Abstract
Effects of a bacterial probiotic (BP) on ruminal fermentation and plasma metabolites were evaluated in four Holstein cattle (body weight, 645 ± 62 kg; mean ± SD) with induced subacute ruminal acidosis (SARA). SARA was induced by feeding a SARA-inducing diet, and thereafter, 20, 50 or 100 g per head of a commercial BP was administered for 7 consecutive days during the morning feeding. Cattle without BP served as the control. The 24-hr mean ruminal pH in the control was lower, whereas those in the BP groups administered 20 or 50 g were significantly higher compared to the control from days 2 to 7. Circadian patterns of the 1-hr mean ruminal pH were identical (6.4–6.8) among all cattle receiving BP. Although the mean minimum pH in the control on day –7 and day 0 was <5.8, the pH in the treatment groups on day 7 was >5.8 and significantly higher than that of the control group ( >5.2). Ruminal volatile fatty acid (VFA) concentrations were not affected by BP treatment; however, the BP groups had lower lactic acid levels compared with the control group at 20:00 on day 7. Additionally, non-esterified fatty acid levels decreased from 8:00 to 20:00 in all BP groups on day 7. These results suggest that administration of 20 to 50 g of a multi-strain BP for 7 days might improve the low pH and high lactic acid level of the ruminal fluid in SARA cattle.
Keywords: bacterial probiotic, cattle, ruminal pH, subacute ruminal acidosis, VFA
Probiotics composed of various microbial components are known to improve ruminal fermentation by activating rumen microbiota [6, 12, 17, 26] and directly increasing ruminal performance and dry matter intake in dairy cattle [4, 28]. Among bacterial probiotics (BPs), lactic acid bacteria (LAB), including Lactobacillus plantarum (L. plantarum) and Enterococcus faecium (E. faecium), are the most frequent bacterial species used in ruminants [7, 28]. Repeated administration of a BP could be the optimal solution for countering decreases in ruminal bacteria, particularly in animals with digestive disorders [12, 14]. Current research on the microbial composition and functional diversity of ruminant digestive ecosystems suggests that consecutive BP supplementation improves the performance of animals by altering their ruminal microbiota and increasing their digestion capability [4, 17, 26]. BP reduces organic acid accumulation and might decrease the risk of subacute ruminal acidosis (SARA) [19, 22]. However, there is little information on the effects of a multi-strain BP containing LAB on SARA in cattle.
SARA occurs when dairy cattle are fed large quantities of rapidly fermentable carbohydrates that exceed the buffering capacity of the rumen [22]. When ruminal volatile fatty acids (VFAs) and lactate accumulate, the ruminal pH is decreased [11, 22]. In cattle, if the ruminal pH decreases below 6.0, fiber digestibility is decreased, and animals may show clinical signs of SARA [16, 19]. To diagnose SARA, continuous pH measurements of the ruminal fluid using automated pH measurement systems developed for this purpose are required [10, 24]. In addition to ruminal pH, VFA and lactic acid concentrations are important indicators of ruminal fermentation for diagnosing SARA in dairy cattle [2, 5, 14].
To prevent and treat SARA, the use of live yeast, such as Saccharomyces cerevisiae, as a probiotic has been studied in cattle fed high-concentrate diets. S. cerevisiae increases the pH and decreases lactic acid concentrations in the rumen [2, 8, 25]. However, preventing and treating SARA are still challenges in clinical veterinary medicine. Various BP products have been investigated for their ability to modulate rumen fermentation characteristics in cattle fed high-concentrate diets [7, 12, 17], although the effects of BP on SARA in cattle have not been studied extensively. We previously reported the effects of a BP containing L. plantarum, E. faecium and Clostridium butyricum on the ruminal components of weaned calves [20]. In this study, we examined the effects of different doses of a multi-strain BP on ruminal pH and VFA, lactic acid, ammonia nitrogen (NH3-N) and plasma metabolite concentrations in cattle with SARA.
MATERIALS AND METHODS
Animals and treatment: All procedures in this experiment were conducted following protocols approved by the Iwate University Laboratory Animal Care and Use Committee. Four primiparous non-lactating, rumen-cannulated healthy Holstein cattle, weighing 645 ± 62 (mean ± SD) kg, were housed in a stanchion barn at the Cattle Research Center of Iwate University. During the experimental period, the cattle were fed a SARA-inducing diet 2 weeks before and 1 week after first administration of BP. The SARA-inducing diet was composed of a mix of orchard grass and timothy hay with an equivalent amount of flaked barley and corn. Each cattle was fed 5.5–6.5 kg dry matter twice daily. The ratio of roughage-to-concentrate was 3:7, and the percentages of total digestible nutrients, crude protein, neutral detergent fiber, non-fiber carbohydrates and starch in the dry matter were adjusted to 75.1, 12.2, 37.7, 42.4 and 37.0%, respectively. Cattle were fed daily at 9:00 and 17:00 and allowed free access to fresh water. Cattle were assigned randomly to a 4 × 4 Latin square experimental design containing three treatments and a control. The BP (Miyarisan Pharmaceutical Co., Ltd., Tokyo, Japan) included L. plantarum strain 220 (9 × 106 colony-forming units (CFU)/g), E. faecium strain 26 (9 × 105 CFU/g) and C. butyricum strain Miyari (9 × 104 CFU/g) was administered as a daily single dose of 20, 50 or 100 g per head for 7 consecutive days. Cows fed the SARA-inducing diet without BP served as a control. Based on the effects of the treatment, the experimental design consisted of 2 weeks of an adaptation period to the respective BP treatment during which cattle were fed only hay. The BP was stored at 4°C, and each dose was mixed with their diet during the morning feeding. No clinical disorders were observed in the cattle during the experimental period.
Ruminal pH measurements: Ruminal pH was measured with a radio transmission pH measurement system (YCOW-S; DKK-TOA Yamagata, Yamagata, Japan). The pH sensor was calibrated with pH 4 and 7 buffer solutions at the start of each experiment and was placed in the ventral sac of the rumen via the rumen cannula, as the ventral sac of the rumen has more stable pH values than the other ruminal site [10]. Ruminal pH was recorded continuously every 10 min throughout the experimental period. The pH sensors remained in the ventral sac of the rumen throughout the trial.
Ruminal fluid sampling and VFA, lactic acid and NH3-N assays: Ruminal fluids were collected from the same location as the pH sensor through the rumen cannula at 8:00, 11:00, 14:00, 17:00 and 20:00 on 7 days before (day −7) and 7 days after (day 7) the first BP administration (day 0). These sampling times (8:00–20:00) were chosen based on our previous report [23]. Samples for the VFA, lactic acid and NH3-N measurements were filtered immediately through two layers of cheesecloth. For the VFA analysis, 10 ml of ruminal fluid was added to 2 ml of 25% metaphosphoric acid in 3 N sulfuric acid. Total VFAs and three individual VFAs (acetic acid, propionic acid and butyric acid) were separated and quantified with gas chromatography (Model 135, Hitachi, Tokyo, Japan) using a packed glass column (3% Thermon-3000) on a Shimalite TPA 60–80 support (Shinwa Chemical Industries Ltd., Kyoto, Japan). For the lactic acid analysis, the ruminal fluid was centrifuged immediately at 2,000 × g for 15 min, and concentrations in the supernatant were determined using a commercially available kit (F-kit; D-lactate/L-lactate, J. K. International Co., Tokyo, Japan). To measure NH3-N concentrations, ruminal fluid was analyzed using the steam distillation method with an NH3-N analyzer (Kjeltec Auto Sampler System 1035 Analyzer, Tecator, Sweden).
Blood sampling and plasma metabolite profiles: Blood samples were collected from the jugular vein into 10-ml evacuated serum-separating tubes and tubes containing sodium fluoride (BD Vacutainer, Franklin Lakes, NJ, U.S.A.). Blood samples were collected at the same time as the ruminal fluid samples (days −7 and 7), centrifuged (1,500 × g, 15 min, 4°C) immediately to separate the serum and plasma, and preserved at −80°C until the analysis. Glucose (GLU), non-esterified fatty acids (NEFA) and β-hydroxybutyrate acid (BHBA) in plasma, as well as total cholesterol (T-Chol), triglycerides (TG) and blood urea nitrogen (BUN) in sera were analyzed using an automated biochemistry analyzer (Accute, Toshiba Ltd., Tokyo, Japan).
Statistical analysis: Quantitative data are expressed as means ± standard errors (SEs). The main effects included the SARA challenge and BP treatment, day of the experiment, and hours after administration and feeding. Diurnal measurements of the ruminal pH were analyzed as the 24-hr mean pH from day −7 to day 7. The pH data collected in 10-min intervals were summarized as a 1-hr mean from 9:00 to 8:00 of the following day to assess circadian changes. The minimum and maximum pH in one day was determined for days −7, 0 and 7. Graph Pad Prism ver. 5.01 (La Jolla, CA, U.S.A.) was used for the statistical calculations, and two-way repeated-measures analysis of variance (ANOVA) followed by the Bonferroni post hoc test was used to evaluate the differences between the treatment and control groups. P-values <0.05 were considered to be significant.
RESULTS
Ruminal pH: SARA was successfully induced in cattle fed the SARA-inducing diet. According to representative pH data on 3 days after beginning the diet, the 1-hr mean ruminal pH decreased rapidly and slowly after the morning and evening feedings, which was indicative of SARA (Fig. 1). Considerable disparities in the ruminal pH among the treatment and control groups were observed and continued throughout the treatment period (Fig. 2). The 24-hr mean ruminal pH in the control was generally lower, whereas that in the BP groups receiving 20 or 50 g was significantly higher from days 2 to 7 compared with the control. The 24-hr mean pH in the 100 g group was also significantly higher from days 2 to 4 compared with the control; however, it decreased on days 5 to 7. Among the treatment groups, the 20 g BP group maintained a constant pH (6.4–6.5) from days 3 to 7. Circadian changes in the 1-hr mean ruminal pH were almost identical among the treatment groups on days 0 and 7. However, the 1-hr mean pHs in the treatment groups were slightly higher than those in the control group on day 7 (Fig. 3). Additionally, the mean minimum pH in the control on day 7 was <5.0, which was significantly lower than in the treatment groups (Fig. 4). The mean maximum pH approached >6.8 in the treatment groups, which did not differ from that in the control.
Fig. 1.
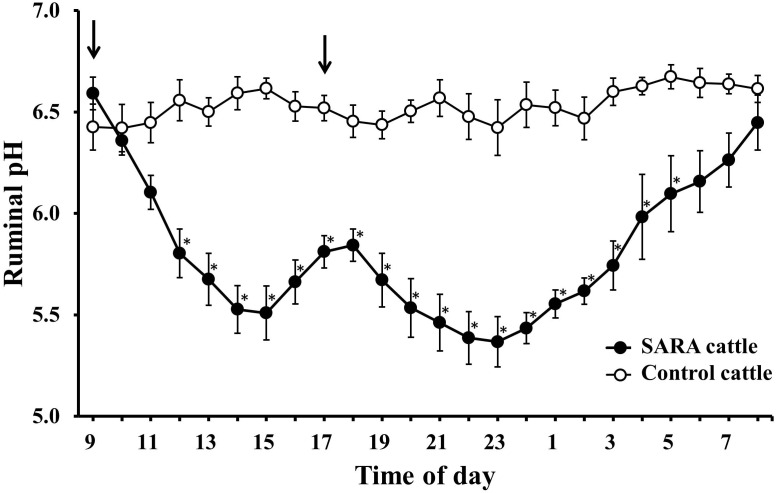
Circadian changes in the 1-hr mean ruminal pH at control day (n=4; ○) and 3 days after beginning the SARA-inducing diet (n=4; ●). Cattle satiated with hay served as the control. The 1-hr mean pH in cattle fed SARA-inducing diet decreased after the morning and evening feedings, which was indicative of successfully induced SARA. Data are means ± SE. * Significant difference compared with a control on the same day (P<0.05). The arrows indicate the feeding times.
Fig. 2.
Changes in the 24-hr mean ruminal pH in cattle administered 20 g (n=4; ●), 50 g (n=4; ♦) or 100 g (n=4; ■) of a bacterial probiotic for 7 consecutive days. Cattle not administered the probiotic served as a control (n=4; ○). Data are means ± SE. * Significant difference compared with a control on the same day (P<0.05). The first day of probiotic administration was regarded as day 0.
Fig. 3.
Circadian changes in the 1-hr mean ruminal pH on days 0 and 7 in cattle administered 20 g (n=4; ●), 50 g (n=4; ♦) or 100 g (n=4; ■) of a bacterial probiotic for 7 consecutive days. Cattle not administered the probiotic served as a control (n=4; ○). Data are means ± SE. The arrows indicate feeding times.
Fig. 4.
Box plots of the maximum and minimum ruminal pH on days –7, 0 and 7 in the treatment groups administered 20 g (n=4; light gray boxes), 50 g (n=4; gray boxes) and 100 g (n=4; dark boxes) of a bacterial probiotic for 7 consecutive days. Cattle not administered the probiotic served as a control (n=4; white boxes). The median and quartiles are displayed in the box. The upper and lower bars represent the maximum and minimum values, respectively. * Significant difference compared with a control on the same day (P<0.05). # Significant difference compared with the same group on day 7 (P<0.05).
Ruminal VFA, lactic acid and NH3-N concentrations: Total VFA increased from 8:00 to 20:00 in the treatment and control groups on days 0 and 7 (Fig. 5). However, no difference was observed in the total or individual VFA concentrations between the treatment and control groups. The acetic acid-to-propionic acid ratio (A:P) was almost identical among the treatment groups. Lactic acid concentrations remained stable in the treatment groups, and the concentrations at 20:00 on day 7 were significantly lower than that in the control. No difference was observed in NH3-N concentrations among the treatment groups, although the NH3-N concentrations in the 50 g and 100 g groups at 8:00 on day 7 were significantly higher than that in the control.
Fig. 5.
Circadian changes in the ruminal VFA, A:P ratio, lactic acid and NH3-N concentrations on days 0 and 7 in cattle administered 20 g (n=4; ●), 50 g (n=4; ♦) or 100 g (n=4; ■) of a bacterial probiotic for 7 consecutive days. Cattle not administered the probiotic served as a control (n=4; ○). Data are means ± SE. * Significant difference compared with a control on the same day (P<0.05).
Blood metabolite profiles: GLU concentrations decreased from 8:00 to 20:00, but were almost the same among the treatment and control groups (Fig. 6). NEFA levels were affected by feeding time and were significantly lower at 20:00 compared with at 8:00 in the treatment groups on days 0 and 7. Furthermore, NEFA level in the 50 g group at 20:00 on day 7 was significantly lower than that in the control. In contrast, BHBA and BUN concentrations were higher and lower, respectively, in all groups from 8:00 to 20:00. BHBA concentrations in the 20 g and 50 g at 17:00 and 20:00, and BUN concentrations in the treatment groups at 8:00 were significantly higher than that in the controls, respectively. T-Chol and TG concentrations were unaffected by BP treatment.
Fig. 6.
Circadian changes in the blood GLU, NEFA, BHBA and BUN concentrations on days 0 and 7 in cattle administered 20 g (n=4; ●), 50 g (n=4; ♦) or 100 g (n=4; ■) of a bacterial probiotic for 7 consecutive days. Cattle not administered the probiotic served as a control (n=4; ○). Data are means ± SE. * Significant difference compared with a control on the same day (P<0.05).
DISCUSSION
SARA occurs when the ruminal pH decreases due to a combination of overproduction of VFA and lactic acid and a decrease in VFA absorption in the rumen [1, 22]. SARA is diagnosed when the pH in the ruminal fluid is <5.6 for at least 3 hr per day [5, 17, 21]. In this study, the ruminal pH measured continuously indicated that SARA was successfully induced during the experimental period, especially during the first week of the experiment, by feeding a SARA-inducing diet.
Studies have shown that the main ruminal bacteria affecting the ruminal fermentative capacity are classified as lactic acid-producers and consumers [4, 15], and both of these bacterial groups have been used as BPs [12]. Ghorbani et al. (2002) [12] reported that a BP containing both lactate-consuming (Propionibacterium) and lactate-producing (Enterococcus) bacteria activated ruminal fermentation and reduced the risk of acidosis in cattle. In another study, a BP consisting of L. plantarum and E. faecium induced changes in the ruminal pH of cows fed a high-grain diet [17]. However, the mechanism of the effects of BP on ruminal fermentation is unclear, and the effects of BP on SARA in cattle are unknown. In this study, the 24-hr mean ruminal pH was higher in the BP-treated groups compared with in the control group during SARA challenge. This was indicative of increased ruminal fermentation in the cows, as reported previously [12, 27, 28]. Chiquette et al. (2012) [7] examined the effects of administering BP in SARA-challenged cows and found no effects on ruminal pH when BP was administered as a single strain; however, ruminal pH increased compared over that in the control group when using a combination of E. faecium and yeast. We used the same BP in a previous study on weaned calves fed a high-concentrate diet, and the mean ruminal pH was significantly higher in the treatment group compared with in the control group [20]. In this study, different doses of a multi-strain BP were administered to SARA-challenged cows, and the 24-hr mean ruminal pH was notably higher on days 3 to 7, whereas the minimum pH increased on day 7 in the treatment groups.
BP appears to increase the ability of ruminal bacteria to metabolize lactic acid and regulate ruminal pH [20, 21]. It has been hypothesized that the functionality and efficacy of BPs can be determined based on their effects on the predominant rumen microbiota [7, 12]. In this study, cattle receiving lower BP doses (20 g per head) had a constant mean pH of 6.4–6.6. In contrast, cattle receiving higher BP doses (100 g per head) had higher mean pH on days 2 and 3, which decreased on days 5 to 7. Previous reports have indicated that changes in rumen bacterial composition and diversity, increased activities of lactate-consuming bacteria and greater lactate absorption affect ruminal pH [13, 15]. The higher BP dose might increase LAB numbers in the rumen, which could be related to increased ruminal fermentation capacity, higher carbohydrate fermentation and increased ruminal pH [22]. Therefore, ruminal LAB might have been overly increased due to the higher BP dose, causing the ruminal fermentation and pH to decrease on day 5. These results are consistent with previous reports that lower BP doses improve gastrointestinal tract microbiota in calves [26]. Furthermore, weaned calves administered lower BP doses showed a higher mean ruminal pH compared with controls [20]. Based on our results, administration of an extremely high dose (100 g per head) of LAB could cause decreased ruminal fermentation and ruminal pH.
Decreases in ruminal pH are related to VFA production and lactic acid accumulation after feeding a high-grain diet [1, 18]. In this study, although ruminal VFA concentrations were not affected by BP treatment, they were affected by feeding time. This is in agreement with previous reports in which ruminal VFAs were not affected by probiotics including LAB [6, 20]. Administration of LAB probiotics is thought to help rumen microbiota adapt to the presence of lactic acid [12] and prevent lactate accumulation in the rumen [22]. Russell et al. (1992) [21] reported that lactate-consuming bacteria increased only when lactic acid accumulated and ruminal pH decreased. Based on these results, LAB probiotics might affect ruminal pH by increasing the activity of lactate-consuming bacteria [7]. In our previous study, significantly lower lactic acid concentrations were observed in the ruminal fluid of weaned calves receiving BP compared with the control [20]. In this study, lactic acid concentrations were stable in the treatment groups; however, lactic acid was higher and correlated with a lower mean ruminal pH in the control group. Gradually increasing ruminal pH might be due to time after feeding and the absorption capacity of the rumen [9]. BPs may prevent a decline in ruminal pH by increasing the lactic acid consumption by some microbes [3, 6]. A combination of certain probiotic bacteria that synthesize lactic acid may sustain a tonic level of lactic acid in the rumen, stimulating rumen microbial communities that consume lactic acid and reducing acidity, causing the ruminal pH to remain constant and stable.
Conversely, the NH3-N concentrations in the ruminal fluid of the BP groups remained stable in this study, although significant difference was observed at 8:00 on day 7 between the BP-treated and control groups. BP has no effect on the NH3-N concentrations in the rumen [7, 12]. Decreases in NH3-N levels after morning feeding in this study were in agreement with the previous study, which found that NH3-N concentrations decreased with decreasing ruminal pH [7, 13]. Furthermore, NEFA levels decreased after the morning feeding in this study. It has been reported that NEFA levels are lower in steers with SARA compared with in the control [5]; therefore, the decrease in NEFA levels indicates a more efficient use of dietary energy and greater dry matter intake in the BP-treated groups.
In conclusion, repeated administration of a BP comprised of L. plantarum, E. faecium and C. butyricum improved the 24-hr mean ruminal pH in cattle with experimentally induced SARA at doses of 20 or 50 g per head. Diurnal patterns of the 1-hr mean ruminal pH were identical among the treatment and control groups. Ruminal VFA was not affected by BP treatment; however, lactic acid was lower in the treatment groups than in the control group. Based on these results, BP might affect ruminal pH by increasing lactate consumption and decreasing lactic acid concentrations, resulting in a consistently higher ruminal pH in SARA cattle. These results suggest that repeated administration of a multi-strain BP might reduce the risk of SARA in cattle and that consecutive treatment with 20 or 50 g of a BP containing LAB during high-concentrate feeding might reduce the incidence of SARA in dairy cattle.
REFERENCES
- 1.Aschenbach J. R., Penner G. B., Stumpff F., Gäbel G.2011. Ruminant Nutrition Symposium: Role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 89: 1092–1107. doi: 10.2527/jas.2010-3301 [DOI] [PubMed] [Google Scholar]
- 2.Bach A., Iglesias C., Devant M.2007. Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Anim. Feed Sci. Technol. 136: 146–153. doi: 10.1016/j.anifeedsci.2006.09.011 [DOI] [Google Scholar]
- 3.Beauchemin K. A., Yang W. Z., Morgavi D. P., Ghorbani G. R., Kautz W., Leedle J. A.2003. Effects of bacterial direct-fed microbials and yeast on site and extent of digestion, blood chemistry, and subclinical ruminal acidosis in feedlot cattle. J. Anim. Sci. 81: 1628–1640. [DOI] [PubMed] [Google Scholar]
- 4.Belanche A., Doreau M., Edwards J. E., Moorby J. M., Pinloche E., Newbold C. J.2012. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr. 142: 1684–1692. doi: 10.3945/jn.112.159574 [DOI] [PubMed] [Google Scholar]
- 5.Brown M. S., Krehbiel C. R., Galyean M. L., Remmenga M. D., Peters J. P., Hibbard B., Robinson J., Moseley W. M.2000. Evaluation of models of acute and subacute acidosis on dry matter intake, ruminal fermentation, blood chemistry, and endocrine profiles of beef steers. J. Anim. Sci. 78: 3155–3168. [DOI] [PubMed] [Google Scholar]
- 6.Chiquette J., Allison M. J., Rasmussen M. A.2008. Prevotella bryantii 25A used as a probiotic in early-lactation dairy cows: effect on ruminal fermentation characteristics, milk production, and milk composition. J. Dairy Sci. 91: 3536–3543. doi: 10.3168/jds.2007-0849 [DOI] [PubMed] [Google Scholar]
- 7.Chiquette J., Allison M. J., Rasmussen M.2012. Use of Prevotella bryantii 25A and a commercial probiotic during subacute acidosis challenge in midlactation dairy cows. J. Dairy Sci. 95: 5985–5995. doi: 10.3168/jds.2012-5511 [DOI] [PubMed] [Google Scholar]
- 8.Chung Y. H., Walker N. D., McGinn S. M., Beauchemin K. A.2011. Differing effects of 2 active dried yeast (Saccharomyces cerevisiae) strains on ruminal acidosis and methane production in nonlactating dairy cows. J. Dairy Sci. 94: 2431–2439. doi: 10.3168/jds.2010-3277 [DOI] [PubMed] [Google Scholar]
- 9.Dohme F., DeVries T. J., Beauchemin K. A.2008. Repeated ruminal acidosis challenges in lactating dairy cows at high and low risk for developing acidosis: ruminal pH. J. Dairy Sci. 91: 3554–3567. doi: 10.3168/jds.2008-1264 [DOI] [PubMed] [Google Scholar]
- 10.Duffield T., Plaizier J. C., Fairfield A., Bagg R., Vessie G., Dick P., Wilson J., Aramini J., McBride B.2004. Comparison of techniques for measurement of rumen pH in lactating dairy cows. J. Dairy Sci. 87: 59–66. doi: 10.3168/jds.S0022-0302(04)73142-2 [DOI] [PubMed] [Google Scholar]
- 11.Garrett E. F., Pereira M. N., Nordlund K. V., Armentano L. E., Goodger W. J., Oetzel G. R.1999. Diagnostic methods for the detection of subacute ruminal acidosis in dairy cows. J. Dairy Sci. 82: 1170–1178. doi: 10.3168/jds.S0022-0302(99)75340-3 [DOI] [PubMed] [Google Scholar]
- 12.Ghorbani G. R., Morgavi D. P., Beauchemin K. A., Leedle J. A. Z.2002. Effects of bacterial direct-fed microbials on ruminal fermentation, blood variables, and the microbial populations of feedlot cattle. J. Anim. Sci. 80: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 13.Khafipour E., Li S., Plaizier J. C., Krause D. O.2009. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 75: 7115–7124. doi: 10.1128/AEM.00739-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause K. M., Oetzel G. R.2005. Inducing subacute ruminal acidosis in lactating dairy cows. J. Dairy Sci. 88: 3633–3639. doi: 10.3168/jds.S0022-0302(05)73048-4 [DOI] [PubMed] [Google Scholar]
- 15.Mackie R. I., Gilchrist F. M. C.1979. Changes in lactate-producing and lactate-utilizing bacteria in relation to pH in the rumen of sheep during stepwise adaptation to a high-concentrate diet. Appl. Environ. Microbiol. 38: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nocek J. E., Kautz W. P.2006. Direct-fed microbial supplementation on ruminal digestion, health, and performance of pre- and postpartum dairy cattle. J. Dairy Sci. 89: 260–266. doi: 10.3168/jds.S0022-0302(06)72090-2 [DOI] [PubMed] [Google Scholar]
- 17.Nocek J. E., Kautz W. P., Leedle J. A., Allman J. G.2002. Ruminal supplementation of direct-fed microbials on diurnal pH variation and in situ digestion in dairy cattle. J. Dairy Sci. 85: 429–433. doi: 10.3168/jds.S0022-0302(02)74091-5 [DOI] [PubMed] [Google Scholar]
- 18.Pitt R. E., Pell A. N.1997. Modeling ruminal pH fluctuations: interactions between meal frequency and digestion rate. J. Dairy Sci. 80: 2429–2441. doi: 10.3168/jds.S0022-0302(97)76195-2 [DOI] [PubMed] [Google Scholar]
- 19.Plaizier J. C., Krause D. O., Gozho G. N., McBride B. W.2008. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet. J. 176: 21–31. doi: 10.1016/j.tvjl.2007.12.016 [DOI] [PubMed] [Google Scholar]
- 20.Qadis A. Q., Goya S., Ikuta K., Yatsu M., Kimura A., Nakanishi S., Sato S.2014. Effects of a bacteria-based probiotic on ruminal pH, volatile fatty acids and bacterial flora of Holstein calves. J. Vet. Med. Sci. 76: 877–885. doi: 10.1292/jvms.14-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell J. B., O’Connor J. D., Fox D. G., Van Soest P. J., Sniffen C. J.1992. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 70: 3551–3561. [DOI] [PubMed] [Google Scholar]
- 22.Russell J. B., Wilson D. B.1996. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 79: 1503–1509. doi: 10.3168/jds.S0022-0302(96)76510-4 [DOI] [PubMed] [Google Scholar]
- 23.Sato S., Kimura A., Anan T., Yamagishi N., Okada K., Mizuguchi H., Ito K.2012. A radio transmission pH measurement system for continuous evaluation of fluid pH in the rumen of cows. Vet. Res. Commun. 36: 85–89. doi: 10.1007/s11259-012-9518-x [DOI] [PubMed] [Google Scholar]
- 24.Sato S., Mizuguchi H., Ito K., Ikuta K., Kimura A., Okada K.2012. Technical note: development and testing of a radio transmission pH measurement system for continuous monitoring of ruminal pH in cows. Prev. Vet. Med. 103: 274–279. doi: 10.1016/j.prevetmed.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 25.Thrune M., Bach A., Ruiz Moreno M., Stern D., Linn J. G.2009. Effects of Saccharomyces cerevisiae on ruminal pH and microbial fermentation in dairy cows. Livest. Sci. 124: 261–265. doi: 10.1016/j.livsci.2009.02.007 [DOI] [Google Scholar]
- 26.Timmerman H. M., Mulder L., Everts H., van Espen D. C., van der Wal E., Klaassen G., Rouwers S. M. G., Hartemink R., Rombouts F. M., Beynen A. C.2005. Health and growth of veal calves fed milk replacers with or without probiotics. J. Dairy Sci. 88: 2154–2165. doi: 10.3168/jds.S0022-0302(05)72891-5 [DOI] [PubMed] [Google Scholar]
- 27.Weinberg Z. G., Muck R. E., Weimer P. J.2003. The survival of silage inoculant lactic acid bacteria in rumen fluid. J. Appl. Microbiol. 94: 1066–1071. doi: 10.1046/j.1365-2672.2003.01942.x [DOI] [PubMed] [Google Scholar]
- 28.Weinberg Z. G., Muck R. E., Weimer P. J., Chen Y., Gamburg M.2004. Lactic acid bacteria used in inoculants for silage as probiotics for ruminants. Appl. Biochem. Biotechnol. 118: 1–9. doi: 10.1385/ABAB:118:1-3:001 [DOI] [PubMed] [Google Scholar]