Abstract
Objective
To investigate the safety, feasibility and efficacy of delayed cord clamping (DCC) compared with immediate cord clamping (ICC) at delivery among infants born at 22 to 27 weeks' gestation.
Study Design
This was a pilot, randomized, controlled trial in which women in labor with singleton pregnancies at 22 to 27 weeks' gestation were randomly assigned to ICC (cord clamped at 5 to 10 s) or DCC (30 to 45 s).
Results
Forty mother–infant pairs were randomized. Infants in the ICC and DCC groups had mean gestational ages (GA) of 24.6 and 24.4 weeks, respectively. No differences were observed between the groups across all available safety measures, although infants in the DCC group had higher admission temperatures than infants in the ICC group (97.4 vs 96.2 °F, P =0.04). During the first 24 h of life, blood pressures were lower in the ICC group than in the DCC group (P < 0.05), despite a threefold greater incidence of treatment for hypotension (45% vs 12%, P < 0.01). Infants in the ICC group had increased numbers of red blood transfusions (in first 28 days of life) than infants in DCC group (4.1 ± 3.9 vs 2.8 ± 2.2, P = 0.04).
Conclusion
Among infants born at an average GA of 24 weeks', DCC appears safe, logistically feasible, and offers hematological and circulatory advantages compared with ICC. A more comprehensive appraisal of this practice is needed.
Introduction
In most medical settings, clamping and cutting of the umbilical cord occurs within seconds following delivery.1 Suggested advantages of this immediate separation include active management of the third stage of labor, prompt resuscitation of the neonate and less volume overload.2,3 However, over the past decade, several important benefits of delayed umbilical cord clamping (DCC) have been observed for preterm infants.4–6 A recent Cochrane review (15 trials, 738 preterm infants) showed that DCC, compared with immediate cord clamping (ICC), resulted in higher hematocrits, less exposure to blood products, lower incidence of necrotizing enterocolitis and an almost 50% lower risk of intraventricular hemorrhage (IVH).7 However, the majority of evidence supporting DCC in preterm infants comes from infants >28 weeks' gestation and concerns that DCC will delay resuscitation and may worsen outcomes have precluded enrollment of infants born at lower gestational ages (GAs).8
Recently, the American College of Obstetricians and Gynecologists (ACOG), the American Academy of Pediatrics (AAP), and the World Health Organization (WHO) all issued statements supporting the practice of DCC at birth in preterm infants, but called for the need for a better understanding of the safety and feasibility of the practice among preterm infants born <28 weeks' gestation.9–11 Without evidence-based recommendations to guide clinical care, many obstetricians perform ICC for infants delivered <28 weeks' gestation.1 This study was designed to evaluate the safety and feasibility of DCC among infants born at 22 through 27 weeks' gestation.
The secondary objective was to evaluate the potential efficacy of DCC in comparison with ICC in this subgroup of preterm infants.
Methods
This was a randomized, controlled trial conducted at a single academic hospital (The Ohio State University Wexner Medical Center) between August 2009 and December 2013. All women with singleton pregnancies between 22.5 and 27.6 weeks' gestation who were admitted to the labor and delivery service were candidates for inclusion in the study. The determination of GA was based on best obstetrical estimate, including ultrasound performed by obstetrician or maternal–fetal medicine specialist. Women whose pregnancies were complicated by placental abruption, placental previa, multiple gestations, chromosomal abnormalities (including trisomy 21), known major congenital malformations, attending obstetrician refusal to participate or intent to withhold care were not eligible for enrollment. To ensure adequate time for patient consent and enrollment in the study, women enrolled were required to be admitted to the hospital at least 3 h before delivery. Each mother was provided a written document outlining the nature of the study. Written informed consent was obtained for all patients before their participation according to a protocol approved by The Ohio State University Wexner Medical Center institutional review board (IRB #09-00298).
Throughout the study, the principal investigator met weekly with nursing and ancillary staff to review the study protocol. The primary purpose of the sessions was to minimize communication errors among staff members regarding study procedures. A random number system was generated by a statistician not involved in the study. Laminated cards for randomization were maintained in sealed, opaque envelopes. Study personnel provided contact information to labor and delivery staff to notify them of potential study participants or the impending delivery of previously enrolled subjects. When called for a subject's impending delivery, the team member opened the next randomization card, notified the obstetrician performing the delivery of the group assignment, reviewed the study protocol, attended the delivery and timed the cord clamping.
Women were assigned to two groups: ICC or DCC. For the ICC group, the obstetrician clamped the umbilical cord immediately following delivery of the infant (<10 s). For the DCC, the obstetrician clamped the umbilical cord 30 to 45 s following delivery of the infant. During the delay, the infant was held in a sterile towel approximately 10 to 15 inches below the mother's introitus at vaginal delivery or below the level of the incision at cesarean section. A member of the research team notified the delivering physician regarding time elapsed in 5-s intervals. Following clamping of the umbilical cord, the infant was handed to the neonatology team for routine infant care. Per standard protocol at our institution, all births before 28 weeks' gestation are attended by a neonatologist.
The subsequent clinical management of infants was at the discretion of the attending neonatologist. As a result of the nature of the intervention, the study could not be blinded. However, the infant's group assignment (DCC or ICC) was not revealed to the primary team.
Prenatal and peripartum data were collected from the mothers' medical records by a study member who was unaware of the treatment group. None of the study members present at the time of randomization or aware of group assignment participated in the daily clinical care of study patients. Infant data were collected until the infants' discharge from the hospital. Time of cord clamping, placement of the infant during the intervention, and time and date of birth were collected in the delivery room. Safety variables obtained in the delivery room and upon admission to the neonatal intensive care unit were recorded. We also recorded maximum serum bilirubin levels and incidence of polycythemia (defined as venous hematocrit >65%).
Data on hematological outcomes included the following: hematocrit values (admission, 72 h, 2 weeks, 4 weeks, 8 weeks, discharge), numbers of blood transfusions at 28 days of life and throughout hospitalization. As the mode of delivery (cesarean section or vaginal delivery) may impact hematological outcomes,12 data on infants delivered by cesarean delivery and vaginal delivery were also considered separately. Phlebotomy loss (ml kg−1 birth weight) throughout first 28 days of life was also recorded. Research personnel recorded hourly mean arterial blood pressure values from umbilical arterial catheters or peripheral arterial lines. Treatment for hypotension was defined as the following: (1) normal saline bolus; (2) dopamine ≥ 5 μg kg−1 min−1; or (3) hydrocortisone.
Neonatal morbidities during initial hospitalization were recorded. Early-onset sepsis was based on identification of positive blood or urine culture within 72 h of birth, while late-onset sepsis after 72 h of birth. Bronchopulmonary dysplasia was defined as requiring oxygen therapy at 28 days of life. Necrotizing enterocolitis was defined as stage 2 or greater, based on grading system developed by Bell et al.13 Retinopathy of prematurity was determined by an attending ophthalmologist during routine screening, per institutional protocol. Head ultrasound readings were interpreted by a pediatric radiologist blinded to the infant's group assignment based on the grading system developed by Papile et al.14
Statistical analysis
Sample sizes of 16 infants in each group were necessary to detect an increase in the hematocrit by 15% with a power of 80% and α of 5%. This calculation was based on the hematocrit differences reported in the trial by Oh et al.15 An oversampling of 25% brought each group to 20 infants for a total of 40 subjects. Group comparisons for continuous variables were made using the Student's t-test. For nonparametric analyses, chi-squared or Fisher's exact tests were used for categorical variables, and the Mann–Whitney test was used for continuous variables. Mean blood pressures were compared by analysis of repeated measures. Data are expressed as means with s.d. or s.e.m. All analyses were conducted with two-tailed tests. P-values <0.05 were considered statistically significant.
Results
There were 362 women with singleton pregnancies who were admitted to labor and delivery service and eligible for study participation during the study period (Figure 1). Forty infants were randomized to DCC or ICC groups at delivery.
Figure 1.
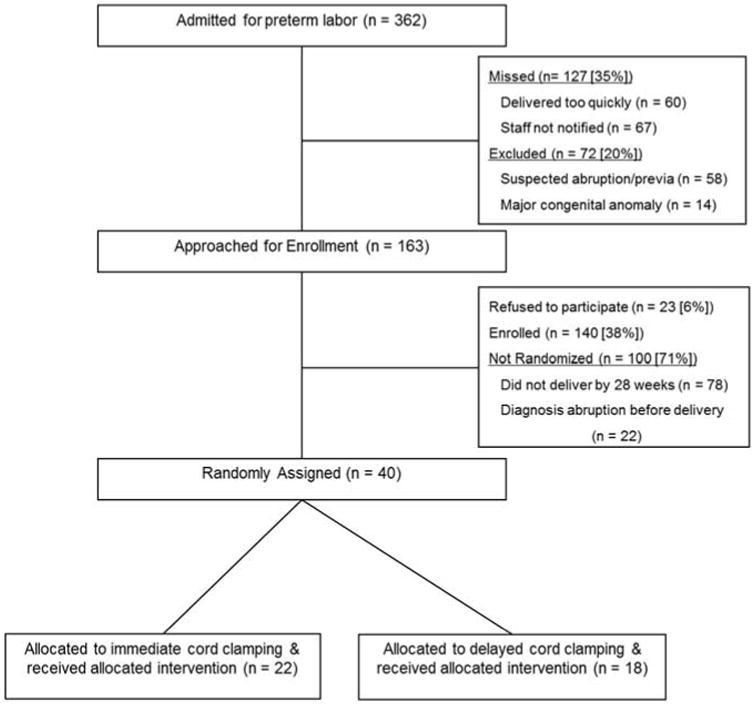
Flow diagram for patient selection, inclusion and exclusion from the study.
No differences in maternal demographics or characteristics between the two groups were observed (Supplementary Table 1). All mothers received at least one dose of antenatal steroids ≥3 h before delivery. In addition, over half of mothers in both groups (59% in ICC and 78% in DCC groups) received antenatal magnesium for neuroprotection. There were no differences in the reasons for preterm birth between the two groups. We observed no differences in risk of adverse events among mothers, including the incidence of postpartum hemorrhage.
No differences in infant characteristics between the two groups were observed (Table 1). The average GAs of infants in the two groups were 24.6 weeks for the ICC group and 24.4 weeks for the DCC group, respectively; 11 (28%) infants were born at 22 to 23 weeks' gestation, 16 (40%) infants were born at 24 to 25 weeks' gestation and 13 (33%) infants were born at 26 to 27 weeks' gestation. Over half (21/40, 53%) of the cohort in both groups had birth weights <600 g. No infants assigned to DCC received ICC to expedite resuscitation, as was specified in the study protocol to be allowed if indicated by clinical appearance of the infant at the time of delivery. As expected, cord clamping times were greater in the DCC group than in the ICC group.
Table 1. Infant characteristics.
| Characteristics | ICC (N = 22) | DCC (N = 18) | P-value |
|---|---|---|---|
| Gestational age (weeks) | 24.6 ± 1.1 | 24.4 ± 1.2 | NS |
| Birth weight (g) | 634 ±160 | 645 ± 193 | NS |
| Male gender, n (%) | 11 (50) | 12 (67) | NS |
| Small for gestational age, n (%) | 6 (27) | 7 (39) | NS |
| Cord clamping time (s) | 3.8 ± 1.0 | 37.4 ± 5.7 | <0.01 |
Abbreviations: DCC, delayed cord clamping; ICC, immediate cord clamping; NS, not statistically different. Data presented as mean ± s.d. unless otherwise stated.
We observed no significant differences in markers of infant safety between the two groups (Table 2). In the delivery room, we observed no differences between the DCC or ICC groups in the proportion of infants who received supplementary oxygen (89% vs 91%, P = 0.83), intubated (83% vs 73%, P =0.68) or received surfactant (78% vs 64%, P = 0.53). Two infants in the ICC and one infant in the DCC group received chest compressions during resuscitation. These three infants, each with a birth weight <400 g, died in the delivery room and were excluded from subsequent analyses. Markers of infant safety on admission to the neonatal intensive care unit were also similar between the two groups, although admission temperatures were higher in the DCC infants than in the ICC group. No infants in either group developed polycythemia.
Table 2. Safety measures.
| Safety measures | ICC (N= 22) | DCC (N= 18) | P-value |
|---|---|---|---|
| Characteristics in delivery room | |||
| Umbilical artery pHa | 7.22 (7.08–7.34) | 7.25 (7.05–7.41) | 0.88 |
| 1-min Apgar scorea | 2 (0–8) | 2 (0–8) | – |
| 5-min Apgar scorea | 6 (0–9) | 6 (0–9) | – |
| Death in delivery room, n (%) | 2 (9) | 1 (6) | 0.67 |
| Characteristics on admissionb | |||
| pHc | 7.22 ± 1.2 | 7.24 ± 1.1 | 0.96 |
| pCO2c | 52.3 ±25.1 | 52.6 ± 20.2 | 0.97 |
| pO2c | 62.1 ± 32.4 | 65.3 ± 38.8 | 0.75 |
| c | 20.2 ± 4.4 | 22.2 ± 3.0 | 0.12 |
| Fraction of inspired oxygen received | 0.45 ± 0.3 | 0.35 ± 0.22 | 0.26 |
| Temperature (°F) | 96.2 ± 1.7 | 97.4 ± 1.8 | 0.04 |
| Maximum serum bilirubin | 5.2 ± 3.2 | 5.8 ± 1.1 | 0.47 |
| Received phototherapy, n (%) | 20 (100) | 17 (100) | – |
Abbreviations: DCC, delayed cord clamping; ICC, immediate cord clamping.
Median (range).
Data among 20 infants in the ICC group and 17 infants in the DCC group who survived the delivery room and were admitted to the neonatal intensive care unit.
Values are from arterial samples. None of the data are statistically different. Data are expressed as mean ± s.d. unless otherwise stated.
Hematological outcomes are listed in Table 3. The samples obtained before 72 h were arterial, whereas the remaining samples were venous. Hematocrit values were higher in DCC infants than in ICC infants during the first 72 h of life, but were not different at later time points. We observed no differences in hematocrit values based on mode of delivery (Supplementary Table 2). The numbers of blood transfusions received during the first 28 days of life was increased among ICC infants than DCC infants, whereas total numbers of blood transfusions received throughout the entire hospitalization were not different between the groups. Phlebotomy losses were similar in the DCC and ICC groups.
Table 3. Hematological outcomes.
| Age | ICC (N= 20)a | DCC (N= 17)b | P-value |
|---|---|---|---|
| Hematocrit at 2 h | 39.2 ± 5.8 | 43.4 ± 4.2 | 0.03 |
| Hematocrit at 72 h | 34.6 ± 4.7 | 40.0 ± 8.6 | 0.03 |
| Hematocrit at 2 weeks | 31.3 ± 5.7 | 33.6 ± 6.1 | 0.32 |
| Hematocrit at 4 weeks | 31.0 ± 5.9 | 31.9 ± 5.4 | 0.65 |
| Hematocrit at discharge | 31.2 ± 5.9 | 31.5 ± 5.6 | 0.88 |
| Number RBC transfusions first 28 days of life | 4.1 ± 3.9 | 2.8 ± 2.2 | 0.04 |
| Total no. of RBC transfusions | 5.6 ± 4.1 | 4.2 ± 3.8 | 0.11 |
| Phlebotomy loss/birth weight (ml kg−1)c | 31.2 ± 21.2 | 30.7 ± 16.9 | 0.84 |
Abbreviations: DCC, delayed cord clamping; ICC, immediate cord clamping; RBC, red blood cell.
Number 20 for the ICC group because two infants died in the delivery room.
Number 17 because one infant died in the delivery room.
Phlebotomy loss throughout first 28 days of life. Data are expressed as mean ± s.d.
Over the first 24 h of life, DCC infants had higher mean arterial blood pressures (mean difference of 4.13 mm Hg, 95% confidence interval 2.0 to 6.2, P < 0.01) than did ICC infants (Figure 2). During that same time period, infants in the ICC infants received treatment for hypotension at almost four times the frequency as infants in the DCC group (ICC 45% vs DCC 12%, P < 0.01). No differences in blood pressure or use of antihypotensive therapies were observed at later time points between the two groups.
Figure 2.
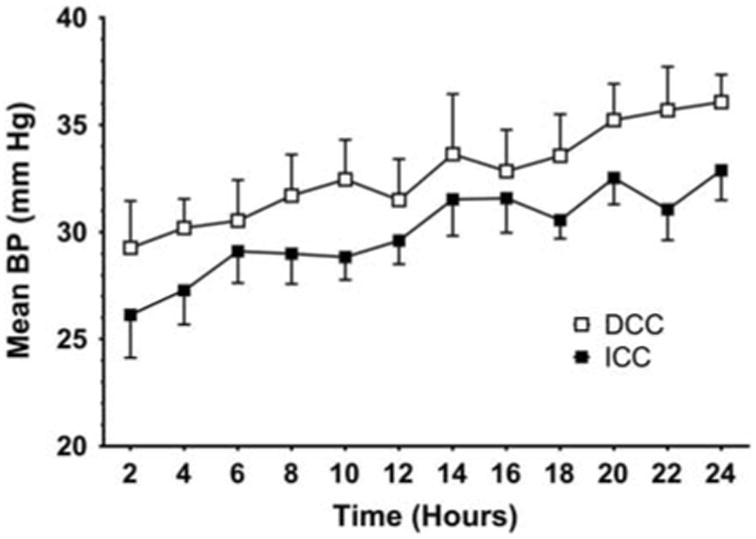
Changes in mean arterial blood pressure (mean ± s.d.) over time in infants receiving delayed cord clamping (DCC) compared with immediate cord clamping (ICC). Infants receiving DCC had significantly higher mean arterial blood pressures over the first 24 h of life than infants receiving ICC (analysis of variance, P = 0.01).
No differences in neonatal morbidities between the two groups were observed (Table 4). Although not statistically significant, there was greater than a twofold lower risk of severe IVH in the DCC group than in the ICC group (6% vs 20%). Similar numbers of infants in both groups received indomethacin for neuroprotection (ICC 8/20, 40% vs DCC 10/18, 55%). Two infants in the ICC group died beyond 30 days of life because of gastrointestinal perforation and Gram-negative sepsis, respectively. One infant in the DCC group died at day of life 49 because of Gram-negative sepsis.
Table 4. Neonatal morbidities.
| ICC (N= 20) | DCC (N= 17) | P-value | |
|---|---|---|---|
| Early-onset sepsis | 1 (5) | 0 (0) | 1.0 |
| Late-onset sepsis | 8 (40) | 8 (47) | 0.75 |
| Bronchopulmonary dysplasiaa | 15 (75) | 10 (59) | 0.48 |
| Necrotizing enterocolitisb | 4 (20) | 4 (24) | 0.79 |
| Spontaneous intestinal perforation | 0 (0) | 1 (6) | 0.46 |
| Medical treatment of PDA | 8 (40) | 10 (59) | 0.33 |
| Surgical treatment of PDA | 4 (20) | 3 (18) | 1.0 |
| Retinopathy of prematurity | 10 (50) | 10 (59) | 0.83 |
| Any IVH | 8 (40) | 6 (35) | 0.77 |
| Severe IVH (≥ grade 3)c | 4 (20) | 1 (6) | 0.34 |
| Periventricular leukomalacia | 3 (15) | 0 (0) | 0.23 |
| Length of initial hospitalization | 93 (69–125) | 99 (78–152) | 0.64 |
| Deathd | 2 (10) | 1 (6) | 1.0 |
Abbreviations: DCC, delayed cord clamping; ICC, immediate cord clamping; IVH, intraventricular hemorrhage; PDA, patent ductus arteriosus.
Oxygen requirement at 36 weeks' corrected gestational age.
Bell's stage 2 or greater.
Two infants in the ICC group had severe IVH and periventricular leukomalacia.
Deaths shown here are separate from those in the delivery room. None of the data are statistically different. Length of hospitalization expressed as median (range). All other data are expressed as no. (%).
Discussion
Evidence for improved outcomes following DCC in older infant groups led us to investigate the intervention among infants born at 22 through 27 weeks' gestation. The main finding of our study is that a brief (30 to 45 s) delay in umbilical cord clamping among infants with an average GA of 24 weeks' is safe, logistically feasible, and appears to offer hematological and circulatory benefits compared with ICC.
Some investigators have expressed concerns on the safety of DCC among neonates <28 weeks' gestation, citing the maneuver may worsen neonatal outcomes by imposing delays in resuscitation.16 A survey of US obstetricians found that 76% of respondents cite concerns regarding delays in neonatal resuscitation as the primary reason to perform ICC.17 Consistent with studies of more mature infants,6 we observed no short-term differences between the two groups across all available safety outcomes. However, the absence of differences between the two groups is not an adequate substitute for long-term studies adequately powered to detect changes in neurocognitive status and mortality.16
Previous studies have described violations of study protocol wherein preterm infants assigned to DCC receive ICC based on clinical signs and the perceived need for expedited resuscitation.18,19 In contrast, our study had no protocol violations at the time of delivery. These differences can be explained by the lack of objective criteria regarding successful transition at birth. Although we observed no differences in variables associated with neonatal transition, recent evidence suggests that enhanced placental transfusion may provide better cardiopulmonary transition at birth than does ICC.20,21 To characterize more robustly the variables associated with physiologic transition at birth, the use of data acquisition systems in the delivery room to monitor regional (cerebral, renal and splanchnic) blood flow and oxygenation status are needed.20 In addition, recent evidence suggests that establishing ventilation before cord clamping improves outcomes;22 thus, careful attention to breathing status during DCC will be important.23 To that end, maneuvers that incorporate active resuscitation with DCC or use umbilical cord milking as a strategy to increase placental transfusion at birth could have value, if appropriate patient groups could be identified and specific maneuvers optimized.24,25 An ongoing trial in the United Kingdom (UK CORD trial) is investigating the use of active resuscitation during DCC among very preterm infants.26
Another important issue to discuss is feasibility. Despite a dedicated staff for patient education and recruitment, a large number of mother–infant dyads who met eligibility criteria were either not approached to participate in the study or were enrolled in the study but not randomized at the time of delivery. Other investigators have noted similar logistical issues enrolling patients in umbilical cord clamping trials, including precipitous delivery of the infant.27 Although logistical issues required to conduct and execute a randomized trial on the timing of umbilical cord clamping are substantial, these issues are separate and distinct from those necessary to perform the intervention at the time of delivery.
Previous investigators have argued that DCC, compared with ICC, may increase the risk of hypothermia; a major risk to the survival or infants born <28 weeks' gestation.28,29 Our observation that infants in the DCC group had higher admission temperatures than did infants in the ICC group was unexpected and warrants explanation. Per institutional protocol, the use of wool hats, polyethylene bags and thermal gel mattresses are used in all deliveries of infants <28 weeks. However, although infants in the ICC group were transferred immediately to the open warmer following delivery, infants in the DCC groups were wrapped in warm sterile towels during the DCC procedure. We speculate that concerns for a potential increased risk of hypothermia among infants in the DCC group, compared with the ICC group, may have led to more vigilance by the medical team in maintaining temperature in the DCC group during all aspects of their immediate postpartum care. Provided the short-and long-term consequences of hypothermia, additional evidence-based guidelines to prevent heat loss among infants born <28 weeks' gestation are clearly needed.30
Consistent with previous studies,24,31 we observed that DCC resulted in higher hematocrits during the first 72 h after birth compared with ICC. This finding suggests that a delay of only 30 to 45 s provides an effective placental transfusion at the time of birth. In addition, DCC resulted in fewer blood transfusions (over first 28 days of life) compared with ICC. Although the decision to transfuse was left up to the practicing clinician, this finding is noteworthy, in view of the adverse effects of blood product exposures on short- and long-term outcomes (IVH and necrotizing enterocolitis) in preterm infants.32 However, even at a single institution with a guideline-driven approach to the use of blood products, lack of an ideal test for transfusion need led to considerable variability in blood transfusion rates in both groups. This may explain why we did not observe a reduction in total blood product exposure between the two groups throughout hospitalization.
We found that infants exposed to DCC, compared with ICC, had higher blood pressure in the first 24 h of life, which may have lowered the frequencies of blood pressures falling below ‘critical threshold values’ necessary for optimal hemodynamics.33 In addition, higher mean blood pressures in the DCC group decreased the proportion of infants exposed to antihypotensive therapy than the ICC group. Although the rationale for blood pressure treatment is based on a reported association between adverse short-term outcomes and hypotension,34 evidence on the benefits of treating hypotension early in life is controversial.35 In fact, a recent study from the National Institute of Child Health and Human Development (NICHD) showed that, among 367 extremely premature infants, infants treated with antihypotensive therapies were more likely to develop severe IVH compared with untreated infants (22% vs 11%, P < 0.01).36
Although our study was not powered to observe differences in neonatal morbidities, the twofold lower rates of severe IVH among infants exposed to DCC than with ICC are consistent with previous observations showing that enhanced placental transfusion may be neuroprotective.5,7 We speculate that DCC, and the provision of additional blood volume, stabilizes systemic and cerebral blood flow; the importance of which is magnified among infants born at the limits of viability who may lack cerebral autoregulation and therefore be uniquely vulnerable to fluctuations in cerebral perfusion and ischemia37,38 However, studies on the value of DCC in the prevention of IVH have been controversial.5,19,39,40 For example, a recent Cochrane review of preterm infants (<37 weeks' gestation) showed that DCC results in a reduction in any grade, but not severe, IVH.41 Safe modalities and strategies that are neuroprotective are desperately needed and warrant significant attention since infants born <28 weeks' gestation age are at highest risk for poor neurodevelopmental outcomes.42 Our findings are promising for this cohort and will be explored further.
Limitations
Our study is limited by a small number of patients, and confirmation of these results in a larger multicenter trial is desirable. A number of factors may limit the generalizability of our findings to all mother–infant dyads born <28 weeks' gestation. This study represents the experience at a single institution with an aggressive approach toward perinatal and neonatal intervention. Not all institutions, practitioners or parents would be comfortable with this level of intervention among neonates born at 22 and 23 weeks' gestations, especially when long-term outcomes among infants born at limits of viability are difficult to predict.43
This study was conducted in a tertiary care facility with highly trained personnel available to attend all deliveries. In an effort to maximize patient safety, we excluded mothers with placental previa/abruption and velamentous cord; therefore, our cohort likely represents a more stable subgroup of premature infants. Although we observed no differences in hematocrit values based on mode of delivery, our limited sample size make conclusions regarding negative results potentially due to lack of statistical power. Although we observed hematological and circulatory benefits in the first few weeks' of life for the present cohort, it will be important to assess whether these short-term benefits translate into a reduction in long-term morbidity.
Conclusion
In summary, our results suggest that a brief delay in cord clamping among infants with an average GA of 24 weeks is logistically feasible, appears to be safe, and offers a number of hematological and circulatory benefits. The results of this pilot study justify the need for a larger multicenter study to evaluate the potential benefits of enhanced placental transfusion among infants born at <28 weeks' gestation.
Supplementary Material
Acknowledgments
The present work is supported in part by a grant from the American Heart Association (# 10CRP3730033, CHB) and by internal funding provided by Nationwide Children's Hospital Research Institute. We wish to thank Jessica Deverse, BA, and all the nurses in the neonatal intensive care unit at The Ohio State University Wexner Medical Center for their efforts in patient recruitment.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Perinatology website (http://www.nature.com/jp)
References
- 1.Ononeze ABO, Hutchon DJR. Attitude of obstetricians towards delayed cord clamping: a questionnaire-based study. J Obstet Gynaecol. 2009;29(3):223–224. doi: 10.1080/01443610802712918. [DOI] [PubMed] [Google Scholar]
- 2.Maughan KL, Heim SW, Galazka SS. Preventing postpartum hemorrhage: managing the third stage of labor. Am Fam Physician. 2006;73(6):1025–1028. [PubMed] [Google Scholar]
- 3.Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labour. Cochrane Database Syst Rev. 2000;(3):CD000007. doi: 10.1002/14651858.CD000007. [DOI] [PubMed] [Google Scholar]
- 4.Baenziger O, Stolkin F, Keel M, von Siebenthal K, Fauchere JC, Das Kundu S, et al. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics. 2007;119(3):455–459. doi: 10.1542/peds.2006-2725. [DOI] [PubMed] [Google Scholar]
- 5.Mercer JS, Vohr BR, McGrath MM, Padbury JF, Wallach M, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late-onset sepsis: a randomized, controlled trial. Pediatrics. 2006;117(4):1235–1242. doi: 10.1542/peds.2005-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercer JS, McGrath MM, Hensman A, Silver H, Oh W. Immediate and delayed cord clamping in infants born between 24 and 32 weeks: a pilot randomized controlled trial. J Perinatol. 2003;23(6):466–472. doi: 10.1038/sj.jp.7210970. [DOI] [PubMed] [Google Scholar]
- 7.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012;8:CD003248. doi: 10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Strauss RG, Mock DM, Johnson KJ, Cress GA, Burmeister LF, Zimmerman MB, et al. A randomized clinical trial comparing immediate versus delayed clamping of the umbilical cord in preterm infants: short-term clinical and laboratory endpoints. Transfusion. 2008;48(4):658–665. doi: 10.1111/j.1537-2995.2007.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AAP. Timing of umbilical cord clamping after birth. Pediatrics. 2013;131(4):e1323. [Google Scholar]
- 10.Gynecology AACoOa. Committee opinion no. 543: timing of umbilical cord clamping after birth. Obstet Gynecol. 2012;120(6):1522–1526. doi: 10.1097/01.AOG.0000423817.47165.48. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Guidelines on Basic Newborn Resuscitation 2012. World Health Organization Press; Geneva: 2012. [PubMed] [Google Scholar]
- 12.Katheria AC, Truong G, Cousins L, Oshiro B, Finer NN. Umbilical cord milking versus delayed cord clamping in preterm infants. Pediatrics. 2015;136(1):61–69. doi: 10.1542/peds.2015-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 15.Oh W, Fanaroff AA, Carlo WA, Donovan EF, McDonald SA, Poole WK, et al. Effects of delayed cord clamping in very-low-birth-weight infants. J Perinatol. 2011;31(suppl):S68–S71. doi: 10.1038/jp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarnow-Mordi WO, Duley L, Field D, Marlow N, Morris J, Newnham J, et al. Timing of cord clamping in very preterm infants: more evidence is needed. Am J Obstet Gynecol. 2014;211:118–123. doi: 10.1016/j.ajog.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Jelin AC, Kuppermann M, Erickson K, Clyman R, Schulkin J. Obstetricians' attitudes and beliefs regarding umbilical cord clamping. J Matern Fetal Neonatal Med. 2013;11:11. doi: 10.3109/14767058.2013.864275. [DOI] [PubMed] [Google Scholar]
- 18.Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants' blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117(1):93–98. doi: 10.1542/peds.2004-1773. [DOI] [PubMed] [Google Scholar]
- 19.McDonnell M, Henderson-Smart DJ. Delayed umbilical cord clamping in preterm infants: a feasibility study. J Paediatr Child Health. 1997;33(4):308–310. doi: 10.1111/j.1440-1754.1997.tb01606.x. [DOI] [PubMed] [Google Scholar]
- 20.Katheria A, Blank D, Rich W, Finer N. Umbilical cord milking improves transition in premature infants at birth. PloS One. 2014;9(4):e94085. doi: 10.1371/journal.pone.0094085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katheria AC, Leone TA. Changes in hemodynamics after rescue surfactant administration. J Perinatol. 2013;33(7):525–528. doi: 10.1038/jp.2012.166. [DOI] [PubMed] [Google Scholar]
- 22.Ersdal HL, Linde J, Mduma E, Auestad B, Perlman J. Neonatal outcome following cord clamping after onset of spontaneous respiration. Pediatrics. 2014;134(2):265–272. doi: 10.1542/peds.2014-0467. [DOI] [PubMed] [Google Scholar]
- 23.Nevill E, Meyer MP. Effect of delayed cord clamping (DCC) on breathing and transition at birth in very preterm infants. Early Hum Dev. 2015;91(7):407–411. doi: 10.1016/j.earlhumdev.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, et al. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks' gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F14–F19. doi: 10.1136/adc.2006.108902. [DOI] [PubMed] [Google Scholar]
- 25.Hutchon DJ, Thakur I. Resuscitate with the placental circulation intact. Arch Dis Child. 2008;93(5):451. [PubMed] [Google Scholar]
- 26.Pushpa-Rajah A, Bradshaw L, Dorling J, Gyte G, Mitchell EJ, Thornton J, et al. Cord pilot trial - immediate versus deferred cord clamping for very preterm birth (before 32 weeks gestation): study protocol for a randomized controlled trial. Trials. 2014;15:258. doi: 10.1186/1745-6215-15-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oddie S, Rhodes P on behalf of the ‘Very Preterm Birth Qualitative Collaborative G. Barriers to deferred cord clamping in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2014;99:F391–F394. doi: 10.1136/archdischild-2014-305968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCall EM, Alderdice F, Halliday HL, Jenkins JG, Vohra S. Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst Rev. 2010;(3):CD004210. doi: 10.1002/14651858.CD004210.pub4. [DOI] [PubMed] [Google Scholar]
- 29.Laptook AR, Salhab W, Bhaskar B, Neonatal Research N. Admission temperature of low birth weight infants: predictors and associated morbidities. Pediatrics. 2007;119(3):e643–e649. doi: 10.1542/peds.2006-0943. [DOI] [PubMed] [Google Scholar]
- 30.DeMauro SB, Douglas E, Karp K, Schmidt B, Patel J, Kronberger A, et al. Improving delivery room management for very preterm infants. Pediatrics. 2013;132(4):e1018–e1025. doi: 10.1542/peds.2013-0686. [DOI] [PubMed] [Google Scholar]
- 31.March MI, Hacker MR, Parson AW, Modest AM, de Veciana M. The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. J Perinatol. 2013;33(10):763–767. doi: 10.1038/jp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen RD, Baer VL, Del Vecchio A, Henry E. Unique risks of red blood cell transfusions in very-low-birth-weight neonates: associations between early transfusion and intraventricular hemorrhage and between late transfusion and necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2013;26(Suppl 2):60–63. doi: 10.3109/14767058.2013.830495. [DOI] [PubMed] [Google Scholar]
- 33.Munro MJ, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004;114(6):1591–1596. doi: 10.1542/peds.2004-1073. [DOI] [PubMed] [Google Scholar]
- 34.Watkins AM, West CR, Cooke RW. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum Dev. 1989;19(2):103–110. doi: 10.1016/0378-3782(89)90120-5. [DOI] [PubMed] [Google Scholar]
- 35.Barrington KJ. Low blood pressure in extremely preterm infants: does treatment affect outcome? Arch Dis Child Fetal Neonatal Ed. 2011;96(5):F316–F317. doi: 10.1136/adc.2010.206599. [DOI] [PubMed] [Google Scholar]
- 36.Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Use of antihypotensive therapies in extremely preterm infants. Pediatrics. 2013;131(6):e1865–e1873. doi: 10.1542/peds.2012-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer MP, Mildenhall L. Delayed cord clamping and blood flow in the superior vena cava in preterm infants: an observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F484–F486. doi: 10.1136/adc.2010.199703. [DOI] [PubMed] [Google Scholar]
- 38.Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, Raker C, et al. Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics. 2012;129(3):e667–e672. doi: 10.1542/peds.2011-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaempf JW, Tomlinson MW, Kaempf AJ, Wu Y, Wang L, Tipping N, et al. Delayed umbilical cord clamping in premature neonates. Obstetrics and gynecology. 2012;120(2 Pt 1):325–330. doi: 10.1097/AOG.0b013e31825f269f. [DOI] [PubMed] [Google Scholar]
- 40.Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology. 2008;93(2):138–144. doi: 10.1159/000108764. [DOI] [PubMed] [Google Scholar]
- 41.Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst Rev. 2004;(4):CD003248. doi: 10.1002/14651858.CD003248.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Hack M. Survival and neurodevelopmental outcomes of preterm infants. J Pediatr Gastroenterol Nutr. 2007;45(Suppl 3):S141–S142. doi: 10.1097/01.mpg.0000302959.55428.05. [DOI] [PubMed] [Google Scholar]
- 43.Raju TN, Mercer BM, Burchfield DJ, Joseph GF., Jr Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(5):1083–1096. doi: 10.1097/AOG.0000000000000243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


