Abstract
Methylmercury (MeHg) is a persistent environmental contaminant that has been reported worldwide. MeHg exposure has been reported to lead to increased risk of cardiovascular diseases; however, the mechanisms underlying the toxic effects of MeHg on the cardiovascular system have not been well elucidated. We have previously reported that mice exposed to MeHg had increased blood pressure along with impaired endothelium-dependent vasodilation. In this study, we investigated the toxic effects of MeHg on a human endothelial cell line, EA.hy926. In addition, we have tried to elucidate the role of myristoylated alanine-rich C kinase substrate (MARCKS) in the MeHg toxicity mechanism in EA.hy926 cells. Cells exposed to MeHg (0.1–10 µM) for 24 hr showed decreased cell viability in a dose-dependent manner. Treatment with submaximal concentrations of MeHg decreased cell migration in the wound healing assay, tube formation on Matrigel and spontaneous nitric oxide (NO) production of EA.hy926 cells. MeHg exposure also elicited a decrease in MARCKS expression and an increase in MARCKS phosphorylation. MARCKS knockdown or MARCKS overexpression in EA.hy926 cells altered not only cell functions, such as migration, tube formation and NO production, but also MeHg-induced decrease in cell viability and NO production. These results suggest the broad role played by MARCKS in endothelial cell functions and the involvement of MARCKS in MeHg-induced toxicity.
Keywords: EA.hy926 cells, endothelium, MARCKS, methylmercury, nitric oxide
The myristoylated alanine-rich C kinase substrate (MARCKS) is a major protein kinase C substrate that is expressed in many tissues [1], including brain and endothelial cells [17, 24, 38]. Homozygous mutant mice with targeted deletion of the Marcks gene showed morphological abnormalities in the central nervous system and perinatal death [39], suggesting the essential role of MARCKS in brain development. MARCKS plays roles in cellular functions, such as adhesion, migration, proliferation and fusion in multiple types of cells through its interaction with the membrane phospholipids and actin, which is regulated by phosphorylation at the central polybasic region of MARCKS called the effector domain [2, 3, 27, 48]. In vascular smooth muscle and endothelial cells, MARCKS has been shown to regulate proliferation [46], cell migration [17, 26, 47] and endothelial cell permeability [16]. These studies have shown that MARCKS also plays an important role in the cardiovascular system.
Methylmercury (MeHg) is a ubiquitous and potent environmental pollutant [8]. The central nervous system is the main target of MeHg toxicity [6, 7, 42]. The cardiovascular system has also been reported as a target of MeHg [4, 31]. In humans, MeHg exposure has been reported to cause cardiovascular dysfunctions, including myocardial infarction [30], heart rate variability, atherosclerosis, coronary heart disease and hypertension [35, 45]. In animal experimental models, in vivo treatment of MeHg has been reported to induce hypertension [10, 43, 44]. However, the exact mechanism by which MeHg induces a toxic effect on the cardiovascular system is not yet fully understood.
We recently demonstrated that mice exposed to MeHg in vivo developed increased blood pressure and impaired endothelium-dependent vasodilation [15]. Although it has been reported that the alteration in MARCKS expression or phosphorylation affects MeHg-induced neurotoxicity in neuroblastoma cells [37], the relationship between MeHg toxicity and MARCKS has not yet been determined in vascular endothelial cells. Therefore, in this study, we investigated the role of MARCKS in MeHg-induced toxicity in the EA.hy926 endothelial cell line. We observed that MeHg exposure induced decrease in cell viability, migration in wound healing assay, tube formation on Matrigel and nitric oxide (NO) production, and this was accompanied by an increase in MARCKS phosphorylation in EA.hy926 cells. Furthermore, the involvement of MARCKS in MeHg toxicity was studied by using cells with MARCKS knockdown or MARCKS overexpression.
MATERIALS AND METHODS
Cell viability assay: A human endothelial cell line, EA.hy926 cells (ATCC, Manassas, VA, U.S.A.), was grown in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO, U.S.A.) containing 10% fetal bovine serum at 37°C in a humidified atmosphere with 5% CO2. To evaluate MeHg cytotoxicity, cell viability was measured using the WST-8 assay Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) in accordance with the manufacturer’s instructions. Two days before experiments, the cells were seeded at a density of 1 × 104 cells/cm2 in a 96-well plate. Cells were serum-starved for 4 hr before the addition of MeHg chloride (Kanto Chemical, Tokyo, Japan) dissolved in distilled water. The absorbance of formazan dye solution in the WST-8 assay was measured using an Infinite M200 FA plate reader (TECAN, Männedorf, Switzerland).
Cell cycle analysis by flow cytometry: One day before the experiments, cells were seeded on 35-mm dishes at a density of 2.5 × 104 cells/cm2. After 4 hr of serum starvation, the cells were treated with MeHg for 24 hr. Then, the cells were harvested by using Accumax (Innovative Cell Technologies, San Diego, CA, U.S.A.) and then fixed with 4% paraformaldehyde. The cell cycle was analyzed by flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA, U.S.A.) by using cells stained with propidium iodide.
Wound healing assay: Two days before the experiments, cells were seeded on 35-mm dishes at a density of 1.5 × 104 cells/cm2. After 4 hr of serum starvation, confluent cells were scraped with sterile 200-µl pipette tips. These cells were treated with MeHg for 24 hr, after which the images of the wound areas were obtained by using an inverted microscope IX70 (Olympus, Tokyo, Japan). The percentage of area covered by the migrated cells was measured using ImageJ software (NIH, Bethesda, MD, U.S.A.).
Tube formation assay: Tube formation assay was performed as previously reported [20, 21], with slight modifications. In brief, the surface of 24-well plates was coated with 100 µl of Corning Matrigel Basement Membrane Matrix (BD Biosciences), which was allowed to polymerize at 37°C for 30 min. EA.hy926 cells were seeded on to the Matrigel-coated wells (3 × 104 cells/cm2) with or without MeHg. The images were taken at 12 hr after seeding. The length of the tube was measured by using ImageJ software (NIH).
Measurement of NO production: NO production was measured as previously described [13, 25]. Two days before the experiments, cells were seeded at a density of 8.8 × 104 cells/cm2 in a 100-mm dish. After changing the medium to DMEM without phenol red, the medium was collected from the dish at 24 hr after addition of MeHg. Accumulated NO2 in the medium was measured using the NO2/NO3 Assay Kit-FX (Dojindo) in accordance with the manufacturer’s instructions. The fluorescence intensity of the sample was measured using an Infinite M200 FA plate reader (TECAN).
Transfection of siRNA and plasmid DNA: ScreenFectA (Wako, Osaka, Japan) was used for both siRNA and plasmid DNA transfections. MARCKS siRNA (HSS180966) and negative control siRNA were purchased from Invitrogen (Carlsbad, CA, U.S.A.). EA.hy926 cells were mixed with siRNA and then seeded on 35-mm dishes (1 × 104 cells/cm2) at 48 hr before the experiments, according to the manufacturer’s instructions. For plasmid DNA transfection, cells were seeded on 35-mm dishes at a density of 2.5 × 104 cells/cm2. After 24 hr incubation, GFP-fused wild-type MARCKS-expression plasmids [36] or control pEGFP-N1 (Clontech, Palo Alto, CA, U.S.A.) was transfected to the cells for 24 hr.
Western blotting: Western blotting was performed as previously described [36, 37]. In brief, two days before the experiments, cells were seeded at a density of 1 × 104 cells/cm2. Cells were treated with MeHg after 4 hr of starvation. The primary antibodies used were anti-MARCKS, anti-NOS3 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), anti-pS159/163 MARCKS (Cell Signaling Technology, Danvers, MA, U.S.A.) and anti-β-actin antibody (Sigma-Aldrich). Immunoreactive proteins were detected using Luminata Forte Western HRP substrate (Millipore, Billerica, MA, U.S.A.) and quantified by densitometric analysis using Image J software (NIH). The MARCKS and eNOS expression or MARCKS phosphorylation was normalized to the amount of β-actin or pan-MARCKS, respectively.
Statistical analysis: All values are expressed as the means ± SEM of the number of independent experiments. Statistical differences between two means were evaluated by the Student’s t-test. Multiple comparisons were performed using one-way analysis of variance followed by Dunnett’s test. Differences were considered significant at P<0.05.
RESULTS
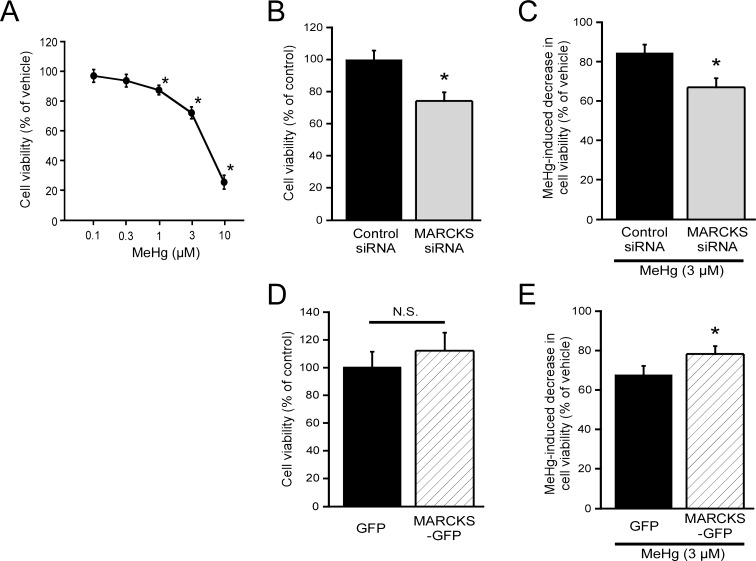
Effect of MeHg on endothelial cell viability: To determine the effect of MeHg on cell viability, EA.hy926 cells were treated with 0.1–10 µM MeHg for 24 hr. MeHg elicited a decrease in cell viability in a dose-dependent manner (Fig. 1A). At MeHg concentration higher than 1 µM, significant decrease in cell viability was observed. We assessed the involvement of MARCKS in MeHg-induced decrease in cell viability by using EA.hy926 cells with MARCKS knockdown or MARCKS overexpression. Transfection of siRNA for MARCKS or MARCKS-expression plasmid caused decrease in MARCKS expression to 36.0 ± 8.4% (n=4) or increase in MARCKS expression to 148.0 ± 7.9% (n=4), respectively, in comparison with control mock-transfected cells. In cells with MARCKS knockdown, cell viability was decreased in comparison with control siRNA-transfected cells (Fig. 1B), suggesting the involvement of MARCKS in endothelial cell proliferation. In addition, decrease in cell viability induced by 3 µM MeHg for 24 hr was significantly augmented in cells with MARCKS knockdown (Fig. 1C). Although cells with MARCKS overexpression showed similar cell viability as control cells (GFP) (Fig. 1D), MeHg-induced decrease in cell viability was significantly suppressed in cells with MARCKS overexpression (Fig. 1E). Flow cytometric analysis of the cell cycle of the cells treated with MeHg (0.1–3 µM) showed that there was no alteration in the distribution of cells in the G1, S or G2/M phase (data not shown).
Fig. 1.
Effect of MeHg on cell viability and involvement of MARCKS. Effect of MeHg on cell viability (A, n=9), effect of MARCKS knockdown on cell viability (B, n=9) or MeHg-induced decrease in cell viability (C, n=9), and effect of MARCKS overexpression on cell viability (D, n=8) or MeHg-induced decrease in cell viability (E, n=8) were examined 24 hr after addition of MeHg by cell viability assay in EA.hy926 cells. Data are expressed as a percentage of vehicle-treated or mock-transfected cells (control). Results shown are the mean ± SEM. *P<0.05, as compared with vehicle-treated or mock-transfected cells. N.S.; not significant
Effect of MeHg on cell migration: To determine the effect of MeHg on cell functions, we first observed the effect of MeHg on cell migration by a wound healing assay. Incubation of cells with 0.1–3 µM MeHg for 24 hr showed dose-dependent inhibition of cell migration of EA.hy926 cells (Fig. 2A). Significant inhibition by MeHg was observed at concentrations higher than 0.3 µM. In cells with MARCKS knockdown or overexpression, the cell migration was significantly suppressed or augmented, respectively (Fig. 2B and 2D), suggesting the role of MARCKS in the migration of endothelial cells as reported previously [17, 46]. However, 0.3 µM MeHg-induced inhibition of cell migrations was not altered in both cells with MARCKS knockdown or overexpression (Fig. 2C and 2E).
Fig. 2.
Effect of MeHg on cell migration and involvement of MARCKS. Effect of MeHg on cell migration (A, n=5), effect of MARCKS knockdown on cell migration (B, n=10) or MeHg-induced decrease in cell migration (C, n=10), and effect of MARCKS overexpression on cell migration (D, n=8) or MeHg-induced decrease in cell migration (E, n=8) were examined 24 hr after addition of MeHg by wound healing assay in EA.hy926 cells. Results shown are the mean ± SEM. *P<0.05, as compared with vehicle-treated or mock-transfected cells. N.S.; not significant
Effect of MeHg on tube formation: EA.hy926 cells were seeded onto Matrigel-coated plates, and then, the tube formation of EA.hy926 cells was analyzed by measurement of the tube length. In the presence of 0.1–1 µM MeHg, tube length was significantly decreased in a dose-dependent manner (Fig. 3A). Although MARCKS knockdown or overexpression in EA.hy926 cells significantly decreased or increased the tube length on Matrigel (Fig. 3B and 3D), respectively, the modification of MARCKS expression did not alter the tube length in the presence of 1 µM MeHg (Fig. 3C and 3E).
Fig. 3.
Effect of MeHg on tube formation and involvement of MARCKS. Effect of MeHg on tube formation (A, n=9), effect of MARCKS knockdown on tube formation (B, n=5) or MeHg-induced decrease in tube formation (C, n=5), and effect of MARCKS overexpression on tube formation (D, n=4) or MeHg-induced decrease in tube formation (E, n=4) were examined 12 hr after seeding of cells with or without MeHg by measurement of tube formation of EA.hy926 cells. Results shown are the mean ± SEM. *P<0.05, as compared with vehicle-treated or mock-transfected cells. N.S.; not significant.
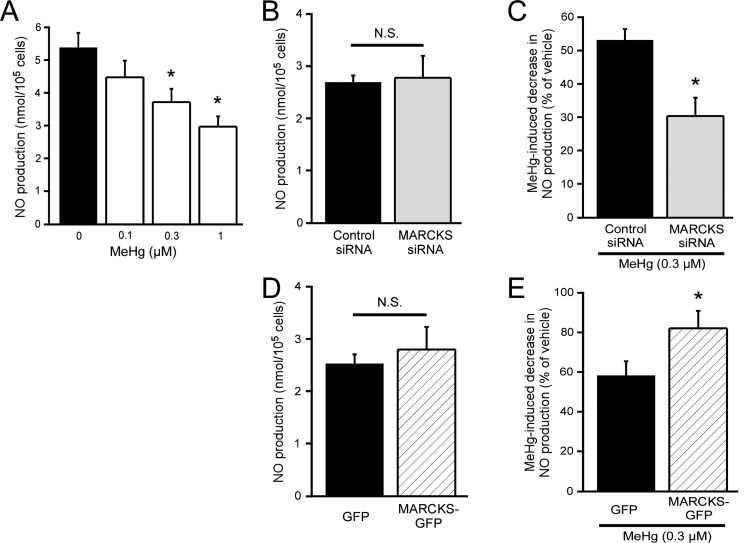
Effect of MeHg on NO production: Next, we examined the effect of MeHg on NO production by EA.hy926 cells, because NO has been shown to play an important role in the regulation of vascular tones [23, 41]. In the presence of 0.1–1 µM MeHg, spontaneous NO production by EA.hy926 cells for 24 hr was significantly inhibited in a dose-dependent manner (Fig. 4A). MARCKS knockdown or overexpression did not change the spontaneous NO production of EA.hy926 cells during the 24 hr observation (Fig. 4B and 4D). In contrast, in cells with MARCKS knockdown, 0.3 µM MeHg-induced inhibition of spontaneous NO production was significantly augmented (Fig. 4C). Furthermore, MARCKS overexpression in EA.hy926 cells significantly suppressed the inhibition of NO production by MeHg (Fig. 4E).
Fig. 4.
Effect of MeHg on NO production and involvement of MARCKS. Effect of MeHg on NO production (A), effect of MARCKS knockdown on NO production (B) or MeHg-induced decrease in NO production (C), and effect of MARCKS overexpression on NO production (D) or MeHg-induced decrease in NO production (E) were examined 24 hr after addition of MeHg by measurement of spontaneous NO production. Results shown are the mean ± SEM (n=6). *P<0.05, as compared with vehicle-treated or mock-transfected cells. N.S.; not significant.
Effect of MeHg on expression of MARCKS, eNOS and phosphorylation of MARCKS: Finally, we observed the effect of MeHg on MARCKS expression or phosphorylation, since alteration of MARCKS expression/phosphorylation has been reported in MeHg-treated neuroblastoma cells [37]. Western blotting using specific antibodies (Fig. 5A) showed a decrease in MARCKS expression (Fig. 5B) and biphasic increase in MARCKS phosphorylation by MeHg in a dose-dependent manner (Fig. 5C). At 24 hr after exposure to MeHg, significant differences were observed in the MARCKS expression in cells exposed to 3 µM MeHg and in the MARCKS phosphorylation in cells exposed to concentrations higher than 0.3 µM MeHg. In contrast, there was no alteration in the expression of eNOS by treatment of MeHg (Fig. 5D and 5E).
Fig. 5.
Effect of MeHg on expression of MARCKS, eNOS and phosphorylation of MARCKS. Representative immunoblots of MARCKS, phosphorylated-MARCKS (P-MARCKS) (A) and eNOS (D) by specific antibodies. Changes in MARCKS expression (B, n=9), MARCKS phosphorylation (C, n=9) and eNOS expression (E, n=4) induced by MeHg were determined by densitometric analysis. Data are expressed as a percentage of vehicle-treated cells (control). Results shown are the mean ± SEM. *P<0.05, as compared with control.
DISCUSSION
EA.hy926 cells exposed to MeHg for 24 hr showed a dose-dependent decrease in cell viability. Significant decrease in cell viability was observed at concentrations higher than 1 µM MeHg. The concentration of MeHg that caused significant decrease in cell viability was in accordance with that reported previously in neuroblastoma SH-SY5Y cells and primary human endothelial cells, such as brain microvascular endothelial cells and umbilical vein endothelial cells [11, 21, 37]. MeHg has been reported to elicit cell growth inhibition by interfering with the cell cycle process [19]. However, in this study, flow cytometric analysis of the cell cycle showed that there were no significant differences between control and MeHg-treated cells, suggesting that the decrease in the cell viability cannot be attributed to the toxic effect of MeHg on the cell cycle process. We have previously reported that MARCKS knockdown accelerates MeHg-induced decrease in cell viability in neuroblastoma SH-SY5Y cells [37]. Thus, in this study, we studied the effect of MeHg on cell viability by using MARCKS knockdown/overexpression experiments in EA.hy926 cells. Although MARCKS overexpression did not alter the cell viability of EA.hy926 cells, MARCKS knockdown caused significant decrease in the cell viability in comparison with control siRNA-transfected cells. The observed decrease in the cell viability may be due to the suppression of cell proliferation, which is regulated by MARCKS [32, 33, 46]. MARCKS knockdown, as previously reported in neuroblastoma cells, significantly accelerated MeHg-induced decrease in cell viability in EA.hy926 cells. In addition, in cells with MARCKS overexpression, suppression of the MeHg toxicity was observed. These results support the fact that MARCKS is involved in MeHg toxicity not only in neuronal cells but also in endothelial cells.
The migration of endothelial cells is one of the key processes in angiogenesis, which is involved in a wide range of physiological and pathophysiological events, such as wound healing, cancer and cardiovascular diseases. Treatment of cells with MeHg significantly and dose-dependently inhibited EA.hy926 cell migration in the wound healing assay and tube formation on the Matrigel. These observations are in agreement with a previous report using primary human endothelial cells [11, 12, 20, 21]. In the wound healing assay, we observed significant inhibition of migration at 0.3 µM MeHg, which is a lower concentration than that which induced significant decrease in the cell viability assay, suggesting that the inhibition of migration may be one of the principal toxic actions of MeHg on EA.hy926 cells. Since the involvement of MARCKS in cell migration has been reported in many types of cells, including endothelial cells [9, 17, 28, 47], we observed the effects of MARCKS knockdown/overexpression on EA.hy926 cell migration and the effects of MeHg exposure on the cell migration. In cells with MARCKS knockdown by siRNA, cell migration was significantly suppressed in comparison with control cells, whereas overexpression of MARCKS accelerated cell migration in the wound healing assay. These results indicated the role of MARCKS in cell migration of EA.hy926 cells. However, the effects of MARCKS knockdown/overexpression on MeHg-induced inhibition of migration were not observed. Furthermore, we observed similar results for the tube formation of EA.hy926 cells on Matrigel. Therefore, it seems likely that MARCKS is not involved in the MeHg toxic effect on cell migration and tube formation of EA.hy926 cells under our experimental conditions.
Next, we examined the effect of MeHg on spontaneous NO production by EA.hy926 cells, because NO has been shown to play an important role in the regulation of vascular tones [23, 41]. We have previously reported that vasodilation induced by acetylcholine, which is dependent on NO production from endothelial cells, was decreased in a basilar artery isolated from MeHg-exposed mice [14, 15]. In this study, we showed that treatment of 0.3 µM MeHg significantly inhibited NO production, but not expression of eNOS, in a dose-dependent manner. Taken together, these results indicate that the inhibition of NO production in endothelial cells is one of the principal toxic actions of MeHg. Although MARCKS knockdown/overexpression did not change spontaneous NO production, MeHg-induced decrease in NO production in EA.hy926 cells was significantly accelerated or inhibited by MARCKS knockdown or overexpression, respectively, suggesting the involvement of MARCKS in MeHg-induced toxicity on NO production in EA.hy926 cells. Although the role of MARCKS in the transport of extracellular l-arginine, which is the immediate substrate for NO synthesis in bovine aortic endothelial cells, has been reported [40], further studies are needed to determine whether MARCKS directly functions as a regulator of NO production in endothelial cells.
Finally, we examined the effects of MeHg on MARCKS expression and phosphorylation in EA.hy926 cells, since we reported that alteration in MARCKS expression or phosphorylation has consequences on the MeHg-induced neurotoxicity in neuroblastoma cells [37]. EA.hy926 cells exposed to MeHg showed a dose-dependent decrease in MARCKS expression, although a significant difference was only found at higher (3 µM) concentrations of MeHg. However, MeHg exposure elicited a biphasic increase in MARCKS phosphorylation, and significant differences were observed at concentrations higher than 0.3 µM at 24 hr after the treatment. Since the interactions between MARCKS and its target molecules, such as actin and phosphatidylinositol 4,5-bisphosphate, are regulated by phosphorylation at the effector domain of MARCKS [3, 17], it is likely that the phosphorylation of MARCKS induced by MeHg is directly involved in the MeHg toxicity on EA.hy926 cells. MeHg is known to induce reactive oxygen species (ROS) production, including hydrogen peroxide (H2O2). Since the distinct role of MARCKS accompanying its phosphorylation in H2O2-mediated signaling pathway in bovine aortic endothelial cells has been reported [16, 18], MARCKS is possibly phosphorylated through mechanisms associated with MeHg-induced H2O2 production in EA.hy926 cells. Although we previously reported that, in neuroblastoma cells, the MARCKS phosphorylation by MeHg exposure was mediated by protein kinase C activation and occurred in a Ca2+-dependent manner, the phosphorylation mechanisms in EA.hy926 cells are still not clear and remain to be elucidated. MeHg has been reported to elicit calpain activation accompanying intracellular Ca2+ elevation, and calpain inhibitor suppresses MeHg-induced decrease in cell viability in neuroblastoma cells and rat cerebellar neurons [29, 34]. Since the regulation of MARCKS functions by calpain proteolytic cleavage has also been reported, it is possible that calpain activation induced by MeHg exposure causes alteration in the MARCKS functions in a phosphorylation-independent manner [5, 22].
In summary, we showed that MeHg exposure induced a dose-dependent decrease in cell viability, migration, tube formation on Matrigel and NO production. MeHg exposure also elicited a decrease in MARCKS expression and an increase in MARCKS phosphorylation in EA.hy926 cells. Furthermore, alteration of MeHg-induced decrease in cell viability and NO production was observed in cells with MARCKS knockdown or overexpression. The findings of our study suggest the broad role of MARCKS in endothelial cell functions and show that MARCKS is involved in MeHg-induced toxicity in endothelial cells. It has been reported that MARCKS plays roles in cell proliferation, migration and tube formation of endothelial cells through the regulation of actin polymerization and sequestering phospholipid phosphatidylinositol 4,5-bisphosphate [17, 48]. Future studies are needed to determine the precise roles of MARCKS on the toxicity of MeHg on endothelial cells.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (B) (No. 23780298) and Scientific Research (C) (No. 26450407) from the Japan Society for the Promotion of Sciences (JSPS).
REFERENCES
- 1.Albert K. A., Walaas S. I., Wang J. K., Greengard P.1986. Widespread occurrence of “87 kDa,” a major specific substrate for protein kinase C. Proc. Natl. Acad. Sci. U.S.A. 83: 2822–2826. doi: 10.1073/pnas.83.9.2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbuzova A., Schmitz A. A., Vergères G.2002. Cross-talk unfolded: MARCKS proteins. Biochem. J. 362: 1–12. doi: 10.1042/bj3620001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brudvig J. J., Weimer J. M.2015. X MARCKS the spot: myristoylated alanine-rich C kinase substrate in neuronal function and disease. Front. Cell. Neurosci. 9: 407. doi: 10.3389/fncel.2015.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi A. L., Weihe P., Budtz-Jørgensen E., Jørgensen P. J., Salonen J. T., Tuomainen T. P., Murata K., Nielsen H. P., Petersen M. S., Askham J., Grandjean P.2009. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ. Health Perspect. 117: 367–372. doi: 10.1289/ehp.11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulong S., Goudenege S., Vuillier-Devillers K., Manenti S., Poussard S., Cottin P.2004. Myristoylated alanine-rich C kinase substrate (MARCKS) is involved in myoblast fusion through its regulation by protein kinase Calpha and calpain proteolytic cleavage. Biochem. J. 382: 1015–1023. doi: 10.1042/BJ20040347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eto K.1997. Pathology of Minamata disease. Toxicol. Pathol. 25: 614–623. doi: 10.1177/019262339702500612 [DOI] [PubMed] [Google Scholar]
- 7.Eto K., Tokunaga H., Nagashima K., Takeuchi T.2002. An autopsy case of minamata disease (methylmercury poisoning)--pathological viewpoints of peripheral nerves. Toxicol. Pathol. 30: 714–722. doi: 10.1080/01926230290166805 [DOI] [PubMed] [Google Scholar]
- 8.Fujimura M., Usuki F., Kawamura M., Izumo S.2011. Inhibition of the Rho/ROCK pathway prevents neuronal degeneration in vitro and in vivo following methylmercury exposure. Toxicol. Appl. Pharmacol. 250: 1–9. doi: 10.1016/j.taap.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 9.Green T. D., Park J., Yin Q., Fang S., Crews A. L., Jones S. L., Adler K. B.2012. Directed migration of mouse macrophages in vitro involves myristoylated alanine-rich C-kinase substrate (MARCKS) protein. J. Leukoc. Biol. 92: 633–639. doi: 10.1189/jlb.1211604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grotto D., de Castro M. M., Barcelos G. R., Garcia S. C., Barbosa F., Jr2009. Low level and sub-chronic exposure to methylmercury induces hypertension in rats: nitric oxide depletion and oxidative damage as possible mechanisms. Arch. Toxicol. 83: 653–662. doi: 10.1007/s00204-009-0437-8 [DOI] [PubMed] [Google Scholar]
- 11.Hirooka T., Fujiwara Y., Yamamoto C., Yasutake A., Kaji T.2007. Methylmercury retards the repair of wounded monolayer of human brain microvascular endothelial cells by inhibiting their proliferation without nonspecific cell damage. J. Health Sci. 53: 450–456. doi: 10.1248/jhs.53.450 [DOI] [Google Scholar]
- 12.Hirooka T., Fujiwara Y., Inoue S., Shinkai Y., Yamamoto C., Satoh M., Yasutake A., Eto K., Kaji T.2009. Suppression of fibroblast growth factor-2 expression: possible mechanism underlying methylmercury-induced inhibition of the repair of wounded monolayers of cultured human brain microvascular endothelial cells. J. Toxicol. Sci. 34: 433–439. doi: 10.2131/jts.34.433 [DOI] [PubMed] [Google Scholar]
- 13.Islam M. Z., Miyagi K., Matsumoto T., Nguyen H. T. T., Yamazaki-Himeno E., Shiraishi M., Miyamoto A.2014a. Bradykinin induces NO and PGF2α production via B2 receptor activation from cultured porcine basilar arterial endothelial cells. Naunyn Schmiedebergs Arch. Pharmacol. 387: 697–702. doi: 10.1007/s00210-014-0989-x [DOI] [PubMed] [Google Scholar]
- 14.Islam M. Z., Watanabe Y., Nguyen H. T. T., Yamazaki-Himeno E., Obi T., Shiraishi M., Miyamoto A.2014b. Vasomotor effects of acetylcholine, bradykinin, noradrenaline, 5-hydroxytryptamine, histamine and angiotensin II on the mouse basilar artery. J. Vet. Med. Sci. 76: 1339–1345. doi: 10.1292/jvms.14-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Islam M. Z., Van Dao C., Shiraishi M., Miyamoto A.2016. Methylmercury affects cerebrovascular reactivity to angiotensin II and acetylcholine via Rho-kinase and nitric oxide pathways in mice. Life Sci. 147: 30–38. doi: 10.1016/j.lfs.2016.01.033 [DOI] [PubMed] [Google Scholar]
- 16.Jin B. Y., Lin A. J., Golan D. E., Michel T.2012. MARCKS protein mediates hydrogen peroxide regulation of endothelial permeability. Proc. Natl. Acad. Sci. U.S.A. 109: 14864–14869. doi: 10.1073/pnas.1204974109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalwa H., Michel T.2011. The MARCKS protein plays a critical role in phosphatidylinositol 4,5-bisphosphate metabolism and directed cell movement in vascular endothelial cells. J. Biol. Chem. 286: 2320–2330. doi: 10.1074/jbc.M110.196022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalwa H., Sartoretto J. L., Sartoretto S. M., Michel T.2012. Angiotensin-II and MARCKS: a hydrogen peroxide- and RAC1-dependent signaling pathway in vascular endothelium. J. Biol. Chem. 287: 29147–29158. doi: 10.1074/jbc.M112.381517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y. J., Kim Y. S., Kim M. S., Ryu J. C.2007. The inhibitory mechanism of methylmercury on differentiation of human neuroblastoma cells. Toxicology 234: 1–9. doi: 10.1016/j.tox.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto T., Oguri T., Abe M., Kajitani H., Tada M.1995a. Inhibitory effect of methylmercury on migration and tube formation by cultured human vascular endothelial cells. Arch. Toxicol. 69: 357–361. doi: 10.1007/s002040050184 [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto T., Oguri T., Tada M.1995b. Methylmercury-injury effect on tube formation by cultured human vascular endothelial cells. Cell Biol. Toxicol. 11: 29–36. [DOI] [PubMed] [Google Scholar]
- 22.Lampe W. R., Park J., Fang S., Crews A. L., Adler K. B.2012. Calpain and MARCKS protein regulation of airway mucin secretion. Pulm. Pharmacol. Ther. 25: 427–431. doi: 10.1016/j.pupt.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mas M.2009. A Close Look at the Endothelium: Its Role in the Regulation of Vasomotor Tone. Eur. Urol. Suppl. 8: 48–57. doi: 10.1016/j.eursup.2008.10.011 [DOI] [Google Scholar]
- 24.McNamara R. K., Lenox R. H.1997. Comparative distribution of myristoylated alanine-rich C kinase substrate (MARCKS) and F1/GAP-43 gene expression in the adult rat brain. J. Comp. Neurol. 379: 48–71. doi: [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto A., Hashiguchi Y., Obi T., Ishiguro S., Nishio A.2007. Ibuprofen or ozagrel increases NO release and l-nitro arginine induces TXA2 release from cultured porcine basilar arterial endothelial cells. Vascul. Pharmacol. 46: 85–90. doi: 10.1016/j.vph.2006.06.018 [DOI] [PubMed] [Google Scholar]
- 26.Monahan T. S., Andersen N. D., Martin M. C., Malek J. Y., Shrikhande G. V., Pradhan L., Ferran C., LoGerfo F. W.2009. MARCKS silencing differentially affects human vascular smooth muscle and endothelial cell phenotypes to inhibit neointimal hyperplasia in saphenous vein. FASEB J. 23: 557–564. doi: 10.1096/fj.08-114173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myat M. M., Anderson S., Allen L. A., Aderem A.1997. MARCKS regulates membrane ruffling and cell spreading. Curr. Biol. 7: 611–614. doi: 10.1016/S0960-9822(06)00262-4 [DOI] [PubMed] [Google Scholar]
- 28.Ott L. E., Sung E. J., Melvin A. T., Sheats M. K., Haugh J. M., Adler K. B., Jones S. L.2013. Fibroblast migration is regulated by myristoylated alanine-rich C-kinase substrate (MARCKS) protein. PLoS ONE 8: e66512. doi: 10.1371/journal.pone.0066512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petroni D., Tsai J., Agrawal K., Mondal D., George W.2012. Low-dose methylmercury-induced oxidative stress, cytotoxicity, and tau-hyperphosphorylation in human neuroblastoma (SH-SY5Y) cells. Environ. Toxicol. 27: 549–555. doi: 10.1002/tox.20672 [DOI] [PubMed] [Google Scholar]
- 30.Rissanen T., Voutilainen S., Nyyssönen K., Lakka T. A., Salonen J. T.2000. Fish oil-derived fatty acids, docosahexaenoic acid and docosapentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation 102: 2677–2679. doi: 10.1161/01.CIR.102.22.2677 [DOI] [PubMed] [Google Scholar]
- 31.Roman H. A., Walsh T. L., Coull B. A., Dewailly É., Guallar E., Hattis D., Mariën K., Schwartz J., Stern A. H., Virtanen J. K., Rice G.2011. Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environ. Health Perspect. 119: 607–614. doi: 10.1289/ehp.1003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rombouts K., Mello T., Liotta F., Galli A., Caligiuri A., Annunziato F., Pinzani M.2012. MARCKS actin-binding capacity mediates actin filament assembly during mitosis in human hepatic stellate cells. Am. J. Physiol. Cell Physiol. 303: C357–C367. doi: 10.1152/ajpcell.00093.2012 [DOI] [PubMed] [Google Scholar]
- 33.Rombouts K., Carloni V., Mello T., Omenetti S., Galastri S., Madiai S., Galli A., Pinzani M.2013. Myristoylated Alanine-Rich protein Kinase C Substrate (MARCKS) expression modulates the metastatic phenotype in human and murine colon carcinoma in vitro and in vivo. Cancer Lett. 333: 244–252. doi: 10.1016/j.canlet.2013.01.040 [DOI] [PubMed] [Google Scholar]
- 34.Sakaue M., Okazaki M., Hara S.2005. Very low levels of methylmercury induce cell death of cultured rat cerebellar neurons via calpain activation. Toxicology 213: 97–106. doi: 10.1016/j.tox.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 35.Salonen J. T., Seppänen K., Lakka T. A., Salonen R., Kaplan G. A.2000. Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis 148: 265–273. doi: 10.1016/S0021-9150(99)00272-5 [DOI] [PubMed] [Google Scholar]
- 36.Shiraishi M., Tanabe A., Saito N., Sasaki Y.2006. Unphosphorylated MARCKS is involved in neurite initiation induced by insulin-like growth factor-I in SH-SY5Y cells. J. Cell. Physiol. 209: 1029–1038. doi: 10.1002/jcp.20814 [DOI] [PubMed] [Google Scholar]
- 37.Shiraishi M., Hangai M., Yamamoto M., Sasaki M., Tanabe A., Sasaki Y., Miyamoto A.2014. Alteration in MARCKS phosphorylation and expression by methylmercury in SH-SY5Y cells and rat brain. Environ. Toxicol. Pharmacol. 37: 1256–1263. doi: 10.1016/j.etap.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 38.Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J.1989. Molecular cloning, characterization, and expression of a cDNA encoding the “80- to 87-kDa” myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc. Natl. Acad. Sci. U.S.A. 86: 4012–4016. doi: 10.1073/pnas.86.11.4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stumpo D. J., Bock C. B., Tuttle J. S., Blackshear P. J.1995. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc. Natl. Acad. Sci. U.S.A. 92: 944–948. doi: 10.1073/pnas.92.4.944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venardos K., Enriquez C., Marshall T., Chin-Dusting J. P., Ahlers B., Kaye D. M.2009. Protein kinase C mediated inhibition of endothelial L-arginine transport is mediated by MARCKS protein. J. Mol. Cell. Cardiol. 46: 86–92. doi: 10.1016/j.yjmcc.2008.09.712 [DOI] [PubMed] [Google Scholar]
- 41.Villar I. C., Francis S., Webb A., Hobbs A. J., Ahluwalia A.2006. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int. 70: 840–853. doi: 10.1038/sj.ki.5001680 [DOI] [PubMed] [Google Scholar]
- 42.Wakabayashi K., Kakita A., Sakamoto M., Su M., Iwanaga K., Ikuta F.1995. Variability of brain lesions in rats administered methylmercury at various postnatal development phases. Brain Res. 705: 267–272. doi: 10.1016/0006-8993(95)01208-7 [DOI] [PubMed] [Google Scholar]
- 43.Wakita Y.1987. Hypertension induced by methyl mercury in rats. Toxicol. Appl. Pharmacol. 89: 144–147. doi: 10.1016/0041-008X(87)90185-2 [DOI] [PubMed] [Google Scholar]
- 44.Wildemann T. M., Mirhosseini N., Siciliano S. D., Weber L. P.2015. Cardiovascular responses to lead are biphasic, while methylmercury, but not inorganic mercury, monotonically increases blood pressure in rats. Toxicology 328: 1–11. doi: 10.1016/j.tox.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 45.Yorifuji T., Tsuda T., Kashima S., Takao S., Harada M.2010. Long-term exposure to methylmercury and its effects on hypertension in Minamata. Environ. Res. 110: 40–46. doi: 10.1016/j.envres.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 46.Yu D., Makkar G., Dong T., Strickland D. K., Sarkar R., Monahan T. S.2015a. MARCKS signaling differentially regulates vascular smooth muscle and endothelial cell proliferation through a KIS-, p27kip1- dependent mechanism. PLOS ONE 10: e0141397. doi: 10.1371/journal.pone.0141397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu D., Makkar G., Strickland D. K., Blanpied T. A., Stumpo D. J., Blackshear P. J., Sarkar R., Monahan T. S.2015b. Myristoylated alanine-rich protein kinase substrate (MARCKS) regulates small GTPase Rac1 and Cdc42 activity and is a critical mediator of vascular smooth muscle cell migration in intimal hyperplasia formation. J. Am. Heart Assoc. 4: e002255. doi: 10.1161/JAHA.115.002255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y., Neltner B. S., Davis H. W.2000. Role of MARCKS in regulating endothelial cell proliferation. Am. J. Physiol. Cell Physiol. 279: C1611–C1620. [DOI] [PubMed] [Google Scholar]