypothesis for the ‘intestine-kidney connection’ in IgAN17,26. A defective immune tolerance might favour an abnormal response to microbiota with alterations of the intestinal barrier, including increased alimentary antigens and bacterial toxins absorption, triggering mucosal-associated lymphoid tissue activation and subclinical intestinal inflammation26. This can produce an abnormal response to alimentary antigens or commensal microbes with synthesis of aberrantly glycosylated polymeric IgA1, which eventually enters the circulation with renal deposit formation26. Because S. mutans is a major pathogen of dental caries, our data provide evidence for a new concept of ‘oral-kidney association’. Future experimental approaches should focus on determining how oral infectious pathogens induce or aggravate IgAN. We believe that the strong correlation between S. mutans and IgAN shown in the present study could provide a new breakthrough for future prevention and intervention for IgAN.
Interestingly, we found that a high DMFT value (designated as values of 15 or greater) was associated with high urinary protein levels and low renal function in IgAN patients, suggesting that IgAN patients who have elevated dental caries experience could have higher urinary protein levels and lower renal function. When analysing possible factors that influence proteinuria (i.e., cnm positivity and high DMFT index value), both factors were clearly important. Because it is true that older subjects tend to have higher DMFT index values, it is possible that age itself may influence IgAN severity. However, the cnm-positive and high DMFT group was not significantly associated with higher age compared with all other groups (Fig. 3B). We also performed subsequent logistic regression analyses adjusted for age and sex, and our findings led us to consider that patients with these two factors may exhibit severe IgAN aggravation.
Unfortunately, the present study did not demonstrate sufficient evidence to determine the possible mechanism by which S. mutans strains, especially cnm-positive strains, contribute to the development of IgAN. However, we speculate that this may involve the following hypotheses. First, frequent, repeated cnm-positive S. mutans immunoreactions with IgA in mucosal tissues of the oral cavity might induce a glycosylation defect in serum IgA1 molecules, which play an important role in the pathogenesis of IgAN. Many studies have revealed that a glycosylation deficiency in IgA1 molecules, usually with reduced galactose and sialic acid content but increased exposure of N-acetylgalactosamine, is a primary characteristic of IgAN4,27,28. This pattern of glycosylation mostly affects polymeric IgA1 produced in mucosal tissues4. Second, cnm-positive S. mutans strains or Cnm antigen itself may bind to mesangial cells, endothelial cells or glomerular basement membrane directly and induce mesangial cell proliferation and extracellular matrix expansion or endocapillary damage transiently. In general, severe dental caries results in the destruction of enamel and/or dentine on the tooth surface, enabling cnm-positive S. mutans access to the bloodstream. Further studies are required to elucidate the detailed mechanisms involved.
It is unclear why age appeared to be a significant factor in the cnm-positive IgAN group compared with the cnm-negative IgAN group. Generally, S. mutans is acquired in the oral cavity from mother’s saliva in early childhood, and cnm-positive S. mutans strains were demonstrated to be transmitted from mothers to their children29. It is possible that cnm-positive strains could be transmitted from those in close contact via saliva in adults. Although there are no reports investigating age as a factor in the distribution rates of cnm-positive strains, it is reasonable to speculate that the distribution rate could increase in older-aged subjects. In fact, it has been suggested that the positivity rate of cnm-positive S. mutans in older people (mean 70.3 years) was relatively higher (36.7%) than in the general population30. We collected specimens from outpatients in our hospital and thus selection bias may exist. For instance, stable IgAN patients were not included in these studies, but rather aged, severe long-term sufferers. However, we attempted to avoid such bias by performing logistic regression analyses adjusted for age.
It should be noted that there are some limitations associated with this study. First, we only showed that cnm positivity and dental caries status were associated with increased urinary protein levels in IgAN patients. However, it is possible that IgAN patients are susceptible to cnm-positive S. mutans strains. Whether persistent cnm-positive S. mutans infection can induce IgAN must be confirmed using experimental rodent models. Second, it remains unclear whether S. mutans directly contributes to the development of IgAN. Ultimately, the precise pathogenic mechanism of IgAN induction must be determined. Third, we were unable to perform additional analyses to evaluate potential confounders, such as diet and socioeconomic status, in the present study. Fourth, the cnm-positive S. mutans rate in other kidney diseases remains unclear. Finally, this study enrolled a relatively small number of IgAN patients from a single ethnic group. Prospective and larger studies should be designed to confirm our results.
Methods
Subjects and clinical data
The subjects were IgAN patients who are outpatients of Seirei Hamamatsu General Hospital, Hamamatsu, Japan. These patients were diagnosed with IgAN by previous renal biopsies. The histological diagnosis was made based on light microscopy and immunohistochemistry findings. Patients who had secondary IgAN diseases, such as IgA vasculitis (Henoch-Schonlein purpura nephritis) or lupus nephritis, were excluded from this study. IgAN patients undergoing steroid or immunosuppression therapy were also excluded from this study. The clinical data (urinary protein excretion and urinary occult blood by dipsticks, urinary protein excretion/urinary creatinine excretion, urinary red blood cell, serum creatinine, estimated glomerular filtration rate (eGFR), serum IgA, serum albumin, total cholesterol, blood pressure, height and body weight) were collected at the time of informed consent.
Age-matched healthy subjects were included as the non-diseased control group. All subjects were confirmed to have normal kidney function (serum Cr < 1.2 mg/dl, no proteinuria and no haematuria), were not taking regular medications and had no history of any other diseases, such as diabetes mellitus, hypertension, stroke, heart failure, rheumatoid arthritis, liver disease, gastrointestinal disease or anaemia.
Isolation of S. mutans strains from saliva specimens
Non-stimulated expectorated whole saliva specimens were collected from a total of 109 IgAN patients and 61 saliva specimens from control subjects from October 2013 to March 2016 using a sterile plastic tube and stored at −20 °C. S. mutans strains were isolated within a month after collection and confirmed as previously described31. Briefly, saliva specimens (approximately 1 ml from each subject) were diluted and streaked onto Mitis Salivarius agar plates (Difco Laboratories, Detroit, MI, USA) containing bacitracin (0.2 U/ml; Sigma-Aldrich, St. Louis, MO, USA) and 15% (wt/vol) sucrose and incubated at 37 °C for 2 days anaerobically. Five colonies from each plate were randomly selected on the basis of rough colony morphology and stocked. To differentiate S. mutans from Streptococcus sobrinus, colony morphology on agar plates was initially evaluated. Rough and smooth colonies were considered to be S. mutans and S. sobrinus, respectively. S. mutans strains were then confirmed via PCR using glucosyltransferase (gtf)-based primer sets as described below.
Bacterial DNA extraction and PCR detection of cnm
Strains were grown in brain heart infusion medium (Difco) at 37 °C for 18 hours, and genomic DNA was then extracted using conventional methods. Then, PCR was performed to confirm that these strains were S. mutans using an S. mutans-specific primer set (Forward: 5′–GGC ACC ACA ACA TTG GGA AGC TCA GTT–3′, Reverse: 5′–GGA ATG GCC GCT AAG TCA ACA GGA T–3′), as described previously14. The S. mutans-specific primer set for PCR was designed based on the sequence of glucosyltransferase gtf gene to differentiate S. mutans from S. sobrinus. PCR was also performed to detect cnm-positive strains using a cnm-specific primer set (Forward: 5′–GAC AAA GAA ATG AAA GAT GT–3′, Reverse: 5′–GCA AAG ACT CTT GTC CCT GC–3′), as described previously14.
Evaluation of dental caries status
The dental caries status of 49 IgAN patients and 49 non-diseased control subjects who provided signed informed consent for dental consultation was evaluated by a single dentist (AF) using conventional methods at the Department of Dentistry, Seirei Hamamatsu General Hospital. The clinical dental examination included the number of total teeth present and the presence of decayed teeth (DT), missing teeth (MT) and filled teeth (FT). Next, the DMFT index with the total numbers of D, M and F was calculated as described previously32.
Ethics regarding subjects
This study was conducted in full adherence with the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, 2013), and study protocols were approved by the Ethics Committee of Seirei Hamamatsu General Hospital (approval no. 1807) and Osaka University Graduate School of Dentistry (approval no. H25-E24). All subjects were informed of the protocols and gave their written consent prior to participating in the study.
Statistical analysis
All results are expressed as the mean ± standard deviation (SD). When a significant difference was found, further statistical analysis was performed using Fisher’s PLSD test, the Mann–Whitney U test or Fisher’s exact test and logistic regression analysis between groups. A two-tailed p-value of 0.05 was considered significant. Statistical analyses were performed using STATVIEW software (SAS Institute Inc., Cary, NC, USA) and SAS 9.1 software (SAS Institute Inc.).
Additional Information
How to cite this article: Misaki, T. et al. Presence of Streptococcus mutans strains harbouring the cnm gene correlates with dental caries status and IgA nephropathy conditions. Sci. Rep. 6, 36455; doi: 10.1038/srep36455 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 15K15754. We appreciate the assistance of Prof. Howard K. Kuramitsu (State University of New York at Buffalo) in editing this manuscript and Dr. Yoichiro Homma (Seirei Hamamatsu General Hospital) for assistance with statistical analysis.
Footnotes
Author Contributions T.M. designed the entire study under the supervision of K.N. A.F. evaluated the patients’ dental caries status. S.N., R.H. and R.N. performed molecular biological experiments. T.M., S.N., R.N., T.I. and K.N. interpreted the data. T.M. and K.N. wrote the manuscript, which all authors read and approved.
References
- D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64, 709–727 (1987). [PubMed] [Google Scholar]
- Julian B. A., Waldo F. B., Rifai A. & Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am J Med 84, 129–132 (1988). [DOI] [PubMed] [Google Scholar]
- Donadio J. V. & Grande J. P. IgA nephropathy. New Eng J Med 347, 738–748 (2002). [DOI] [PubMed] [Google Scholar]
- Wyatt R. J. & Julian B. A. IgA nephropathy. New Eng J Med 368, 2402–2414 (2013). [DOI] [PubMed] [Google Scholar]
- Suzuki S., Nakatomi Y., Sato H., Tsukada H. & Arakawa M. Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet 343, 12–16 (1994). [DOI] [PubMed] [Google Scholar]
- Kusano K., Tokunaga O., Ando T. & Inokuchi A. Helicobacter pylori in the palatine tonsils of patients with IgA nephropathy compared with those of patients with recurrent pharyngotonsillitis. Hum pathol 38, 1788–1797 (2007). [DOI] [PubMed] [Google Scholar]
- Iwama H., Horikoshi S., Shirato I. & Tomino Y. Epstein-Barr virus detection in kidney biopsy specimens correlates with glomerular mesangial injury. Am J Kidney Dis 32, 785–793 (1998). [DOI] [PubMed] [Google Scholar]
- Koyama A. et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 66, 121–132 (2004). [DOI] [PubMed] [Google Scholar]
- Rollino C., Vischini G. & Coppo R. IgA nephropathy and infections. J Nephrol 30(3), 360–366 (2016). [DOI] [PubMed] [Google Scholar]
- Nagasawa Y. et al. Periodontal disease bacteria specific to tonsil in IgA nephropathy patients predicts the remission by the treatment. PloS one 9, e81636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima A. et al. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci Rep 2, 332 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K. & Ooshima T. Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol 4, 891–902 (2009). [DOI] [PubMed] [Google Scholar]
- Nomura R. et al. Potential high virulence for infective endocarditis in Streptococcus mutans strains with collagen-binding proteins but lacking PA expression. Arch Oral Biol 58, 1627–1634 (2013). [DOI] [PubMed] [Google Scholar]
- Nakano K. et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun 2, 485 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonomura S. et al. Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. Sci Rep 6, 20074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka S. et al. A specific Streptococcus mutans strain aggravates non-alcoholic fatty liver disease. Oral Dis 20, 700–706 (2014). [DOI] [PubMed] [Google Scholar]
- Floege J. & Feehally J. The mucosa-kidney axis in IgA nephropathy. Nat Rev Nephrol 12, 147–156 (2016). [DOI] [PubMed] [Google Scholar]
- Misaki T. et al. Distribution of Streptococcus mutans strains with collagen-binding proteins in the oral cavity of IgA nephropathy patients. Clin Exp Nephrol 19, 844–850 (2015). [DOI] [PubMed] [Google Scholar]
- Albini B., Nisengard R. J., Glurich I., Neiders M. E. & Stinson M. W. Streptococcus mutans-induced nephritis in rabbits. Am J Pathol 118, 408–418 (1985). [PMC free article] [PubMed] [Google Scholar]
- Miyata M. et al. Streptococcus-mutans-induced nephritis in rabbits: rheumatoid factors and nephritogenicity. Int Arc Allergy Immunol 108, 360–367 (1995). [DOI] [PubMed] [Google Scholar]
- Kim J. K. et al. Clinical features and outcomes of IgA nephropathy with nephrotic syndrome. Clin J Am Soc Nephrol 7, 427–436 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixnerova D. et al. The retrospective analysis of 343 Czech patients with IgA nephropathy–one centre experience. Nephrol Dial Transplant 27, 1492–1498 (2012). [DOI] [PubMed] [Google Scholar]
- Rossi M., Johnson D. W. & Campbell K. L. The Kidney-Gut Axis: Implications for Nutrition Care. J Renal Nutr 25, 399–403 (2015). [DOI] [PubMed] [Google Scholar]
- Aron-Wisnewsky J. & Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol 12, 169–181 (2016). [DOI] [PubMed] [Google Scholar]
- Vaziri N. D. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int 83, 308–315 (2013). [DOI] [PubMed] [Google Scholar]
- Coppo R. The intestine-renal connection in IgA nephropathy. Nephrol Dial Transplant 30, 360–366 (2015). [DOI] [PubMed] [Google Scholar]
- Allen A. C. et al. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int 60, 969–973 (2001). [DOI] [PubMed] [Google Scholar]
- Tomana M. et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. The J Clin Invest 104, 73–81 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapirattanakul J. & Nakano K. Mother-to-child transmission of mutans streptococci. Future Microbiol 9, 807–823 (2014). [DOI] [PubMed] [Google Scholar]
- Miyatani F. et al. Relationship between Cnm-positive Streptococcus mutans and cerebral microbleeds in humans. Oral Dis 21, 886–893 (2015). [DOI] [PubMed] [Google Scholar]
- Nakano K., Nomura R., Nakagawa I., Hamada S. & Ooshima T. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J Clin Microbiol 42, 198–202 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinkhorn A. S. & Davies R. M. Caries prevention. A continued need worldwide. Int Dental J 46, 119–125 (1996). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


