Abstract
OBJECTIVE
Delayed umbilical cord clamping (DCC) at birth may provide a better neonatal health status than early umbilical cord clamping (ECC). However, the safety and feasibility of DCC in infants with congenital heart disease (CHD) have not been tested. This was a pilot, randomized, controlled trial to establish the safety and feasibility of DCC in neonates with CHD.
STUDY DESIGN
Pregnant women admitted >37 weeks gestational age with prenatal diagnosis of critical CHD were enrolled and randomized to ECC or DCC. For ECC, the umbilical cord was clamped <10 s after birth; for DCC, the cord was clamped ~ 120 s after delivery.
RESULTS
Thirty infants were randomized at birth. No differences between the DCC and ECC groups were observed in gestational age at birth or time of surgery. No differences were observed across all safety measures, although a trend for higher peak serum bilirubin levels (9.2 ± 2.2 vs 7.3 ± 3.2 mg dl− 1, P = 0.08) in the DCC group than in the ECC group was noted. Although similar at later time points, hematocrits were higher in the DCC than in the ECC infants during the first 72 h of life. The proportion of infants not receiving blood transfusions throughout hospitalization was higher in the DCC than in the ECC infants (43 vs 7%, log-rank test P = 0.02).
CONCLUSION
DCC in infants with critical CHD appears both safe and feasible, with fewer infants exposed to red blood cell transfusions than with ECC. A more comprehensive appraisal of this practice is warranted.
INTRODUCTION
Physiological studies have shown that delayed umbilical cord clamping (DCC) results in an increase to the neonate of 20–40 ml of blood per kilogram of body weight,1–3 resulting in higher hematocrits,4,5 less need for red blood cell (RBC) transfusions6 and decreased frequency of iron-deficiency anemia7,8 than are observed with early cord clamping (ECC). On the other hand, DCC has been shown to increase the risk of neonatal jaundice,5,9,10 polcythemia4,7 and blood viscocity,5,10,11 compared with ECC. In light of the positive and negative effects on neonatal outcomes, the ideal timing for umbilical cord clamping has not been established. To address the controvery on optimal cord clamping practices, the American Academy of Pediatrics and American Collge of Obstetrics and Gynecology cited the need for trials on subgroups of neonates to better define specific risk/benefit profiles.12 One subgroup where DCC could be particularly beneficial, but also could be problematic, is neonates with congenital heart disease (CHD).
Without evidence-based recommendations on the hematocrit level needed to maintain optimal hemodynamics, many centers transfuse at a higher starting hematocrit for neonates with CHD.13 Although RBC transfusions augment tissue oxygen delivery by increasing the oxygen-carrying capacity of the blood and are an integral part of the perioperative management of infants with CHD,14 exposure to blood transfusion is associated with post-operative mortality and morbidity, including higher rates of infection,15 lengths of mechanical ventilation,16 longer hospital stays and increased hospital costs.17,18 Thus, strategies that can safely improve blood volume status and limit exposure to allogenic RBC transfusions are needed.
Although DCC may improve hematological status among neonates with CHD, these potential benefits must be weighed against the increased risk for volume overload, neonatal jaundice, and polycythemia.19–21 Since previous trials have excluded infants with CHD, a separate consideration of the potential risks and benefits of DCC in this unique subgroup of infants is needed. To minimize potential adverse outcomes, including polycythemia,22 our investigation was focused on neonates who require surgery or catheterization within the first month of life, or critical CHD.23
Here, we present the results of a pilot, randomized, controlled trial in infants with CHD to determine safety and feasibility of DCC compared with standard of care (ECC). A secondary goal was to evaluate if DCC influences hematological outcomes (perioperative hematocrits) and the need for RBC transfusions during surgical interventions and hospital care of the infants.
PATIENTS AND METHODS
The study was approved by the institutional review board (IRB #09-00496) at Nationwide Children’s Hospital (NCH). We enrolled patients at six delivery hospitals in our region. The study protocol was approved by the research ethics board at each participating institution, and for all patients, written informed consent was obtained from a parent.
Entry criteria
The study was conducted between August, 2010 and December, 2013. Criteria for study participation included: (1) singleton pregnancy; (2) term (>37 weeks) gestation; (3) fetus with critical CHD, defined as heart lesions likely to require surgery or cardiac catheterization within the first 30 days based on fetal echocardiogram. All infants were transferred immediately (<1 h following delivery) to NCH for subsequent management.
Maternal exclusion
Maternal exclusion criteria included placental abruption/previa, multiple gestations or a diagnosis of diabetes.
Fetal exclusion
Fetal exclusion criteria included those with known genetic/major extracardiac anomalies, including Trisomy 21. Based on the fetal echocardiogram, fetuses with an expected biventricular repair were enrolled, while those with single ventricle physiology were excluded. This methodology was intended to limit heterogeneity of the patient population.
Study logistics
A random number system was generated by a statistician not involved in the study. The study team shared an on-call schedule to screen potentially eligible mothers, enroll them and attend the delivery.
Placental transfusion technique
Impending deliveries were randomly assigned to the ECC or DCC groups. For the ECC group, the obstetrician clamped the umbilical cord immediately following delivery of the infant (<10 s), per standard protocol. For DCC, the obstetrician clamped the cord 110–130 s following delivery of the infant. During the delay, the infant was held in a sterile towel, ~ 0–15 inches below the mother’s introitus at vaginal delivery or below the level of the incision following cesarean section. After cutting of the cord, the infant was handed to the neonatology team for routine infant care. Health-care professionals who performed neonatal management after birth were not the ones present when the infant delivered and were not aware of the infant’s group assignment. Subsequent clinical management was at the discretion of the attending physicians.
Existing blood-conservation techniques
Strategies to reduce RBC exposure already in place at our institution include use of cell-salvage techniques, re-transfusion of autologous RBCs and avoidance of unnecessary blood sampling. Specifically, body weight-adapted cardiopulmonary bypass (CPB) circuits with a total priming volume of 185 ml were used for all infants in the cohort. The transfusion triggers during CPB were an estimated hemoglobin <7.0 g dl−1 for acyanotic infants and <10 g dl−1 for cyanotic infants. Moderate hypothermia was used, except for cases requiring deep hypothermic circulatory arrest, in which the temperature was decreased to 17–18 °C. Per institutional protocol, all RBC transfusions were fresh (<7 days old), leukoreduced, cytomegalovirus-negative and irradiated.
Data collected
Maternal and infant data were collected until initial discharge from the hospital. Polycythemia was defined as venous hematocrit >65%. Surgical data included CPB time, aortic cross-clamp time and deep hypothermic circulatory arrest time (DHCA). Post-operative data included the modified inotropic score (maximum score for the first 24 h after intervention).24 We recorded the number and types of laboratory tests for each enrolled infant. We assumed that with each blood draw, only the minimal blood volume required to run the tests was drawn and that no blood was wasted at the time of blood draws. Major neonatal morbidities were also recorded.
Statistical analysis
Using hematocrit at 72 h as the primary outcome variable and an expected 10% relative increase by DCC compared with ECC (α = 0.05, 80% power), we estimated the need for 12 infants in each arm of the study. An additional 20% or six subjects were enrolled to allow for attrition. Group comparisons for continuous variables were made using the Student’s t-test. For categorical variables, X2− and Fisher’s exact tests were used. Kaplan–Meier curves were used to evaluate the probability of being free from a blood transfusion in the DCC group and the ICC group, wherein differences were compared using the log-rank test. In patients who did not receive a blood transfusion, the time from birth to hospital discharge was used. Means and s.d./s.e.m. were used as descriptive measures for continuous variables with normal distributions, and medians and quartiles/ranges were used when the normality assumption was not met. Frequencies and percentages were reported for categorical variables. All analyses were conducted with two-tailed tests. A P-value < 0.05 was considered statistically significant.
RESULTS
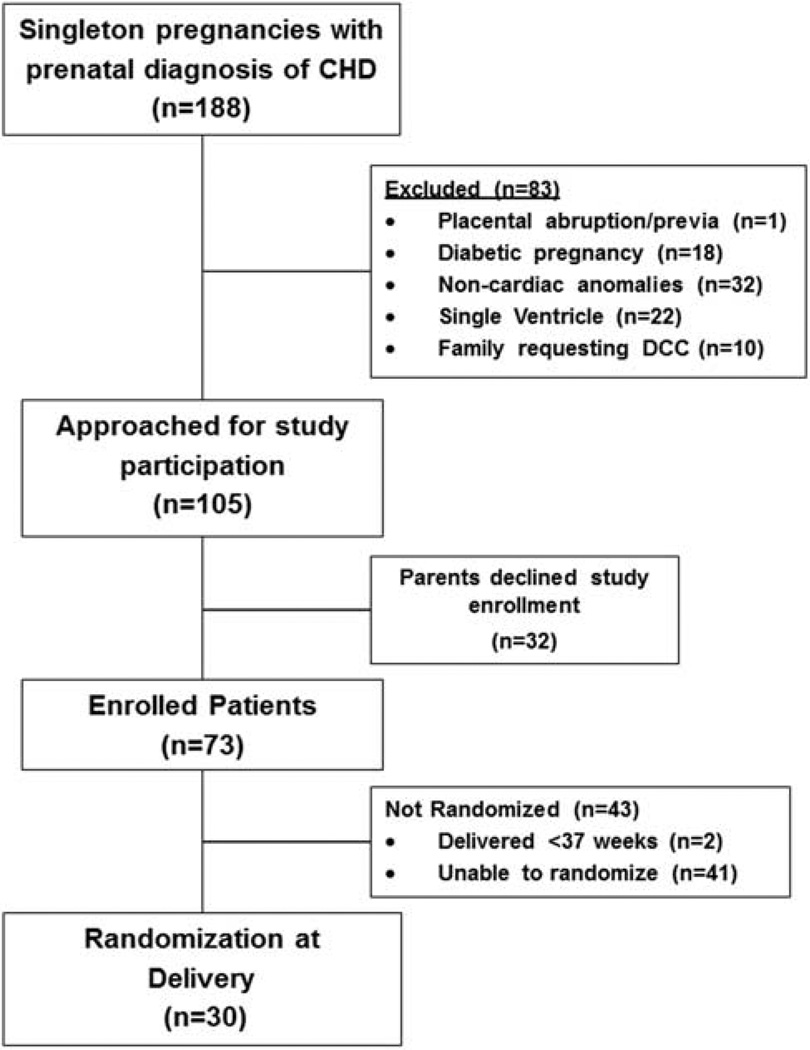
The distribution of 188 women with singleton pregnancies and fetal diagnoses of critical CHD who were admitted to labor and delivery service is shown in Figure 1. During the study’s first year (August 2010 to August 2011), 25 mother–infant dyads were enrolled in the study, but only 2 (8%) were randomized at the time of delivery. Following consultation with nursing leadership and maternal–fetal medicine colleagues, starting in September 2011, a number of steps were implemented to improve communication between clinical and research personnel at the six participating hospitals. First, once an enrolled mother was admitted to labor and delivery, a paging system was activated by clinical leaders to alert the research team of the potential delivery. The primary goal of the paging system was to notify research personnel early in the delivery process to allow sufficient time to attend the delivery. Second, in all delivery hospitals, placards with the principal investigator’s (CHB) contact information and the study protocol were placed in delivery rooms. Third, the principal investigator met monthly with nurse coordinators and nursing leadership at each hospital to review the study protocol to minimize communication errors among staff members regarding study procedure. With these efforts, between September 2011 and March 2013, of the 51 eligible mother–infant dyads consented and enrolled in the study, 28 (55%) were randomized at the time of delivery. Thirty infants were randomized to DCC or ECC groups at delivery.
Figure 1.
Flow diagram for patient selection, inclusion and exclusion from the study.
No differences in maternal demographics or characteristics between the two groups were observed (Table 1). Of note, only one infant in each group was delivered emergently because of fetal distress. We observed no differences between the two groups in risk of adverse events among mothers. Post-partum blood loss was 210 ml (first–third quartiles: 80–460) in the DCC group and 260 ml (first–third quartiles: 120–500) in the ECC group. Post-partum hemorrhage (blood loss > 500 ml) was 13.3% in the DCC group, compared with 6.7% in the ECC group. Severe postpartum hemorrhage (blood loss > 1000 ml) was not observed in either group.
Table 1.
Maternal demographics and clinical characteristics
| ECC (N = 15) | DCC (N = 15) | |
|---|---|---|
| Maternal age (years)a | 28.8 ± 5.7 | 27.9 ± 6.2 |
| Primiparous, n (%) | 6 (40) | 5 (33) |
| Caucasian, n (%) | 5 (33) | 9 (60) |
| Cesarean section, n (%) | 4 (27) | 4 (27) |
| Number of prenatal visitsa | 8 ± 2.4 | 8 ± 3.5 |
| Maternal anemia, n (%) | 1 (7) | 1 (7) |
| Maternal hematocrit before deliverya | 34.8 ± 3.6 | 34.0 ± 4.5 |
| Pregnancy induced hypertension, n (%) | 1 (7) | 0 (0) |
| Delivery due to fetal distress, n (%) | 1 (7) | 1 (7) |
Abbreviations: DCC, delayed umbilical cord clamping; ECC, early umbilical cord clamping.
None of the data are statistically different.
Mean ± s.d.
No differences in infant characteristics between the two groups were observed (Table 2). No infants that were assigned to DCC received immediate cord clamping to expedite resuscitation, as was specified in the study protocol to be allowed if deemed necessary by the attending obstetrician or neonatologist. One infant in each group had a postnatal echocardiogram consistent with noncritical congenital heart disease and thus was excluded from subsequent analysis. All other fetal and postnatal echocardiogram findings were consistent. We observed no differences in measures of infant safety between the two groups. Although not statistically significant, we observed a trend toward higher peak serum bilirubin levels in the DCC group than in ECC infants. No infant in either group had severe hyperbilirubinemia, defined as >20 (mg dl−1).25 Among the two infants in the DCC group who developed polycythemia, no adverse clinical events were reported, including shunt thrombosis, increased perioperative bleeding, or blood product use. Although not statistically significant, receipt of phototherapy exhibited a similar trend for more frequent use in DCC infants than in ECC infants (P = 0.08). The distribution of cardiac lesions was similar in the two groups.
Table 2.
Infant characteristics
| ECC (N = 15) | DCC (N = 15) | P-value | |
|---|---|---|---|
| Birth characteristics | |||
| Birth weight (kg)a | 3.4 ± 1.2 | 3.3 ± 0.7 | 0.78 |
| Gestational age (weeks) at birtha |
38.3 ± 1.4 | 39.2 ± 1.6 | 0.11 |
| Male gender, n (%) | 7 (47) | 5 (33) | 0.71 |
| Cord clamping time (s)a | 3.8 ± 1.0 | 114 ± 10.7 | 0.001 |
| No intervention, n (%) | 1 (7) | 1 (7) | — |
| Safety measuresb | |||
| 5-minute Apgar scorec | 8 (4–9) | 8 (4–9) | — |
| Peak serum bilirubin (mg dl−1)a |
7.3 ± 3.2 | 9.2 ± 2.2 | 0.08 |
| Polycythemia, n (%) | 0 (0) | 2 (17) | 0.48 |
| Phototherapy, n (%) | 1 (7) | 6 (43) | 0.08 |
| Pre-operative mortality, n (%) | 0 (0) | 0 (0) | — |
| Cardiac diagnosisb | |||
| RACHS 4–6, n (%)d | 3 (21) | 2 (14) | — |
| Cardiac lesionsb | |||
| Aortic arch anomaly | 3 (21) | 4 (29) | — |
| Tetralogy of Fallot ± pulmonary atresia |
3 (21) | 2 (14) | — |
| d-Transposition of great arteries |
5 (36) | 4 (29) | — |
| Pulmonary atresia intact ventricular septum |
0 (0) | 1 (7) | — |
| Critical pulmonary stenosis | 2 (14) | 2 (14) | — |
| Truncus arteriosus | 1 (7) | 1 (7) | — |
| Required repair w/CPB, n (%) | 11 (79) | 10 (71) | — |
Abbreviations: CPB, cardiopulmonary bypass; DCC, delayed umbilical cord clamping; ECC, early umbilical cord clamping.
Data are expressed number (percentage within each group) unless otherwise stated. P-value = 1.0.
Mean ± s.d.
Based on infants requiring intervention.
Polycythemia ≡ hematocrit >65%.
Median (range).
RACHS: Risk adjustment cardiac heart score.42
We observed no significant differences in surgical parameters between the two groups (Table 3). No infants in either group died before surgical or catheter-based intervention. A similar proportion of infants in both groups were cyanotic before undergoing intervention. Two infants in each group received initial palliative procedures with a Blalock–Taussig shunt. A similar proportion of infants in both groups required inotropes, cardiopulmonary resuscitation and mechanical ventilation in the pre-operative setting. We observed no differences in the timing of CPB, aortic cross-clamp, DHCA or modified ultrafiltration time between the two groups. A similar proportion of infants in both groups underwent complete repair with CPB. We observed marked variability in the length of intensive-care unit and hospital stays in both groups.
Table 3.
Surgical parameters
| ECC | DCC | P-value | |
|---|---|---|---|
| Pre-operative | |||
| Balloon atrial septostomy, n (%) | 2 (14) | 2 (14) | — |
| Receipt of inotropic medication, n (%) |
6 (43) | 4 (29) | 0.44 |
| Cardiopulmonary resuscitation, n (%) |
2 (14) | 2 (14) | — |
| Duration of mechanical ventilation (days) |
2 (0–15) | 1 (0–19) | 0.78 |
| Intraoperativea | |||
| Age at surgery (days) | 8 (2–28) | 9 (3–34) | 0.82 |
| Cardiopulmonary bypass (min)b | 122 ± 71 | 112 ± 139 | 0.81 |
| Aortic cross-clamp time (min)b | 61 ± 52 | 67 ± 48 | 0.76 |
| Deep hypothermic circulatory arrest, n (%) |
4 (29) | 4 (29) | — |
| Deep hypothermic circulatory arrest time (min)b |
23 ± 14 | 19 ± 10 | 0.39 |
| Modified ultrafiltration time (min)b |
10 ± 2 | 12 ± 3 | 0.07 |
| Post-operative | |||
| Duration of mechanical ventilation (days) |
5 (1–38) | 6 (1–52) | 0.87 |
| Duration of open sternum (days)a | 2 (0–6) | 1 (0–7) | 0.85 |
| Maximum inotropic scoreb | 10.2 ± 4 | 9.4 ± 5.9 | 0.68 |
| Highest lactate (mmol l−1)b | 5.4 ± 2 | 4.2 ± 4 | 0.35 |
| Length of ICU stay (days) | 8 (2–41) | 5 (3–69) | 0.41 |
| Bleeding requiring transfusion, n (%) |
5 (36) | 5 (36) | — |
| Length of hospital stay (days) | 20 (7–69) | 15 (8–112) | 0.78 |
| Unplanned re-interventionsc | 1 (7) | 2 (14) | — |
Abbreviations: DCC, delayed umbilical cord clamping; ECC, early umbilical cord clamping; ICU, intensive-care unit.
Values provided in median (ranges) unless stated otherwise. P-value=1.0.
Among patients undergoing surgical repair.
Mean ± s.d.
Inotropic score as defined by Wernosky et al.24
Included both interventional heart catheterization and cardiac surgeries.
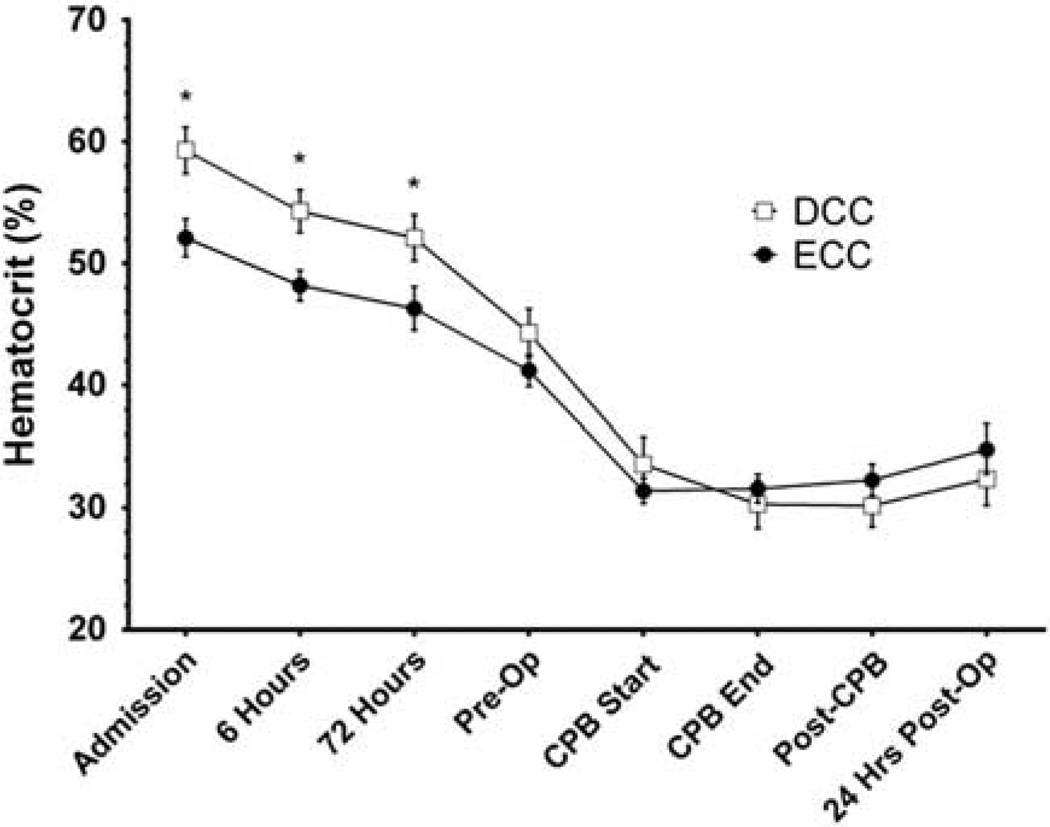
Hematocrits were higher in DCC infants than in ECC infants during the first 72 h of life, but were not different at later time points (Figure 2). In both groups, hematocrits decreased markedly during CPB. Although infants in the DCC group received fewer numbers of blood transfusions per patient before surgery than infants in the ECC group, no differences were observed intra-operatively or post-operatively (Table 4). The total number of blood transfusions per patient throughout hospitalization in the two groups were not different, in large part because one infant received a disproportionally large number of blood transfusion (14 units) while on extracorporeal membrane oxygenation for multisystem organ failure. This infant was the only post-operative patient requiring extracorporeal membrane oxygenation, and post hoc removal of this patient’s data from analysis revealed fewer total numbers of RBC transfusions per patient (2.2 ± 1.4 vs 3.4 ± 1.6, P = 0.048) and total RBC transfusion volumes (66 ± 23 vs 87 ± 29 ml, P = 0.047) in the DCC infants than in the ECC group.
Figure 2.
The course of hematocrit concentrations throughout hospitalization for infants in the delayed umbilical cord clamping and early umbilical cord clamping groups. Data are means ± s.e.m. *P < 0.05. CPB, cardiopulmonary bypass; DCC, delayed umbilical cord clamping; ECC, early umbilical cord clamping; Pre-Op, pre-operative; Post-Op, post-operative.
Table 4.
Hematological data
| RBC exposure (no. transfusions/patient) | ECC | DCC | P-value |
|---|---|---|---|
| Pre-operative | 2.4 ± 2.1 | 1.0 ± 1.1 | 0.04 |
| Intraoperative | 1.3 ± 0.9 | 1.4 ± 0.8 | 0.79 |
| Post-operatively | 2.9 ± 1.2 | 2.1 ± 1.9 | 0.25 |
Abbreviations: DCC, delayed umbilical cord clamping; ECC, early umbilical cord clamping; RBC, red blood cell.
Data are mean ± s.d.
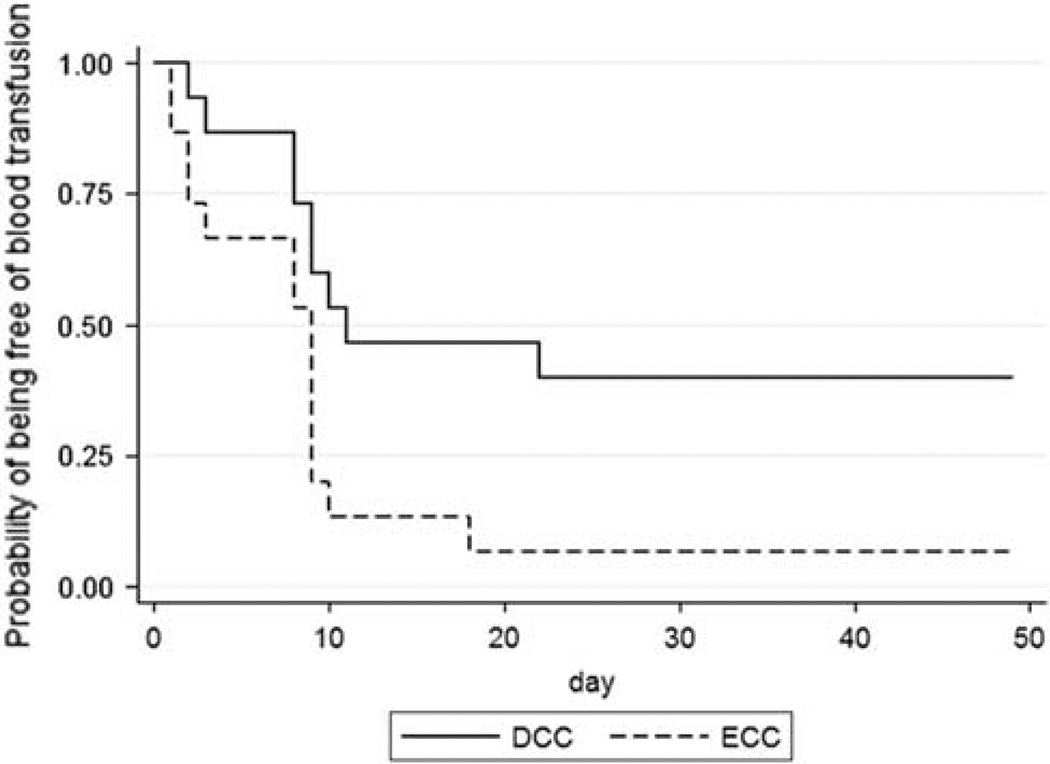
The probability of not having received a blood transfusion throughout the hospitalization was higher in in the DCC infants than in the ECC group (43 vs 7%; P = 0.02, Figure 3). We observed no difference in the DCC or ECC groups in the proportion of infants administered Epoetin alfa (21 vs 29%, P = 1.0), estimated phlebotomy loss (32.2 ± 26 vs 29.7 ± 17 ml, P = 0.77) or post-operative bleeding (13.6 ± 22 vs 15.4 ± 32 ml, P = 0.86). We observed no differences between the two groups in the proportion of neonates exposed to platelet transfusions (28 vs 43%, P = 0.69), cryoprecipitate (21 vs 35%, P = 0.68) or fresh frozen plasma (21 vs 21%).
Figure 3.
Kaplan–Meier curves of the probability of being free from a blood transfusion in the delayed umbilical cord clamping group and the early umbilical cord clamping group. Statistically different by log-rank test (P = 0.02). DCC, delayed umbilical cord clamping; ECC, early umbilical cord clamping.
No differences in neonatal morbidities between the two groups were observed (Table 5). One infant in the ECC group died before hospital discharge, due to complications of necrotizing enterocolitis, while one infant in the DCC group died due to gram-negative sepsis and multiorgan system failure.
Table 5.
Neonatal morbidities
| ECC | DCC | |
|---|---|---|
| Pre-operative infectiona | 1 (7) | 1 (7) |
| Post-operative infectiona | 2 (14) | 3 (21) |
| Necrotizing enterocolitisb | 2 (14) | 1 (7) |
| Arrhythmiac | 1 (7) | 2 (14) |
| Central nervous system lesiond | 1 (7) | 0 (0) |
| Post-operative mortality | 1 (7) | 1 (7) |
Abbreviations: DCC, delayed umbilical cord clamping; ECC, early umbilical cord clamping.
None of the data are statistically different. Data are expressed number (percentage within each group).
Infections include pneumonia, bloodstream and urinary tract infections throughout the infants’ hospitalization.
Bell’s stage 2 or greater throughout hospital course.43
Defined as rhythm necessitating antiarrhythmic therapy.
Lesion defined as any intraventricular/intraparenchymal/cerebellar hemorrhage (IVH) or periventricular leukomalacia on head ultrasound.
DISCUSSION
Evidence for improved hematological status following DCC, including higher hematocrits and less RBC transfusion, in other infant groups led us to investigate the intervention among infants with CHD.7,8,26 Given the absence of safety data on the use of DCC among infants with CHD, our inclusion of infants with critical CHD was intended to minimize the risks of adverse outcomes. Thus, this pilot study was designed to determine the safety and feasibility of DCC among infants with critical CHD.
The main finding of our study is that, among infants with critical CHD, DCC appears safe and is logistically feasible. Consistent with a recent meta-analysis of term infants without CHD,26 we observed similar short-term safety profiles between the two groups across all measured outcomes. However, short-term safety profiles are not substitutes for long-term studies adequately powered to detect changes in neurodevelopment or death. Although evidence on the association between peak serum bilirubin levels and adverse neurodevelopmental outcomes is limited,27 the observed trend towards higher bilirubin levels and greater phototherapy use in DCC than in ECC infants may have important implications on resource utilization and medical costs.28 In turn, these considerations must be balanced with the potential for DCC to reduce RBC transfusion exposure, thereby decreasing the direct and indirect costs associated with blood transfusions.17
Previous investigators have raised concerns on the safety of DCC among infants with CHD.19 Phillips et al.21 suggested that DCC in infants with CHD may result in circulatory overload and impair adaptation to extrauterine life. Although we observed no differences in variables associated with neonatal transition, recent evidence suggests that DCC may provide better cardiopulmonary transition at birth than does ECC. A study of over 15 000 term infants showed that the risk of death or hospitalization decreased by 20% for every 10 s delay in clamping.29 Although the physiological mechanisms for the improved transition have not been elucidated, the additional blood volumes accrued following DCC likely increase pulmonary blood flow, augment venous return to the left atrium and maintain cardiac output, thereby stabilizing systemic blood pressure at birth.30 This may be particularly important for infants with critical CHD, where optimizing delivery room management improves outcomes.31
Another important issue to address is feasibility. Even with a dedicated staff for patient education and recruitment, 59% of the infant’s enrolled in the study were not randomized at the time of delivery. Although we attempted to address these issues systematically over the course of the study, other investigators have described similar logistical issues randomizing neonates at the time of birth, including precipitous delivery of the infant.32 Although randomizing neonates at time of delivery was often not feasible, these issues are separate and distinct from those necessary to perform DCC at birth. In fact, we observed that obstetricians were consistently able to perform the intervention without any complications.
Increasing the percentages of CHD infants who do not require transfusions from 7 to 43% may be important for a number of reasons, from patient safety to health care costs.17 Nevertheless, the majority of infants in both groups received a transfusion during their hospitalization, which, in turn, means that more work is needed to test our initial observation and to identify ways to improve blood conservation even further. Although a number of centers have described comprehensive blood-conservation strategies for infants undergoing CHD repair,33,34 over 90% of operated infants weighing < 4 kg continue to be exposed to autologous RBC transfusions.34 Furthermore, in the absence of objective clinical indications for RBC transfusion in neonates with CHD,14 it was difficult to identify neonates in whom transfusion was truly necessary. This reinforces the need for large, high-quality trials that focus on specific types of heart disease and have sufficient power to assess clinically relevant differences reliably in outcomes based on RBC transfusion thresholds (restrictive vs liberal).35
Although DCC infants had higher hematocrits than did ECC infants in the pre-operative setting, no differences were observed in the perioperative or post-operative settings. This suggests that despite our efforts to investigate a subgroup of neonates with critical CHD, significant heterogeneity in cardiac disease, including anatomic lesions (cyanotic and acyanotic), cardiac physiologies and timing of intervention exist in our cohort. Although increased hematocrit levels are clinically advantageous in most medical settings, higher hematocrits may increase the risk of adverse events among infants undergoing palliative procedures, including modified Blalock–Taussig shunt.36 Sahoo et al.37 showed that, compared with standard practice of maintaining intraoperative hematocrit values ~ 55%, artificially lowering the hematocrit to 40–45% increased shunt patency rates from 84–100%. Additionally, high pre-operative hemoglobin levels (>18 g dl−1) are associated with greater risks of shunt thrombosis, particularly among infants < 3 kg.36 Although we observed no evidence of shunt thrombosis in either the DCC or ECC groups, the limited numbers of neonates in the present cohort undergoing palliative procedures are recognized.
Previous authors have suggested that DCC may increase the risk of postpartum bleeding compared with ECC.38 However, our finding of no harmful effects of DCC on maternal health is consistent with more recent evidence.4,8 In light of inconsistent evidence, consideration of maternal outcomes in future studies is warranted.39
LIMITATIONS
A number of factors limit the generalizability of our findings to all infants delivering with CHD. First, we maintained very-strict enrollment criteria, notably the exclusion of mothers with known prenatal risk factors for polycythemia.40 Second, our study only included infants with a prenatal diagnosis of CHD. Third, children with CHD may increase hematocrit levels in the setting of chronic hypoxia.22 These criteria were intended to minimize the potential risk of polycythemia and adverse outcomes.
At our institution, all decisions regarding the need for blood transfusion are based on consideration of both hematocrit levels and noninvasive measurements of tissue oxygenation (near infrared spectroscopy, arterial saturation). However, without evidence-based recommendations to guide clinicians on the optimal hematocrit levels in infants born with heart disease,14 transfusion practice was left to the discretion of the medical team. To minimize potential biases, we concealed group assignments, standardized follow-up and assessed objective clinical outcomes. However, the significant number of infants enrolled but not randomized at birth is a potential source of bias attributable to sample-size slippage. Although similar baseline characteristics between the two groups suggest our randomization process was successful, we recognize the potential for random apparent effect in our small sample size. With limited patient numbers and a heterogeneous patient population, secondary subgroup analyses (cyanotic vs acyanotic) to determine predictors for RBC transfusion were not feasible. Finally, while our goal was to determine safety and feasibility among infants with two-ventricle physiology, we recognize the potential value of DCC among infants with single ventricle physiology, who may have a precarious oxygen supply/demand balance and require frequent transfusions to optimize oxygen delivery.41
CONCLUSION
DCC among infants with critical CHD is safe, logistically feasible and results in a lower proportion of infants exposed to RBC transfusion during hospitalization. While we observed hematological benefits of DCC in the first few weeks of life for infants born with critical CHD, assessing whether these short-term benefits translate into reductions in long-term morbidity will be important.
Acknowledgments
Supported by a grant from The American Heart Association (# 10CRP3730033, CHB).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Linderkamp O. Placental transfusion: determinants and effects. Clin Perinatol. 1982;9(3):559–592. [PubMed] [Google Scholar]
- 2.Usher R, Shephard M, Lind J. The blood volume of the newborn infant and placental transfusion. Acta Paediatr. 1963;52:497–512. doi: 10.1111/j.1651-2227.1963.tb03809.x. [DOI] [PubMed] [Google Scholar]
- 3.Yao AC, Lind J. Effect of gravity on placental transfusion. Lancet. 1969;2(7619):505–508. doi: 10.1016/s0140-6736(69)90213-x. [DOI] [PubMed] [Google Scholar]
- 4.Ceriani Cernadas JM, Carroli G, Pellegrini L, Otaño L, Ferreira M, Ricci C, et al. The effect of timing of cord clamping on neonatal venous hematocrit values and clinical outcome at term: a randomized, controlled trial. Pediatrics. 2006;117(4):e779–e786. doi: 10.1542/peds.2005-1156. [DOI] [PubMed] [Google Scholar]
- 5.Nelle M, Kraus M, Bastert G, Linderkamp O. Effects of Leboyer childbirth on left-and right systolic time intervals in healthy term neonates. J Perinat Med. 1996;24(5):513–520. doi: 10.1515/jpme.1996.24.5.513. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell M, Henderson-Smart DJ. Delayed umbilical cord clamping in preterm infants: a feasibility study. J Paediatr Child Health. 1997;33(4):308–310. doi: 10.1111/j.1440-1754.1997.tb01606.x. [DOI] [PubMed] [Google Scholar]
- 7.Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297(11):1241–1252. doi: 10.1001/jama.297.11.1241. [DOI] [PubMed] [Google Scholar]
- 8.McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD004074.pub2. CD004074. [DOI] [PubMed] [Google Scholar]
- 9.Emhamed MO, van Rheenen P, Brabin BJ. The early effects of delayed cord clamping in term infants born to libyan mothers. Trop Doct. 2004;34(4):218–222. doi: 10.1177/004947550403400410. [DOI] [PubMed] [Google Scholar]
- 10.Linderkamp O, Nelle M, Kraus M, Zilow EP. The effect of early and late cord-clamping on blood viscosity and other hemorheological parameters in full-term neonates. Acta Paediatr. 1992;81(10):745–750. doi: 10.1111/j.1651-2227.1992.tb12095.x. [DOI] [PubMed] [Google Scholar]
- 11.Linderkamp O, Stadler AA, Zilow EP. Blood viscosity and optimal hematocrit in preterm and full-term neonates in 50- to 500-micrometer tubes. Pediatr Res. 1992;32(1):97–102. doi: 10.1203/00006450-199207000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Obstetric Practice American College of Obstetricians and Gynecologists. Committee opinion no. 543: timing of umbilical cord clamping after birth. Obstet Gynecol. 2012;120(6):1522–1526. doi: 10.1097/01.AOG.0000423817.47165.48. [DOI] [PubMed] [Google Scholar]
- 13.Morley SL. Red blood cell transfusions in acute paediatrics. Arch Dis Child Educ Pract Ed. 2009;94(3):65–73. doi: 10.1136/adc.2007.135731. [DOI] [PubMed] [Google Scholar]
- 14.Guzzetta NA. Benefits and risks of red blood cell transfusion in pediatric patients undergoing cardiac surgery. Paediatr Anaesth. 2011;21(5):504–511. doi: 10.1111/j.1460-9592.2010.03464.x. [DOI] [PubMed] [Google Scholar]
- 15.Szekely A, Cserep Z, Sapi E, Breuer T, Nagy CA, Vargha P, et al. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. 2009;87(1):187–197. doi: 10.1016/j.athoracsur.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 16.Kipps AK, Wypij D, Thiagarajan RR, Bacha EA, Newburger JW. Blood transfusion is associated with prolonged duration of mechanical ventilation in infants undergoing reparative cardiac surgery. Pediatr Crit Care Med. 2011;12(1):52–56. doi: 10.1097/PCC.0b013e3181e30d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 18.Salvin JW, Scheurer MA, Laussen PC, Wypij D, Polito A, Bacha EA, et al. Blood transfusion after pediatric cardiac surgery is associated with prolonged hospital stay. Ann Thorac Surg. 2011;91(1):204–210. doi: 10.1016/j.athoracsur.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Oh W, Lind J, Gessner IH. The circulatory and respiratory adaptation to early and late cord clamping in newborn infants. Acta Paediatr Scand. 1966;55(1):17–25. doi: 10.1111/j.1651-2227.1966.tb15204.x. [DOI] [PubMed] [Google Scholar]
- 20.Oh W, Wallgren G, Hanson JS, Lind J. The effects of placental transfusion on respiratory mechanics of normal term newborn infants. Pediatrics. 1967;40(1):6–12. [PubMed] [Google Scholar]
- 21.Phillip AGS, Saigal S. When should we clamp the umbilical cord? NeoReviews. 2004;5:142–153. [Google Scholar]
- 22.Blackburn ST. Maternal, fetal, and neonatal physiology: a clinical perspective. In: Goldsmith JP, Karotkin EH, editors. Sciences. St Louis, MO: Saunders Elsevier; 2007. pp. 260–267. [Google Scholar]
- 23.Schultz AH, Localio AR, Clark BJ, Ravishankar C, Videon N, Kimmel SE. Epidemiologic features of the presentation of critical congenital heart disease: implications for screening. Pediatrics. 2008;121(4):751–757. doi: 10.1542/peds.2007-0421. [DOI] [PubMed] [Google Scholar]
- 24.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Jr, Hanley FL, Hickey PR, et al. Post-operative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92(8):2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 25.Newman TB, Klebanoff MA. Neonatal hyperbilirubinemia and long-term outcome: another look at the collaborative perinatal project. Pediatrics. 1993;92(5):651–657. [PubMed] [Google Scholar]
- 26.McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;(7) doi: 10.1002/14651858.CD004074.pub3. CD004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip S, Chung M, Kulig J, O'Brien R, Sege R, Glicken S, et al. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114(1):e130–e153. doi: 10.1542/peds.114.1.e130. [DOI] [PubMed] [Google Scholar]
- 28.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012;(8) doi: 10.1002/14651858.CD003248.pub3. CD003248. [DOI] [PubMed] [Google Scholar]
- 29.Ersdal HL, Linde J, Mduma E, Auestad B, Perlman J. Neonatal outcome following cord clamping after onset of spontaneous respiration. Pediatrics. 2014;134(2):265–272. doi: 10.1542/peds.2014-0467. [DOI] [PubMed] [Google Scholar]
- 30.Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. 2013;591(Pt 8):2113–2126. doi: 10.1113/jphysiol.2012.250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson BA, Ades A. Delivery room and early postnatal management of neonates who have prenatally diagnosed congenital heart disease. Clin Perinatol. 2005;32(4):921–946. ix. doi: 10.1016/j.clp.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Mercer JS, McGrath MM, Hensman A, Silver H, Oh W. Immediate and delayed cord clamping in infants born between 24 and 32 weeks: a pilot randomized controlled trial. J Perinatol. 2003;23(6):466–472. doi: 10.1038/sj.jp.7210970. [DOI] [PubMed] [Google Scholar]
- 33.Redlin M, Boettcher W, Kukucka M, Kuppe H, Habazettl H. Blood transfusion during versus after cardiopulmonary bypass is associated with postoperative morbidity in neonates undergoing cardiac surgery. Perfusion. 2014;29(4):327–332. doi: 10.1177/0267659113517922. [DOI] [PubMed] [Google Scholar]
- 34.Redlin M, Habazettl H, Boettcher W, Kukucka M, Schoenfeld H, Hetzer R, et al. Effects of a comprehensive blood-sparing approach using body weight-adjusted miniaturized cardiopulmonary bypass circuits on transfusion requirements in pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2012;144(2):493–499. doi: 10.1016/j.jtcvs.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson KL, Brunskill SJ, Doree C, Trivella M, Gill R, Murphy MF. Red cell transfusion management for patients undergoing cardiac surgery for congenital heart disease. Cochrane Database Syst Rev. 2014;(2) doi: 10.1002/14651858.CD009752.pub2. CD009752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gedicke M, Morgan G, Parry A, Martin R, Tulloh R. Risk factors for acute shunt blockage in children after modified blalock-taussig shunt operations. Heart Vessels. 2010;25(5):405–409. doi: 10.1007/s00380-009-1219-1. [DOI] [PubMed] [Google Scholar]
- 37.Sahoo TK, Chauhan S, Sahu M, Bisoi A, Kiran U. Effects of hemodilution on outcome after modified blalock-taussig shunt operation in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 2007;21(2):179–183. doi: 10.1053/j.jvca.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The bristol third stage trial: active versus physiological management of third stage of labour. BMJ. 1988;297(6659):1295–1300. doi: 10.1136/bmj.297.6659.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raju TN, Singhal N. Optimal timing for clamping the umbilical cord after birth. Clin Perinatol. 2012;39(4):889–900. doi: 10.1016/j.clp.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hay WW., Jr Care of the infant of the diabetic mother. Curr Diab Rep. 2012;12(1):4–15. doi: 10.1007/s11892-011-0243-6. [DOI] [PubMed] [Google Scholar]
- 41.Kuo JA, Maher KO, Kirshbom PM, Mahle WT. Red blood cell transfusion for infants with single-ventricle physiology. Pediatr Cardiol. 2011;32(4):461–468. doi: 10.1007/s00246-011-9901-3. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins KJ, Gauvreau K. Center-specific differences in mortality: preliminary analyses using the risk adjustment in congenital heart surgery (rachs-1) method. J Thorac Cardiovasc Surg. 2002;124(1):97–104. doi: 10.1067/mtc.2002.122311. [DOI] [PubMed] [Google Scholar]
- 43.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]