Abstract
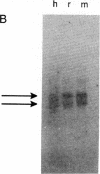
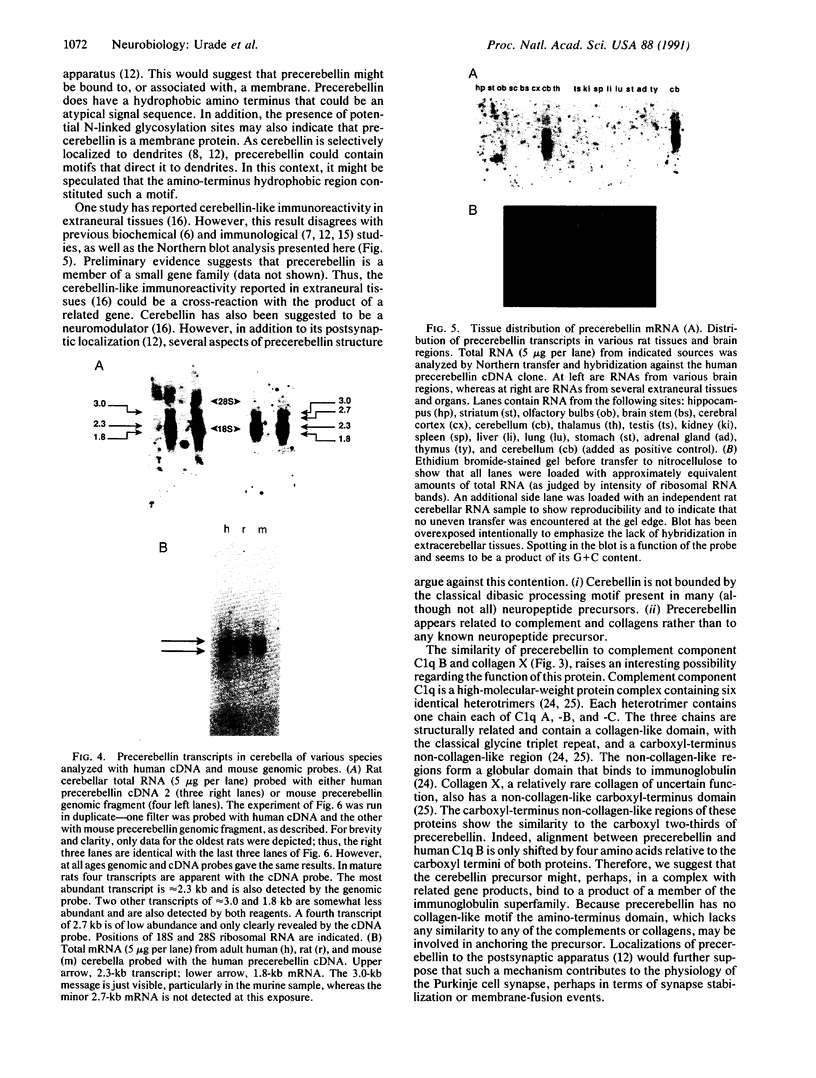
The cerebellum contains a hexadecapeptide, termed cerebellin, that is conserved in sequence from human to chicken. Three independent, overlapping cDNA clones have been isolated from a human cerebellum cDNA library that encode the cerebellin sequence. The longest clone codes for a protein of 193 amino acids that we term precerebellin. This protein has a significant similarity (31.3% identity, 52.2% similarity) to the globular (non-collagen-like) region of the B chain of human complement component C1q. The region of relatedness extends over approximately 145 amino acids located in the carboxyl terminus of both proteins. Unlike C1q B chain, no collagen-like motifs are present in the amino-terminal regions of precerebellin. The amino terminus of precerebellin contains three possible N-linked glycosylation sites. Although hydrophobic amino acids are clustered at the amino terminus, they do not conform to the classical signal-peptide motif, and no other obvious membrane-spanning domains are predicted from the cDNA sequence. The cDNA predicts that the cerebellin peptide is flanked by Val-Arg and Glu-Pro residues. Therefore, cerebellin is not liberated from precerebellin by the classical dibasic amino acid proteolytic-cleavage mechanism seen in many neuropeptide precursors. In Northern (RNA) blots, precerebellin transcripts, with four distinct sizes (1.8, 2.3, 2.7, and 3.0 kilobases), are abundant in cerebellum. These transcripts are present at either very low or undetectable levels in other brain areas and extraneural structures. A similar pattern of cerebellin precursor transcripts are seen in rat, mouse, and human cerebellum. Furthermore, a partial genomic fragment from mouse shows the same bands in Northern blots as the human cDNA clone. During rat development, precerebellin transcripts mirror the level of cerebellin peptide. Low levels of precerebellin mRNA are seen at birth. Levels increase modestly from postpartum day 1 to 8, then increase more dramatically between day 5 and 15, and eventually reach peak values between day 21 and 56. Because cerebellin-like immunoreactivity is associated with Purkinje cell postsynaptic structures, these data raise interesting possibilities concerning the function of the cerebellin precursor in synaptic physiology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972 Aug;145(4):465–513. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Burnet P. W., Bretherton-Watt D., Ghatei M. A., Bloom S. R. Cerebellin-like peptide: tissue distribution in rat and guinea-pig and its release from rat cerebellum, hypothalamus and cerebellar synaptosomes in vitro. Neuroscience. 1988 May;25(2):605–612. doi: 10.1016/0306-4522(88)90262-x. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Slemmon J. R., Danho W., Hempstead J., Berrebi A. S., Mugnaini E. Cerebellin and related postsynaptic peptides in the brain of normal and neurodevelopmentally mutant vertebrates. Synapse. 1988;2(2):117–124. doi: 10.1002/syn.890020203. [DOI] [PubMed] [Google Scholar]
- Mugnaini E., Dahl A. L., Morgan J. I. Cerebellin is a postsynaptic neuropeptide. Synapse. 1988;2(2):125–138. doi: 10.1002/syn.890020204. [DOI] [PubMed] [Google Scholar]
- Mugnaini E., Morgan J. I. The neuropeptide cerebellin is a marker for two similar neuronal circuits in rat brain. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8692–8696. doi: 10.1073/pnas.84.23.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y., Gordon M., van der Rest M., Schmid T., Linsenmayer T., Olsen B. R. The developmentally regulated type X collagen gene contains a long open reading frame without introns. J Biol Chem. 1986 Apr 15;261(11):5041–5050. [PubMed] [Google Scholar]
- Nishikawa K., Nakashima H., Kanehisa M., Ooi T. Detection of weak sequence homology of proteins for tertiary structure prediction. Protein Seq Data Anal. 1987;1(2):107–116. [PubMed] [Google Scholar]
- Oberdick J., Smeyne R. J., Mann J. R., Zackson S., Morgan J. I. A promoter that drives transgene expression in cerebellar Purkinje and retinal bipolar neurons. Science. 1990 Apr 13;248(4952):223–226. doi: 10.1126/science.2109351. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Gagnon J., Frampton J. Completion of the amino acid sequences of the A and B chains of subcomponent C1q of the first component of human complement. Biochem J. 1982 Jun 1;203(3):559–569. doi: 10.1042/bj2030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Molecular cloning and characterization of the complementary DNA and gene coding for the B-chain of subcomponent C1q of the human complement system. Biochem J. 1985 Nov 1;231(3):729–735. doi: 10.1042/bj2310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L., Hempstead J., Morgan J. I. Molecular cloning of a neuron-specific transcript and its regulation during normal and aberrant cerebellar development. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5651–5655. doi: 10.1073/pnas.86.14.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmon J. R., Blacher R., Danho W., Hempstead J. L., Morgan J. I. Isolation and sequencing of two cerebellum-specific peptides. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6866–6870. doi: 10.1073/pnas.81.21.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmon J. R., Danho W., Hempstead J. L., Morgan J. I. Cerebellin: a quantifiable marker for Purkinje cell maturation. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7145–7148. doi: 10.1073/pnas.82.20.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmon J. R., Goldowitz D., Blacher R., Morgan J. I. Evidence for the transneuronal regulation of cerebellin biosynthesis in developing Purkinje cells. J Neurosci. 1988 Dec;8(12):4603–4611. doi: 10.1523/JNEUROSCI.08-12-04603.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A strategy of DNA sequencing employing computer programs. Nucleic Acids Res. 1979 Jun 11;6(7):2601–2610. doi: 10.1093/nar/6.7.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Yiangou Y., Burnet P., Nikou G., Chrysanthou B. J., Bloom S. R. Purification and characterisation of cerebellins from human and porcine cerebellum. J Neurochem. 1989 Sep;53(3):886–889. doi: 10.1111/j.1471-4159.1989.tb11787.x. [DOI] [PubMed] [Google Scholar]
- Ziai M. R., Sangameswaran L., Hempstead J. L., Danho W., Morgan J. I. An immunochemical analysis of the distribution of a brain-specific polypeptide, PEP-19. J Neurochem. 1988 Dec;51(6):1771–1776. doi: 10.1111/j.1471-4159.1988.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Ziai R., Pan Y. C., Hulmes J. D., Sangameswaran L., Morgan J. I. Isolation, sequence, and developmental profile of a brain-specific polypeptide, PEP-19. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8420–8423. doi: 10.1073/pnas.83.21.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]