Abstract
Two Cheviot ewes homozygous for the A136L141R154Q171 (AL141RQ) prion protein (PrP) genotype were exposed intracerebrally to brain pools prepared using four field cases of atypical scrapie from the United Kingdom. Animals were clinically normal until the end of the experiment, when they were culled 7 years post-inoculation. Limited accumulation of disease-associated PrP (PrPSc) was observed in the cerebellar molecular layer by immunohistochemistry, but not by western blot or enzyme-linked immunosorbent assay. In addition, PrPSc was partially localized in astrocytes and microglia, suggesting that these cells have a role in PrPSc processing, degradation or both. Our results indicate that atypical scrapie is transmissible to AL141RQ sheep, but these animals act as clinically silent carriers with long incubation times.
Keywords: ARQ allele, atypical scrapie, prion, sheep, transmission
Scrapie is a neurodegenerative and fatal disorder of small ruminants included in a group of transmissible spongiform encephalopathies (TSEs) or prion diseases. Scrapie prions are classified into more than 20 strains on the basis of their characteristics in an inbred mouse model, namely, incubation period, lesion profile and distribution pattern of disease-associated prion protein (PrPSc), which is a misfolded variant of the cellular form (PrPC) [5]. A distinct scrapie phenotype, named atypical or Nor98 scrapie, was first recorded in Norwegian sheep in 1998 [3]. Atypical scrapie has since been sporadically detected in most European [2, 6, 9, 28, 32, 37, 38, 40, 49] and North American countries [25, 29], the Falkland Islands [11], and New Zealand [20]. However, its origin remains obscure. Atypical scrapie occurs in sheep over 6 years old and has been proposed to be a spontaneous/sporadic form of the disease or an infectious type with very low infection rates under normal conditions [2, 3, 23]. Despite the ongoing TSE surveillance program concerning fallen sheep and goats conducted by the Japanese Ministry of Agriculture, Forestry and Fisheries, no cases of atypical scrapie have been identified in this country to date.
The susceptibility of sheep to scrapie is strongly dependent on alleles of the host prion protein (PrP) gene (PRNP), involving polymorphisms at codons 136 (alanine [A136] or valine [V136]), 154 (arginine [R154] or histidine [H154]) and 171 (glutamine [Q171], R171 or H171) [4, 8, 17]. For instance, V136R154Q171 (shortened to VRQ) sheep are known to be highly susceptible to classical scrapie, whereas animals carrying the ARR genotype are resistant to this disease. In contrast, high incidence of atypical scrapie appears to correlate with homo- or heterozygous combinations of four alleles (AHQ, ARR, ARH and ARQ), and the VRQ variant is rarely present in affected sheep [1, 3, 6, 31, 42]. An ovine PrP variant containing a phenylalanine (F) residue at codon 141 has only been detected in sheep carrying the ARQ allele (designated AF141RQ), which exhibit greater susceptibility to atypical scrapie than wild-type AL141RQ animals and those with the AHQ genotype [26, 31, 42]. These data suggest that atypical scrapie may be influenced by genetic factors [12]. However, no obvious association has been identified between age distributions and PRNP genotypes [2, 26, 30, 42, 45].
Atypical scrapie has been experimentally transmitted to homozygous AHQ sheep by intracerebral [45, 46] and peroral challenge [47], and by the intracerebral route to mice of the ovinized transgenic lines tg338, which overexpresses VRQ PrP [14, 15, 23], and TgOvPrP4, which expresses the AL141RQ form [1]. In Japan, the classical scrapie-susceptible ARQ/ARQ and ARQ/VRQ PRNP genotypes predominate among Suffolk sheep [33]. However, the risk to homozygous ARQ sheep of contracting atypical scrapie remains unknown. The aim of this study was to investigate the transmissibility of this disease to sheep carrying only the AL141RQ PRNP allele by intracerebral inoculation.
Animal experiments were carried out in accordance with the regulations outlined in the Guide for the Care and Use of Laboratory Animals of the National Institute of Animal Health and the Guidelines for Proper Conduct of Animal Experiments, 2006, by the Science Council of Japan [43]. Procedures involving animals were approved by the Institutional Animal Care and Use Committee of the National Institute of Animal Health (approval ID: 08-093), and all possible effort was made to minimize any pain and discomfort. All surgery was performed under ketamine anesthesia. Two PRNP AL141RQ homozygous Cheviot ewes, aged 6 months, were inoculated in the right side of the midbrain with 1 ml of a 10% mixed brain homogenate prepared from four field cases of atypical scrapie, comprising one homologous (ARQ/ARQ), two heterologous (AHQ/AHQ) and one cross-genotype (ARR/ARQ) sheep, the brains of which were kindly supplied by the Veterinary Laboratories Agency (VLA; currently the Animal and Plant Health Agency, Addlestone, Surrey, UK). Each experimental animal was reared in a separate, single pen and clinically monitored throughout the study. The sheep exhibited no abnormal behavior or movement, nor loss of appetite or weight during the experiment and were euthanized for diagnostic confirmation of atypical scrapie at the end of the project (85 months post-inoculation). At necropsy, a range of neural and non-neural tissue samples were fixed in 10% neutral buffered formalin containing 10% methanol for histopathology and PrPSc immunohistochemistry (IHC). Paraffin-embedded brains were sectioned, stained with hematoxylin and eosin, and immunostained with the monoclonal antibodies (mAbs) 2G11, F89/160.1.5 and T1 (Table 1), in combination with the horseradish peroxidase polymer system and diaminobenzidine, as previously described [36]. In addition, dual immunofluorescence was performed using mAb 2G11 for PrPSc staining and either polyclonal rabbit anti-glial fibrillary acid protein (GFAP; Dako, Carpinteria, CA, U.S.A.) for astrocytes or polyclonal rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba-1; Wako Pure Chemical, Osaka, Japan) for microglia, as reported previously [34, 35]. For enzyme-linked immunosorbent assay (ELISA) and western blot (WB) analyses, tissue samples were frozen at −80°C until use. A NippIBL ELISA kit (Nippi, Tokyo, Japan) [50] was used, with several modifications according to a new protocol for atypical scrapie (M. Imamura, manuscript in preparation). The WB procedure for detection of proteinase K (PK)-resistant PrPSc involved pretreating samples with 40 μg/ml PK for 30 min at 37°C and probing membranes with the anti-PrP mAbs P4, 8G8, F89/160.1.5, Sha31 or 2G11 (Table 1), according to the previously described method [30]. Brains of ARQ/ARQ sheep affected with atypical scrapie obtained from the VLA and with classical scrapie by NIAH archive were served as positive controls in ELISA, WB and IHC tests. A known scrapie-negative sample was also used as the negative control.
Table 1. Characteristics of the monoclonal antibodies used in this study.
| Clone | Epitope location in ovine amino acid sequence | Source |
|---|---|---|
| P4 | 93-WGQGGSH-99 | R-Biopharm (Darmstadt, Germany) |
| 8G8 | 98-THSQWNKPSKPKTNMK-113 | SPI-Bio (Montigny le Bretonneux, France) |
| T2 | Discontinuous, unknown (132–156)a) | Shimizu et al. [44] |
| T1 | 141-LIHFGND-147 | Shimizu et al. [44] |
| F89/160.1.5 | 142-IHFG-145 | VMRD (Pullman, WA, U.S.A.) |
| Sha31 | 148-YEDRYYRE-155 | Bertin Pharma (Montigny le Bretonneux, France) |
| 2G11 | 153-YRENMY-158 | SPI-Bio (Montigny le Bretonneux, France) |
a) Mouse PrP amino acid residues.
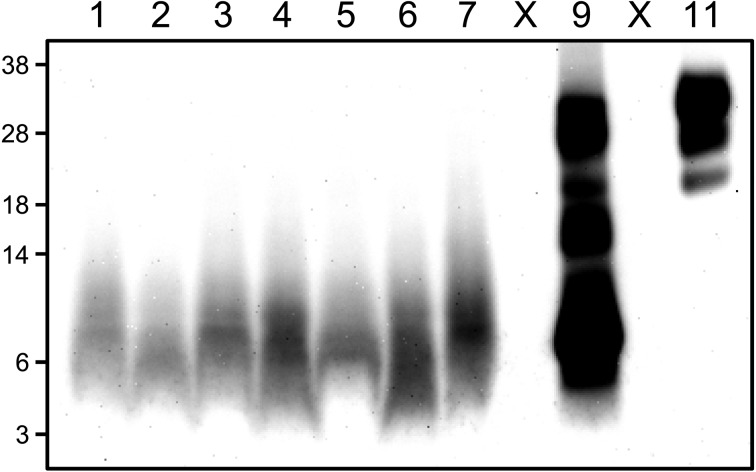
Histopathologic evaluation revealed no vacuolation in any of the brain sections from either ewe. Limited numbers of fine punctate to coarse granular PrPSc deposits were identified by IHC staining. However, these were restricted to the neuropil of the subpial molecular layer of the cerebellar cortex, and specific PrPSc signals were not detected in any other brain region or peripheral tissue, including the lymphoid organs (Fig. 1). In addition, PrPSc granules were occasionally discerned in the cytoplasm and at the periphery of cytoplasmic protrusions of astrocytes and microglia by dual immunofluorescence staining (Fig. 2). No other intraneuronal or extracellular PrPSc staining patterns were visible in any brain area. IHC features, such as PrPSc staining type, neuroanatomical PrPSc distribution pattern and the magnitude of PrPSc deposition in the brain, were similar between the two animals using different antibodies (T1, F89/160.1.5 and 2G11). Surprisingly, the optical density values of five samples prepared from different cerebellar cortex areas of each animal were under the cutoff value (0.37) for the detection of atypical scrapie by the modified NippIBL test using mAb T2. In addition, WB tests employing the five different mAbs mentioned above failed to detect PrPSc in the samples, which were the same as those used for the ELISA (Fig. 3).
Fig. 1.
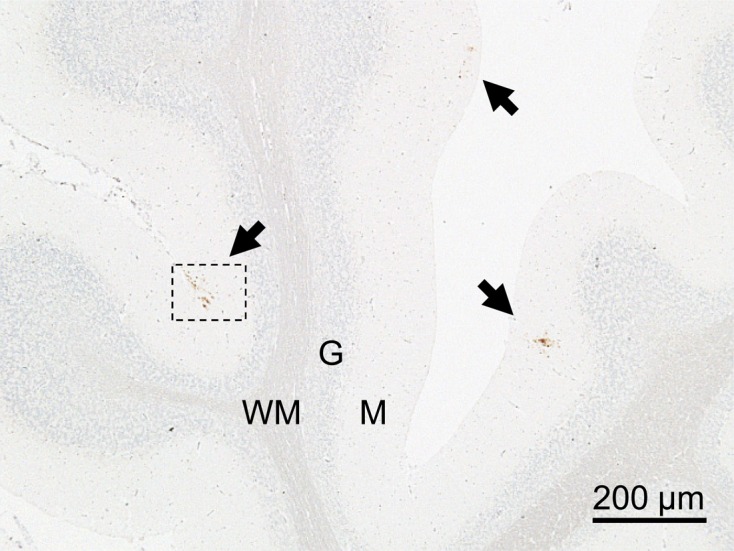
Features of IHC PrPSc staining in the cerebellum of a sheep (case 2) with subclinical infection 85 months after inoculation with atypical scrapie. Arrows show accumulation of PrPSc in the cerebellar molecular layer. Tissue was stained with the mAb 2G11 and counterstained with hematoxylin. M: molecular layer, G: granule cell layer, WM: medulla of white matter.
Fig. 2.
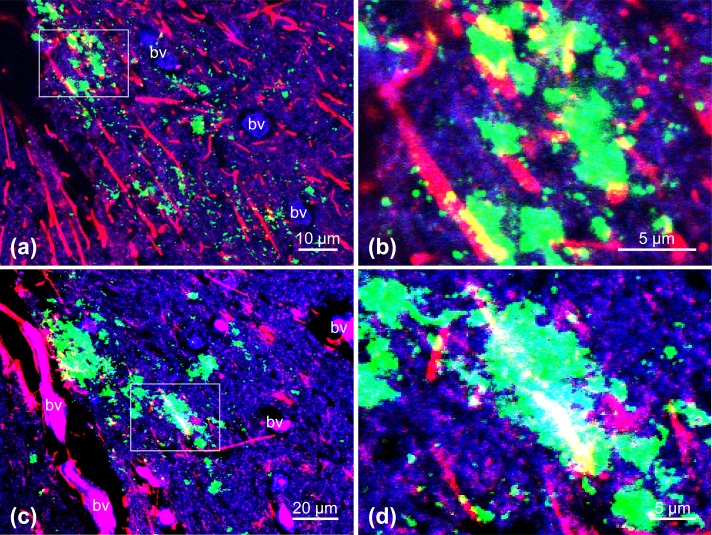
Dual immunofluorescence labeling with mAbs against PrPSc (2G11; a–d, green) and GFAP (a and b) or Iba-1 (c and d). Astrocytes (a and b) and microglia (c and d) are labeled in red. The panels show higher magnification images of the area shown in Fig. 1, obtained from serial sections of the sample. Fine to coarse granular PrPSc deposits can be observed principally in the neuropil and associated with the cytoplasmic protrusions (yellow) of astrocytes (a) and microglia (c). Boxed regions in panels (a) and (c) are shown at higher magnification in panels (b) and (d), respectively. PrPSc deposits can be discerned within astrocytes and microglia or at the periphery of these cells. The section was counterstained with TO-PRO-3. bv: Blood vessel.
Fig. 3.
Western blotting using mAb P4 assessing the presence of PK-resistant PrPSc in the brain of a sheep (case 1) inoculated with atypical scrapie. Compared to the sample from a classical scrapie-affected sheep (lane 11), an additional smaller fragment (~7 kDa) is detected in an atypical scrapie case (lane 9). Lanes 1–2: cerebellum samples from negative sheep [10 mg of tissue equivalent (eq.)]; lanes 3–7: samples from five different areas of the cerebellum from case 1 (10 mg tissue eq.); lane 9: cerebellum sample from an ARQ/ARQ atypical scrapie natural case as a positive control (2 mg tissue eq.); lane 11: brainstem sample from a classical scrapie affected sheep as a positive control (5 μg tissue eq.); lane X: empty lanes. The nonspecific staining band migrates around ~7–9 kDa at bottom of lanes 1–7. Molecular markers are shown on the left (kDa).
Although a limited number of animals were used in this study, we were able to demonstrate the restricted accumulation of PrPSc in the molecular layer of the cerebellum by IHC and dual immunofluorescence staining, indicating the potential transmissibility of atypical scrapie to AL141RQ homozygous sheep by the intracerebral route. The topographical distribution and morphology of PrPSc immunostaining observed using different antibodies in the present study are consistent with the PrPSc IHC features of previously reported atypical scrapie cases in that (1) PrPSc staining appeared as fine punctate to coarse granular deposits in the cerebellum; (2) no intraneuronal immunostaining was observed, nor has it been in prior reports; (3) the neuropil was the only brain structure to exhibit PrPSc immunostaining; and (4) the cerebellar and cerebral cortices are generally the most immunostained neuroanatomic areas [2, 3, 6, 13, 37]. In addition, minimal or entirely absent PrPSc accumulation in the cerebellum has been noted in some atypical scrapie cases [32]. These findings suggest that the animals under investigation here were infected with atypical scrapie.
Despite the intracerebral inoculation, an inconsiderable possibility exists that the present cases arose spontaneously. However, rather than being spontaneous events, it seems likely that atypical scrapie was successfully transmitted to the ewes in question, owing to the following considerations: (1) even regarding atypical scrapie to be contagious, each animal was reared separately in a single pen; and (2) according to epidemiological studies, the incidence of this disease among AL141RQ sheep in the field is far too low for this to be a concern, with atypical scrapie naturally occurring as a single case in a flock [1, 2, 6, 26, 31, 39, 42, 48]. Therefore, the likelihood of two ewes contracting this condition at the same time during the investigation in a manner unrelated to the experiment seems negligible. It can thus be concluded that the natural occurrence of atypical scrapie during this study can be excluded for these reasons.
The present work indicates that IHC is the most effective method for detecting minimal levels of PrPSc in the brain. A possible reason for the negative results obtained using the other procedures may be that PrPSc levels in the cerebellum were below the detection limit of conventional WB and ELISA techniques, even at the end of the project, 7 years post-inoculation. Our findings also suggest that the obex region of the brainstem, sampled in standard scrapie diagnosis, might not be the optimal site to confirm the presence of PrPSc during the early phase of atypical scrapie [2, 3].
Astrocytes are the major non-neuronal PrPC expressing cells [22, 24] and may be the initial sites of PrPSc replication in the brain [10, 27, 41]. PrPSc staining has been detected in glial cells infected with atypical scrapie [49]. We demonstrated an association between PrPSc and astrocytes and microglia, suggesting that these cells may play functional roles in processing, degrading or removing PrPSc, and in its release into extracellular areas [18, 19, 21].
It is difficult to directly compare our results with those of previous studies in which atypical scrapie was successfully transmitted from AHQ/AHQ donor sheep to homologous recipient sheep by either intracerebral [45, 46] or peroral challenge [47]. Homozygous AL141RQ sheep infected with atypical scrapie via the intracerebral route appear to undergo an asymptomatic stage consisting of a 7-year silent incubation period. Such animals may thus be clinically silent carriers of atypical scrapie [16]. The incubation periods of animals challenged with brain material from homologous AHQ/AHQ atypical cases range from 378 to 1,057 days post-inoculation (mean 751 days) [45], significantly shorter intervals than the time over which the present experimental cases were observed (2,591 days). Our study appears to suggest that the replication efficiency of PrPSc in homozygous AL141RQ sheep infected with atypical scrapie may not be sufficient to completely overcome the transmission barrier, even using intracerebral challenge. However, careful judgment is required to determine whether PrPSc immunoreactivity is truly positive or negative. In a separate study (K. Miyazawa and M. Imamura, unpublished data), each source inoculum employed here was used to successfully transmit atypical scrapie to TgOvPrP59 mice [7], with an incubation period of 457.4 ± 81.4 days (n=25, mean ± standard deviation), regardless of the PrP genotype of the animal from which the inoculum was prepared (AHQ/AHQ, ARQ/ARQ or ARR/ARQ). Bioassays of cerebellar samples from these mice are ongoing to confirm whether the PrPSc deposits in the molecular layer of sheep cerebella are infectious.
Acknowledgments
Expert technical assistance was provided by Naomi Furuya, Naoko Tabeta, Ritsuko Miwa, Junko Yamada and the animal caretaker. This work was supported by grants-in-aid from the BSE and Other Prion Disease Project and the Improving Food Safety and Animal Health Project of the Ministry of Agriculture, Forestry and Fisheries, Japan.
REFERENCES
- 1.Arsac J. N., Bétemps D., Morignat E., Féraudet C., Bencsik A., Aubert D., Grassi J., Baron T.2009. Transmissibility of atypical scrapie in ovine transgenic mice: major effects of host prion protein expression and donor prion genotype. PLoS ONE 4: e7300. doi: 10.1371/journal.pone.0007300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benestad S. L., Arsac J. N., Goldmann W., Nöremark M.2008. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet. Res. 39: 19. doi: 10.1051/vetres:2007056 [DOI] [PubMed] [Google Scholar]
- 3.Benestad S. L., Sarradin P., Thu B., Schönheit J., Tranulis M. A., Bratberg B.2003. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet. Rec. 153: 202–208. doi: 10.1136/vr.153.7.202 [DOI] [PubMed] [Google Scholar]
- 4.Bossers A., Schreuder B. E., Muileman I. H., Belt P. B., Smits M. A.1996. PrP genotype contributes to determining survival times of sheep with natural scrapie. J. Gen. Virol. 77: 2669–2673. doi: 10.1099/0022-1317-77-10-2669 [DOI] [PubMed] [Google Scholar]
- 5.Bruce M. E.2003. TSE strain variation. Br. Med. Bull. 66: 99–108. doi: 10.1093/bmb/66.1.99 [DOI] [PubMed] [Google Scholar]
- 6.Buschmann A., Biacabe A. G., Ziegler U., Bencsik A., Madec J. Y., Erhardt G., Lühken G., Baron T., Groschup M. H.2004. Atypical scrapie cases in Germany and France are identified by discrepant reaction patterns in BSE rapid tests. J. Virol. Methods 117: 27–36. doi: 10.1016/j.jviromet.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 7.Cordier C., Bencsik A., Philippe S., Bétemps D., Ronzon F., Calavas D., Crozet C., Baron T.2006. Transmission and characterization of bovine spongiform encephalopathy sources in two ovine transgenic mouse lines (TgOvPrP4 and TgOvPrP59). J. Gen. Virol. 87: 3763–3771. doi: 10.1099/vir.0.82062-0 [DOI] [PubMed] [Google Scholar]
- 8.Dawson M., Hoinville L. J., Hosie B. D., Hunter N., Scrapie Information Group1998. Guidance on the use of PrP genotyping as an aid to the control of clinical scrapie. Vet. Rec. 142: 623–625. [PubMed] [Google Scholar]
- 9.De Bosschere H., Roels S., Benestad S. L., Vanopdenbosch E.2004. Scrapie case similar to Nor98 diagnosed in Belgium via active surveillance. Vet. Rec. 155: 707–708. doi: 10.1136/vr.155.22.707 [DOI] [PubMed] [Google Scholar]
- 10.Diedrich J. F., Bendheim P. E., Kim Y. S., Carp R. I., Haase A. T.1991. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc. Natl. Acad. Sci. U.S.A. 88: 375–379. doi: 10.1073/pnas.88.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein V., Pointing S., Halfacre S.2005. Atypical scrapie in the Falkland Islands. Vet. Rec. 157: 667–668. doi: 10.1136/vr.157.21.667-c [DOI] [PubMed] [Google Scholar]
- 12.Fediaevsky A., Morignat E., Ducrot C., Calavas D.2009. A case-control study on the origin of atypical scrapie in sheep, France. Emerg. Infect. Dis. 15: 710–718. doi: 10.3201/eid1505.081119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavier-Widén D., Nöremark M., Benestad S., Simmons M., Renström L., Bratberg B., Elvander M., af Segerstad C. H.2004. Recognition of the Nor98 variant of scrapie in the Swedish sheep population. J. Vet. Diagn. Invest. 16: 562–567. doi: 10.1177/104063870401600611 [DOI] [PubMed] [Google Scholar]
- 14.Götte D. R., Benestad S. L., Laude H., Zurbriggen A., Oevermann A., Seuberlich T.2011. Atypical scrapie isolates involve a uniform prion species with a complex molecular signature. PLoS ONE 6: e27510. doi: 10.1371/journal.pone.0027510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths P. C., Spiropoulos J., Lockey R., Tout A. C., Jayasena D., Plater J. M., Chave A., Green R. B., Simonini S., Thorne L., Dexter I., Balkema-Buschmann A., Groschup M. H., Béringue V., Le Dur A., Laude H., Hope J.2010. Characterization of atypical scrapie cases from Great Britain in transgenic ovine PrP mice. J. Gen. Virol. 91: 2132–2138. doi: 10.1099/vir.0.018986-0 [DOI] [PubMed] [Google Scholar]
- 16.Hill A. F., Collinge J.2003. Subclinical prion infection in humans and animals. Br. Med. Bull. 66: 161–170. doi: 10.1093/bmb/66.1.161 [DOI] [PubMed] [Google Scholar]
- 17.Hunter N., Goldmann W., Smith G., Hope J.1994. The association of a codon 136 PrP gene variant with the occurrence of natural scrapie. Arch. Virol. 137: 171–177. doi: 10.1007/BF01311184 [DOI] [PubMed] [Google Scholar]
- 18.Jeffrey M., Martin S., González L.2003. Cell-associated variants of disease-specific prion protein immunolabelling are found in different sources of sheep transmissible spongiform encephalopathy. J. Gen. Virol. 84: 1033–1045. doi: 10.1099/vir.0.18825-0 [DOI] [PubMed] [Google Scholar]
- 19.Jeffrey M., McGovern G., Sisó S., González L.2011. Cellular and sub-cellular pathology of animal prion diseases: relationship between morphological changes, accumulation of abnormal prion protein and clinical disease. Acta Neuropathol. 121: 113–134. doi: 10.1007/s00401-010-0700-3 [DOI] [PubMed] [Google Scholar]
- 20.Kittelberger R., Chaplin M. J., Simmons M. M., Ramirez-Villaescusa A., McIntyre L., MacDiarmid S. C., Hannah M. J., Jenner J., Bueno R., Bayliss D., Black H., Pigott C. J., O’Keefe J. S.2010. Atypical scrapie/Nor98 in a sheep from New Zealand. J. Vet. Diagn. Invest. 22: 863–875. doi: 10.1177/104063871002200604 [DOI] [PubMed] [Google Scholar]
- 21.Kovács G. G., Preusser M., Strohschneider M., Budka H.2005. Subcellular localization of disease-associated prion protein in the human brain. Am. J. Pathol. 166: 287–294. doi: 10.1016/S0002-9440(10)62252-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lainé J., Marc M. E., Sy M. S., Axelrad H.2001. Cellular and subcellular morphological localization of normal prion protein in rodent cerebellum. Eur. J. Neurosci. 14: 47–56. doi: 10.1046/j.0953-816x.2001.01621.x [DOI] [PubMed] [Google Scholar]
- 23.Le Dur A., Béringue V., Andréoletti O., Reine F., Laï T. L., Baron T., Bratberg B., Vilotte J. L., Sarradin P., Benestad S. L., Laude H.2005. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc. Natl. Acad. Sci. U.S.A. 102: 16031–16036. doi: 10.1073/pnas.0502296102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima F. R., Arantes C. P., Muras A. G., Nomizo R., Brentani R. R., Martins V. R.2007. Cellular prion protein expression in astrocytes modulates neuronal survival and differentiation. J. Neurochem. 103: 2164–2176. doi: 10.1111/j.1471-4159.2007.04904.x [DOI] [PubMed] [Google Scholar]
- 25.Loiacono C. M., Thomsen B. V., Hall S. M., Kiupel M., Sutton D., O’Rourke K., Barr B., Anthenill L., Keane D.2009. Nor98 scrapie identified in the United States. J. Vet. Diagn. Invest. 21: 454–463. doi: 10.1177/104063870902100406 [DOI] [PubMed] [Google Scholar]
- 26.Lühken G., Buschmann A., Brandt H., Eiden M., Groschup M. H., Erhardt G.2007. Epidemiological and genetical differences between classical and atypical scrapie cases. Vet. Res. 38: 65–80. doi: 10.1051/vetres:2006046 [DOI] [PubMed] [Google Scholar]
- 27.Madec J. Y., Simon S., Lezmi S., Bencsik A., Grassi J., Baron T.2004. Abnormal prion protein in genetically resistant sheep from a scrapie-infected flock. J. Gen. Virol. 85: 3483–3486. doi: 10.1099/vir.0.80220-0 [DOI] [PubMed] [Google Scholar]
- 28.Mallucci G., Dickinson A., Linehan J., Klöhn P. C., Brandner S., Collinge J.2003. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302: 871–874. doi: 10.1126/science.1090187 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell G. B., O’Rourke K. I., Harrington N. P., Soutyrine A., Simmons M. M., Dudas S., Zhuang D., Laude H., Balachandran A.2010. Identification of atypical scrapie in Canadian sheep. J. Vet. Diagn. Invest. 22: 408–411. doi: 10.1177/104063871002200310 [DOI] [PubMed] [Google Scholar]
- 30.Miyazawa K., Okada H., Iwamaru Y., Masujin K., Yokoyama T.2014. Susceptibility of GT1-7 cells to mouse-passaged field scrapie isolates with a long incubation. Prion 8: 306–313. doi: 10.4161/pri.32232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moum T., Olsaker I., Hopp P., Moldal T., Valheim M., Moum T., Benestad S. L.2005. Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor98 cases. J. Gen. Virol. 86: 231–235. doi: 10.1099/vir.0.80437-0 [DOI] [PubMed] [Google Scholar]
- 32.Nentwig A., Oevermann A., Heim D., Botteron C., Zellweger K., Drögemüller C., Zurbriggen A., Seuberlich T.2007. Diversity in neuroanatomical distribution of abnormal prion protein in atypical scrapie. PLoS Pathog. 3: e82. doi: 10.1371/journal.ppat.0030082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohara J., Togari T., Kurokawa A., Maeda J., Ishiguro N., Furuoka H., Horiuchi M.2007. Frequencies of PrP genotypes in meat breeds of Japanese sheep and trail of selective breeding in experimental sheep flock. J. Vet. Med. Sci. 69: 1325–1329. doi: 10.1292/jvms.69.1325 [DOI] [PubMed] [Google Scholar]
- 34.Okada H., Iwamaru Y., Yokoyama T., Mohri S.2013. Immunohistochemical detection of disease-associated prion protein in the peripheral nervous system in experimental H-type bovine spongiform encephalopathy. Vet. Pathol. 50: 659–663. doi: 10.1177/0300985812471541 [DOI] [PubMed] [Google Scholar]
- 35.Okada H., Iwamaru Y., Fukuda S., Yokoyama T., Mohri S.2012. Detection of disease-associated prion protein in the optic nerve and the adrenal gland of cattle with bovine spongiform encephalopathy by using highly sensitive immunolabeling procedures. J. Histochem. Cytochem. 60: 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okada H., Sato Y., Sata T., Sakurai M., Endo J., Yokoyama T., Mohri S.2011. Antigen retrieval using sodium hydroxide for prion immunohistochemistry in bovine spongiform encephalopathy and scrapie. J. Comp. Pathol. 144: 251–256. doi: 10.1016/j.jcpa.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 37.Onnasch H., Gunn H. M., Bradshaw B. J., Benestad S. L., Bassett H. F.2004. Two Irish cases of scrapie resembling Nor98. Vet. Rec. 155: 636–637. doi: 10.1136/vr.155.20.636 [DOI] [PubMed] [Google Scholar]
- 38.Orge L., Galo A., Machado C., Lima C., Ochoa C., Silva J., Ramos M., Simas J. P.2004. Identification of putative atypical scrapie in sheep in Portugal. J. Gen. Virol. 85: 3487–3491. doi: 10.1099/vir.0.80246-0 [DOI] [PubMed] [Google Scholar]
- 39.Ortiz-Peláez A., Arnold M. E., Vidal-Diez A.2016. Epidemiological investigations on the potential transmissibility of a rare disease: the case of atypical scrapie in Great Britain. Epidemiol. Infect. 144: 2107–2116. doi: 10.1017/S0950268816000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polak M. P., Larska M., Langeveld J. P., Buschmann A., Groschup M. H., Zmudzinski J. F.2010. Diagnosis of the first cases of scrapie in Poland. Vet. J. 186: 47–52. doi: 10.1016/j.tvjl.2009.07.032 [DOI] [PubMed] [Google Scholar]
- 41.Raeber A. J., Race R. E., Brandner S., Priola S. A., Sailer A., Bessen R. A., Mucke L., Manson J., Aguzzi A., Oldstone M. B., Weissmann C., Chesebro B.1997. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 16: 6057–6065. doi: 10.1093/emboj/16.20.6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saunders G. C., Cawthraw S., Mountjoy S. J., Hope J., Windl O.2006. PrP genotypes of atypical scrapie cases in Great Britain. J. Gen. Virol. 87: 3141–3149. doi: 10.1099/vir.0.81779-0 [DOI] [PubMed] [Google Scholar]
- 43.Science Council of Japan. Guidelines for Proper Conduct of Animal Experiments Science Council of Japan 2006. http://www.scj.go.jp/ja/info/kohyo/pdf/kohyo-20-k16-2e.pdf.
- 44.Shimizu Y., Kaku-Ushiki Y., Iwamaru Y., Muramoto T., Kitamoto T., Yokoyama T., Mohri S., Tagawa Y.2010. A novel anti-prion protein monoclonal antibody and its single-chain fragment variable derivative with ability to inhibit abnormal prion protein accumulation in cultured cells. Microbiol. Immunol. 54: 112–121. doi: 10.1111/j.1348-0421.2009.00190.x [DOI] [PubMed] [Google Scholar]
- 45.Simmons M. M., Konold T., Thurston L., Bellworthy S. J., Chaplin M. J., Moore S. J.2010. The natural atypical scrapie phenotype is preserved on experimental transmission and sub-passage in PRNP homologous sheep. BMC Vet. Res. 6: 14. doi: 10.1186/1746-6148-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmons M. M., Konold T., Simmons H. A., Spencer Y. I., Lockey R., Spiropoulos J., Everitt S., Clifford D.2007. Experimental transmission of atypical scrapie to sheep. BMC Vet. Res. 3: 20. doi: 10.1186/1746-6148-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmons M. M., Moore S. J., Konold T., Thurston L., Terry L. A., Thorne L., Lockey R., Vickery C., Hawkins S. A., Chaplin M. J., Spiropoulos J.2011. Experimental oral transmission of atypical scrapie to sheep. Emerg. Infect. Dis. 17: 848–854. doi: 10.3201/eid1705.101654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tranulis M. A., Benestad S. L., Baron T., Kretzschmar H.2011. Atypical prion diseases in humans and animals. Top. Curr. Chem. 305: 23–50. doi: 10.1007/128_2011_161 [DOI] [PubMed] [Google Scholar]
- 49.Vidal E., Tortosa R., Costa C., Benavides J., Francino O., Sánchez-Robert E., Pérez V., Pumarola M.2008. Lack of PrPsc immunostaining in intracranial ectopic lymphoid follicles in a sheep with concomitant non-suppurative encephalitis and Nor98-like atypical scrapie: a case report. Vet. J. 177: 283–288. doi: 10.1016/j.tvjl.2007.04.014 [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T., Ushiki-Kaku Y., Yokoyama T., Hattori S.2013. Sensitivity and specificity of a commercial BSE kit for the detection of ovine scrapie. Anim. Sci. J. 84: 508–512. doi: 10.1111/asj.12032 [DOI] [PubMed] [Google Scholar]