Abstract
We previously demonstrated that expression of Notch-1 is associated with poor prognosis in lung adenocarcinoma (LAD) patients. The aim of this study is to reveal whether Notch-1 was associated with Taxanes-resistant LAD and, the underlying mechanisms. We collected 39 patients of advanced LAD treated with Taxanes and found that positive Notch-1 expression is closely related to LAD lymph node metastasis, recurrence and poorer prognosis, and Notch-1 acts as an independent poor prognostic factor in LAD by multivariate analysis with Cox regression model. Then, by using the Docetaxel (DTX)-resistant LAD cell lines that we established previously, we found that Notch-1 contributes to resistance of LAD cells to DTX in vitro, and inhibition of Notch-1 sensitizes LAD to DTX in vivo. We further demonstrated that Notch-1 mediates chemoresistance response and strengthens proliferation capacity in LAD cells partially through negative regulation of miR-451 by transcription factor AP-1. Moreover, we found that MDR-1 is a direct target of miR-451 and influences chemoresistance of LAD cells. Taken together, our data revealed a novel Notch-1/AP-1/miR-451/MDR-1 signaling axis, and suggested a new therapeutic strategy of combining DTX with Notch inhibitors to treat DTX-resistant LAD.
Keywords: activator protein-1, chemoresistance, lung adenocarcinoma, microRNA, notch signaling pathway
Introduction
Over the past decades, Taxanes-based chemotherapy is the common standard therapeutic approach to treat patient with lung cancer.1 However, intrinsic or acquired resistance to drugs always generated a high risk for tumor recurrence, distant relapse or other further tumor progression. The development of chemoresistance in lung cancer is considered as a multifactor, stepwise, and complicated process, which involves in dysfunction of oncogenes and tumor suppressors in several signaling pathways, including PTEN,2 Akt,3 mTOR,4 NF-κB,5 EGFR,6 FGFR,7 Raf/MEK/ERK,8 MAPK,9 IGF,10 and Notch.11
Notch pathway plays a remarkable role in cell fate determination and cell signal communication in organisma multicellularis, which relate to the maintenance of stem cell tumor differentiation, proliferation, apoptosis, and chemoresistance.12 To date, four Notch receptors (Notch-1–4) and five ligands (DLL-1, DLL-3, DLL-4, Jagged-1, and Jagged-2) have been identified in mammals. The Notch-1 receptor is the most common of the family, which was detected to express abnormal in many human tumors, such as glioma,13 neck neoplasm,14 medullary thyroid carcinoma,15 pleural mesothelioma,16 breast carcinoma17 as well as lung cancer.18 Recently, more and more attentions have been paid in the roles of Notch-1 in chemoresistance. The hyperactivation of Notch-1 in tumor development is not only mutation-driven but also depends on proteolytic cleavage by the γ-secretase comple.19 Notch-1 signaling pathway has also been demonstrated to cross-talk with many other pathways and genes where microRNAs (miRNAs) involve in epigenetic regulation.
miRNAs, a class of short noncoding RNAs with 18–25 nucleotides in length, function as a characteristic biomarker for diagnosis, treatment, and prognosis in several kinds of cancers.20 Our previous studies demonstrated that dysregulated miRNAs, such as Let-7c,21 miR-200b,22 miR-100,23 miR-650, 24 and miR-451,25 play key roles in chemoresistant phenotype formation of antitumor drugs in lung adenocarcinoma (LAD) cells. It is meaningful to study further, the mechanisms of miRNAs in LAD chemoresistance.
Previously, we have demonstrated that expression of Notch-1 is associated with poor prognosis in LAD patients.26 In this study, we investigated the expression of Notch-1 in LAD patients underwent Taxanes-based chemotherapy and the roles of Notch-1 in LAD cells and xenograft models. We demonstrated that Notch-1 affect LAD proliferation and apoptosis and confer chemoresistance of LAD to Docetaxel (DTX) by regulation of miR-451 through activated protein (AP-1). Our results may suggest novel targets for LAD chemotherapy.
Results
Notch-1 is associated with chemoresistance of LAD to Taxanes
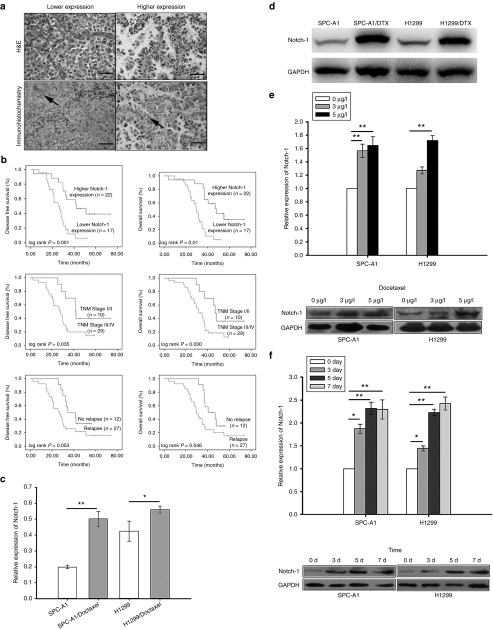
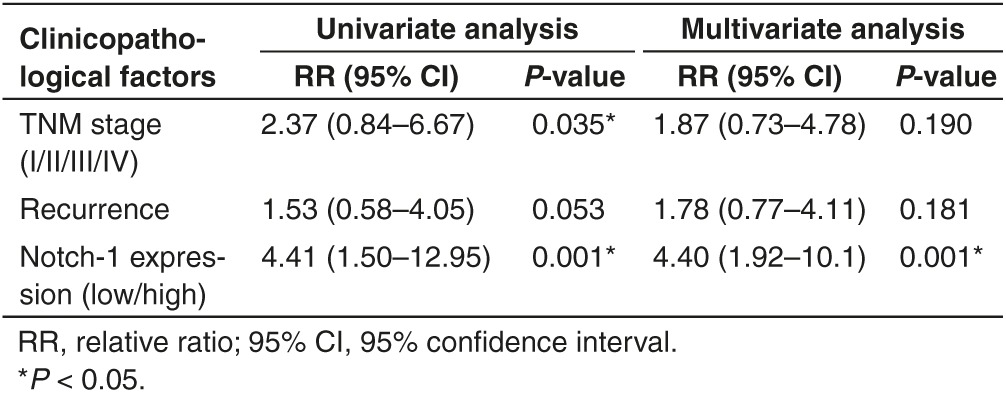
To study how Notch-1 associate with Taxanes chemosensitivity, a total of 39 patients of advanced LAD treated with Taxanes were eligible and enrolled. First of all, Notch-1 expression in surgical resection specimens was tested by immunohistochemistry staining. 17 cases (43.6%) showed stronger Notch-1 expression while 22 cases were weaker (Figure 1a). Positive Notch-1 expression was found to be closely related to lymph node metastasis (P = 0.023), recurrence (P = 0.014) and poorer prognosis (P = 0.026) (Table 1). The Kaplan–Meier univariate analysis revealed the correlation among hyperexpression of Notch-1, advanced TNM stage and poorer survival time, including disease-free survival and overall survival (Figure 1b). Notch-1 was observed to act as an independent poor prognostic factor in LAD by multivariate analysis with Cox regression model (Table 2). To further elucidate the molecular mechanism of LAD chemoresistance, we used the DTX-resistant cell lines SPC-A1/DTX and H1299/DTX which were established from parental LAD cells SPC-A1 and H1299 and published by us previously.21,22,23,24,25 The cDNA gene arrays result was previously told that Notch-1 expression was 34.5-fold higher in SPC-A1/DTX than in parental SPC-A1. Then we investigated the expression levels of Notch-1 in two paired LAD cell lines and noticed that expression of Notch-1 was remarkably increased in DTX-resistant cells when compared with parental ones (Figure 1c,d). Treated parental cells with DTX in different concentration (0, 3, and 5μg/l) or 5μg/l in different time, the results indicated that expression of Notch-1 was elevated along with the concentration increased or time extension (Figure 1e,f), suggesting that the up-regulated Notch-1 might be associated with decreased sensitization to DTX treatment in LAD.
Figure 1.
Notch-1 expression is increased in DTX-treated LAD and associated with poor prognosis LAD patients. (a) Notch-1 expression in surgical resection specimens by immunohistochemistry staining. (b) The Kaplan–Meier univariate analysis for the association of Higher Notch-1 expression, advanced TNM stage and poorer survival time, including disease-free survival (DFS) and overall survival (OS). (c) mRNA and (d) protein levels of Notch-1 in two parental LAD cell lines SPC-A1 and H1299 and their DTX-resistant cell lines. (e) mRNA and protein levels of Notch-1 in two parental LAD cell lines treated with DTX in different concentration (0, 3 or 5μg/l) for 48 hours. (f) mRNA and protein levels of Notch-1 in two parental LAD cell lines treated with 5 μg/l DTX for 0, 3, 5, or 7 days. Scale bar = 50 μm. *P < 0.05 and **P < 0.01. LAD, lung adenocarcinoma; mRNA, microRNA.
Table 1. Correlations between Notch-1 expression and clinicopathological factors of lung adenocarcinoma (LAD) patients.
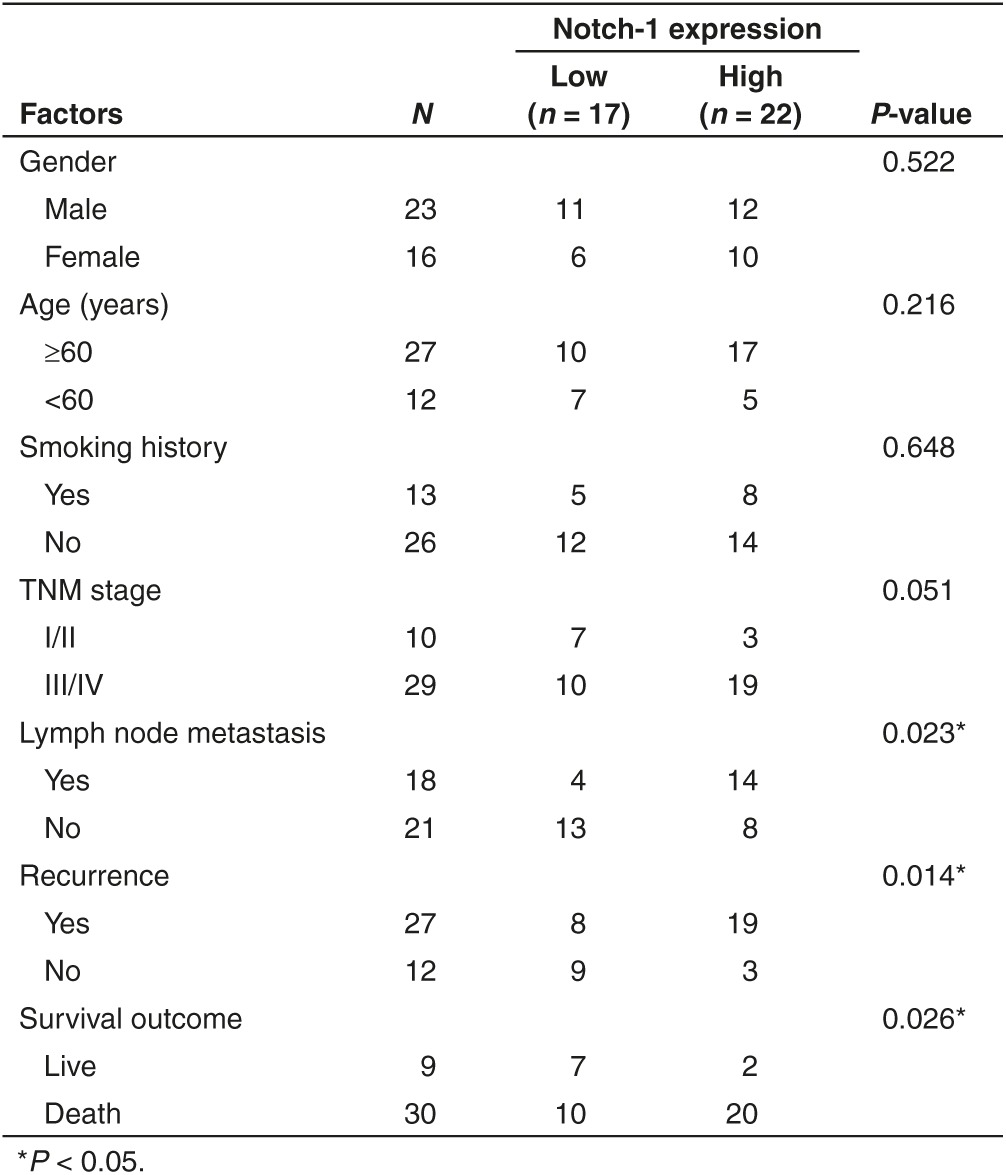
Table 2. Univariate and multivariate analysis of prognostic variables by Cox regression analysis.
Notch-1 contributes to resistance of LAD cells to DTX in vitro
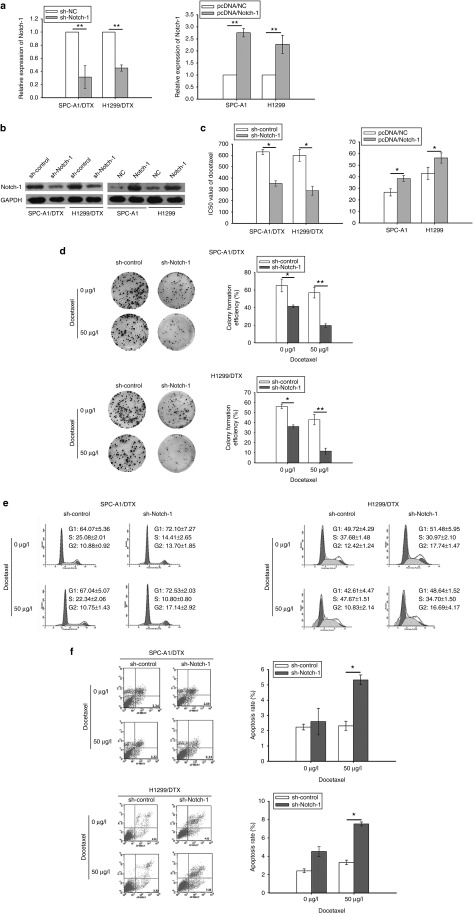
Then, Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1) or overexpression plasmid (pcDNA3/Notch-1) was transfected into DTX-resistant LAD cells or parental cells. Notch-1 expression was sharply decreased in SPC-A1/DTX, H1299/DTX cells transfected with sh-Notch-1, and increased in SPC-A1 and H1299 cells transfected with pcDNA3/Notch-1. Then stably transfected cell lines were chosen and cultured by maintain condition with G418 (Figure 2a,b). In addition, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a widely used γ-secretase inhibitor (GSI) that can effectively suppress the expression of receptors and ligands in Notch signaling pathway (see Supplementary Figure S1a), was used to inhibit Notch-1 expression in our study (see Supplementary Figure S1b).
Figure 2.
Notch-1 promotes proliferation and confers chemoresistance of LAD in vitro. (a) mRNA or (b) protein levels of Notch-1 in two parental LAD cell lines with transfection of Notch-1 overexpression plasmid (pcDNA3/Notch-1) and their DTX-resistant cell lines with transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1). (c) The IC50 value of DTX in two parental LAD cell lines with transfection of Notch-1 overexpression plasmid (pcDNA3/Notch-1) and their DTX-resistant cell lines with transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1). (d) Colony formation assay in two DTX-resistant cell lines with transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1) treated with 50 μg/l DTX. (e) Cell cycle analysis and (f) Cell apoptosis analysis in two DTX-resistant cell lines with transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1) treated with 50 μg/l DTX. *P < 0.05 and **P < 0.01. LAD, lung adenocarcinoma; mRNA, microRNA.
To determine LAD proliferation and chemoresistance, the IC50of DTX was determined by cell counting kit-8 assay. The results revealed that the IC50 of DTX in sh-Notch-1-transfected SPC-A1/DTX (351.86 μg/l ± 23.41 versus control 631.63 μg/l ± 22.36) or H1299/DTX (288.25 μg/l ± 39.52 versus control 599.54 μg/l ± 51.83) was reduced. Conversely, elevated IC50 value was observed in parental SPC-A1 (38.56 μg/l ± 2.49 versus control 26.45 μg/l ± 3.13) or H1299 (56.24 μg/l ± 4.46 versus control 42.93 μg/l ± 5.21) with pcDNA3/Notch-1 transfection (Figure 2c). As well, Notch-1 inhibition by DAPT induced obvious decrease of IC50 (28.86 ± 3.72 for SPC-A1/DTX, 51.24 ± 4.16 for H1299/DTX) compared with control (650.47 ± 33.29 for SPC-A1/DTX, 592.35 ± 47.92 for H1299/DTX) (see Supplementary Figure S1c). The results of colony formation assays showed weaker cell proliferation capacity in SPC-A1/DTX or H1299/DTX cells with downregulated Notch-1 (Figure 2d). On the contrary, overexpression of Notch-1 strengthens colony formation ability in parental LAD cells (see Supplementary Figure S2a). Notch-1 inhibition by DAPT in combination with DTX also induced stronger inhibitory effect of colony formation in SPC-A1/DTX or H1299/DTX cells (see Supplementary Figure S1d).
Then, the flow cytometric analysis was applied to measured cell cycle and apoptosis. The results showed that, knockdown of Notch-1 induces cell percentage increase of G1 and G2/M phase, and also decrease of S phase in DTX-resistant LAD cells (Figure 2e). Contrarily, overexpression of Notch-1 induces cell percentage decrease of G1 and G2/M phase and increase of S phase in parental LAD cells (see Supplementary Figure S2b). Knockdown of Notch-1 also caused increase of apoptosis in SPC-A1/DTX cells from 2.24% ± 0.19 to 5.33% ± 0.32 or H1299/DTX cells from 2.41% ± 0.22 to 7.53% ± 0.27 when they were treated with 50 μg/l DTX (Figure 2f). No significant differences were obtained in parental LAD cells transfected with Notch-1 overexpressed plasmid from when compared with control vector (data not shown). Moreover, the results of Notch-1 inhibition by DAPT showed that, with the treatment of DAPT plus DTX, the percentage of G2/M phase in chemoresistant LAD cells was significantly increased (P< 0.05), while G1 and S phase decreased simultaneously (P< 0.05, Supplementary Figure S1e), whereas combination of DAPT and DTX induces marked increase of apoptosis in SPC-A1/DTX and H1299/DTX cells (see Supplementary Figure S1f). These data recommended that Notch-1 contributes to cell proliferation and chemoresistance in LAD cells.
Inhibition of Notch-1 sensitizes LAD to DTX in vivo
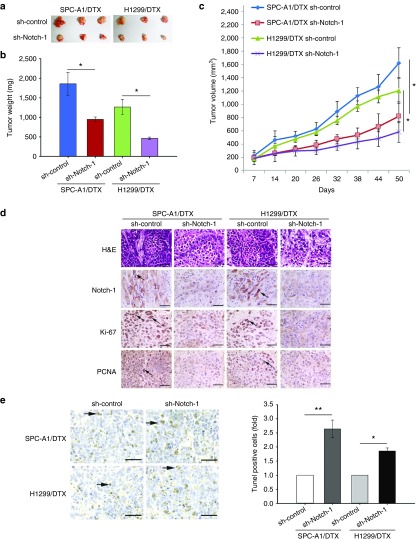
To investigate the effect of Notch-1 knockdown on DTX-resistant LAD cells in vivo, SPC-A1/DTX and H1299/DTX cells with stable transfection of shRNA-Notch-1 were subcutaneously transplanted into nude mice. Two weeks later all tumors were separated after the treatment with DTX in the same way as mentioned above (Figure 3a). With the treatment of DTX, the tumor weight or volume formatted by SPC-A1/DTX and H1299/DTX cells transfected with shRNA-Notch-1 was lighter or smaller than control (Figure 3b,c). Hematoxylin and eosin staining was performed to confirm xenograft tumor morphology, and immunohistochemical staining was performed to show the increased positive rates of proliferation markers ki-67 and proliferating cell nuclear antigen and the downregulation of Notch-1 (Figure 3d). Terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) staining showed more cell apoptosis in tumors with Notch-1 knockdown (Figure 3e).
Figure 3.
Inhibition of Notch-1 sensitizes LAD to DTX in vivo. SPC-A1/DTX and H1299/DTX cells stably interfered Notch-1 expression were subcutaneously explanted into nude mice and DTX-resistant LAD cell stable transfected with sh-control was used as normalized. (a) The xenograft tumors were obtained and measured. (b) The tumor weight of xenograft tumors. (c) The values of tumor volume after transplantation. (d) The immunohistochemistry staining of ki-67, Notch-1 and proliferating cell nuclear antigen (PCNA) in tumor samples. (e) TUNEL staining in tumor samples. Scale bar = 50 μm. *P < 0.05 and **P < 0.01. LAD, lung adenocarcinoma; TUNEL, terminal deoxynucleotidyl transferase-mediated nick end labeling.
Moreover, another xenograft tumor model was built by using SPC-A1/DTX cells injected subcutaneously to demonstrate the effect of Notch-1 inhibition by DAPT on DTX-resistant LAD cells in vivo. 1 mg/kg DTX or/and 100 mg/kg DAPT were intraperitoneally injected, and phosphate buffered saline served as internal control. The final tumor volume and weight of the mice in DTX plus DAPT group were significantly smaller and lighter than those in any other group, indicating that DAPT sensitizes resistant tumor to DTX in vivo (see Supplementary Figure S3a–c). Immunohistochemical staining staining showed the downregulation of Notch-1 and increased expression of proliferating cell nuclear antigen and ki-67 in tumors (see Supplementary Figure S3d). The TUNEL staining was performed to show increased apoptosis cells (with more nuclear fragmentation) in DTX plus DAPT group when compared with other groups (see Supplementary Figure S3e).
miR-451 participates in Notch-1 induced resistance of LAD cells to DTX
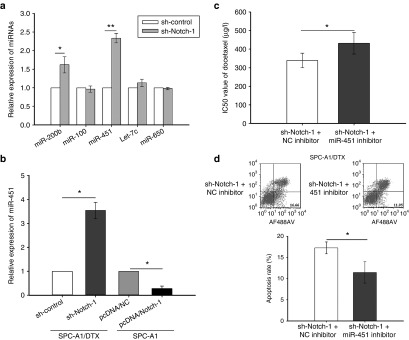
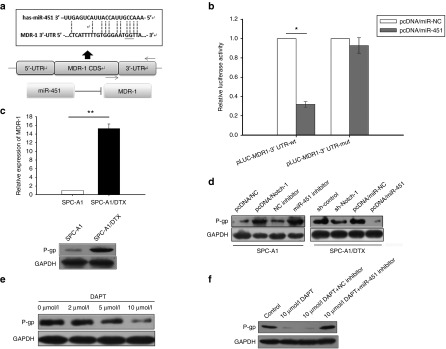
Our research team has mentioned the roles of miR-200b, miR-100, Let-7c, miR-650, and miR-451 in DTX-resistant LAD cells.21,22,23,24,25 In this study, quantitative real time polymerase chain reaction (qRT-PCR) was performed to test the expression of miRNA mentioned above after Notch-1 inhibition. As shown in Figure 4a, expression of miR-451 sharply increased in SPC-A1/DTX transfected with sh-Notch-1. MiR-451 was also hypo-expressed in DTX-resistant LAD cells compared with parental cells (see Supplementary Figure S4a). To investigate how miR-451 affects DTX-resistance, miR-451 inhibitor or pcDNA/miR-451 was transfected in SPC-A1 or SPC-A1/DTX respectively. The transfection efficiency was satisfied about 48 hours after transfection (see Supplementary Figure S4b). The IC50 values of DTX in miR-451 overexpressed DTX-resistant cells are lower than control (see Supplementary Figure S4c). Colony formation assay showed that the proliferation ability of DTX-resistant cells with overexpressed miR-451 diminished significantly (see Supplementary Figure S4d). Moreover, overexpression of miR-451 induced more cell apoptosis and caused G2/M phase arrest in cell cycle distribution (see Supplementary Figure S4e,f). We then tested miR-451 expression by qRT-PCR in Notch-1 upregulated SPC-A1 cells or downregulated SPC-A1/DTX cells, and found that miR-451 is inversely interacted with Notch-1 (Figure 4b). Then, miR-451 inhibitor was found to confer resistance in Notch-1 hypo-expressed SPC-A1/DTX cells by increasing the IC50 value of DTX from 339.47 μg/l ± 39.28 to 431.52 μg/l ± 57.68 (Figure 4c) and decreasing the cell apoptosis percentage from 17.26% ± 1.39 to 11.47% ± 2.52 (P < 0.05) (Figure 4d).
Figure 4.
MiR-451 participates in Notch-1 induced resistance of LAD cells to DTX. (a) The expression of miR-200b, miR-100, Let-7c, miR-650, and miR-451 in SPC-A1/DTX cells with transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1). (b) The expression of miR-451 in SPC-A1/DTX cells with transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1) and SPC-A1 cells with transfection of Notch-1 overexpression plasmid (pcDNA3/Notch-1). (c) The IC50 value of DTX in SPC-A1/DTX cells with transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1) treated with miR-451 inhibitor. (d) The apoptosis assay in SPC-A1/DTX cells with transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1) treated with miR-451 inhibitor. *P < 0.05 and **P < 0.01. LAD, lung adenocarcinoma.
MDR-1 is a direct target of miR-451 and influences chemoresistance of LAD cells
Potential targets of miR-451 were analyzed and chosen by employing open access online miRNA target databases. Multidrug resistant protein-1 (MDR-1), one of the most widely well-known markers reflected in chemoresistance phenotype, was identified as a predictable downstream target. As shown in Figure 5a, the 3′-UTR of MDR-1 is complementary binding site for miR-451. Luciferase reporter containing amplified MDR-1 3′-UTR segment with or without mutated the miR-451 potential binding site were respectively transfected into SPC-A1/DTX cells overexpressed miR-451. The relative luciferase activity was extremely weaker in pLUC-MDR1-3′UTR-wt transfected SPC-A1/DTX, but no statistical differences were collected in pLUC-MDR1-3′UTR-mut transfected cells. The results suggested MDR-1 as a direct downstream target of miR-451(Figure 5b). Then, MDR-1 expression was tested in SPC-A1 and SPC-A1/DTX cells. The results showed that both mRNA and protein level of MDR-1 are higher in SPC-A1/DTX than in SPC-A1 (Figure 5c). Moreover, MDR-1 expression increased in parental SPC-A1 cells with miR-451 inhibition or Notch-1 overexpression and decreased in SPC-A1/DTX cells transfected with pcDNA3/miR-451 or sh-Notch-1 (Figure 5d). The Notch inhibitor DAPT was also showed to suppresses MDR-1 expression in a concentration dependent manner (Figure 5e), which can be partly restored by miR-451 inhibition (Figure 5f).
Figure 5.
MDR-1 is a direct target of miR-451 and influences chemoresistance. (a) The 3′-UTR of MDR-1 is discovered complementary binding site for miR-451. (b) The relative luciferase activity in pLUC-MDR1-3′UTR-wt or pLUC-MDR1-3′UTR-mut transfected SPC-A1/DTX cells with transfection of miR-451 overexpression plasmid (pcDNA/miR-451). (c) The mRNA and protein level of MDR-1 in SPC-A1 cells and SPC-A1/DTX. (d) The protein level of MDR-1 in SPC-A1 cells with transfection of Notch-1 overexpression plasmid (pcDNA3/Notch-1) or treatment of miR-451 inhibitor and SPC-A1/DTX cells with transfection of miR-451 overexpression plasmid (pcDNA/miR-451) or transfection of Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1). (e) The protein level of MDR-1 in SPC-A1/DTX cells treated with DAPT in different concentrations (0, 2, 5 or 10 μmol/l). (f) The protein level of MDR-1 in SPC-A1/DTX cells treated with 10 μmol/l DAPT or 10 μmol/l DAPT plus miR-451 inhibitor. *P < 0.05 and **P < 0.01. DAPT, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester; MDR, multidrug resistant protein-1; mRNA, microRNA.
Notch-1 negatively regulates miR-451 by transcription factor AP-1
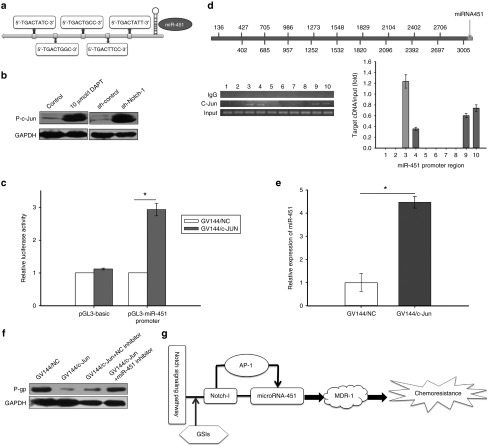
Approximately 3 kb in miR-451 upstream was analyzed as promoter by open online databases, and five complementary binding sites of activator protein (AP-1) were found (Figure 6a). C-Jun, a common plasmid consists of AP-1 complex, can easily combine to the binding sites that AP-1 recognized, and phosphorylated c-Jun involved in activation of AP-1. We found the phosphorylation level of c-Jun was upregulated in DAPT treated or sh-Notch-1 transfected SPC-A1/DTX cells (Figure 6b). The core promoter regions described above of the miR-451 were cloned into the pGL3 basic firefly luciferase reporter, and then the luciferase activity was detected to be elevated in SPC-A1/DTX cells with phosphorylated c-Jun (Figure 6c). Chromatin immunoprecipitation (ChIP) analysis results were shown in Figure 6d, 10 small pieces of segments were separated within miR-451 promoter and primers were correspondingly designed, RNA pol II was used as positive control. Four potential binding sites were noticed by ChIP assays. The possible corresponding locations that c-Jun combined with miR-451 promoter are 705–957 bp, 986–1252 bp, 2402–2697 bp, and 2706–3005 bp. Transfection with c-Jun overexpressed plasmid induces elevated expression of miR-451 (Figure 6e). However, MDR-1 expression was restored by miR-451 inhibitor when c-Jun was upregulated (Figure 6f). Generally, we demonstrated negative regulation in miR-451 and AP-1 by Notch-1 and transcriptional activation in miR-451 by AP-1, which contributes to chemoresistance of LAD by regulating MDR-1 expression (Figure 6g).
Figure 6.
Notch-1 negatively regulates miR-451 by AP-1. (a) There were five complementary binding sites of activator protein-1 (AP-1) in miR-451. (b) The expression of phosphorylated c-jun in SPC-A1/DTX cells treated with 10 μmol/l DAPT, transfected with Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1). GAPDH served as loading control. (c) The luciferase activity of pGL3 basic firefly luciferase reporter or pGL3-miR451 promoter in SPC-A1/DTX cells with or without transfection of c-jun overexpression plasmid (GV144/c-jun). (d) Chromatin Immunoprecipitation (ChIP) analysis for the corresponding location that c-Jun combined with miR-451 promoter. (e) The expression of miR-451 in SPC-A1/DTX cells transfected with c-jun overexpression plasmid (GV144/c-jun). (f) The protein level of MDR-1 in SPC-A1/DTX cells transfected with c-jun overexpression plasmid (GV144/c-jun) or plus miR-451 inhibitor. (g) The Notch-1/AP-1/miR-451/MDR-1 signaling axis. *P < 0.05. DAPT, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester; MDR, multidrug resistant protein-1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
In this study, we demonstrated for the first time that Notch-1 regulates AP-1/miR-451 and confers chemoresistance of LAD to taxanes through MDR-1. Several findings are novel: (i) Notch-1 expression is associated with chemoresistance of LAD to Taxanes; (ii) Combination of Notch-1 inhibition and DTX is more efficient to treat LAD; (iii) miR-451 and its direct target MDR-1 are critical for Notch-1 induced chemoresistance to DTX in LAD; and (iv) Notch-1 triggers AP-1 to negatively regulate miR-451 in DTX-resistant LAD.
Notch-1 has been reported to be involved in the resistance of many anticancer drugs, such as cisplatin, taxol, tamoxifen, oxaliplatin, trastuzumab, gefitinib, taxetere, as well as taxanes.27 Zang et al. reported that down-regulation of Notch-1 signaling increases chemosensitivity to taxotere and doxorubicin in breast cancer.28 Taxotere-resistant DU145 prostate cancer cells have also been reported to hyperexpress Notch-1.11 DTX is a member of the taxane family with high efficacy in the treatment of solid tumors including prostate, breast, lung, and gastric cancer. It has been successfully used in clinical treatment in earlier years. However, the acquired resistance always eliminates the therapeutic efficiency, followed by tumor recurrence and poor prognosis. Our team has focused on study of DTX-resistant LAD for a long time. Herein, we demonstrated that Notch-1 confers resistance to DTX in LAD and is associated with poor prognosis in LAD patients. Targeting Notch-1 for DTX-resistant LAD therapy may be a better strategy. Notch signaling is activated by γ-secretase which can be specifically inhibited by GSIs.29 GSIs possess potent antitumour activity to inhibit cell growth and induce cell apoptosis in many human cancers, such as breast cancer, hepatoma, myeloma, and pancreatic cancer, and some of them have already been tested in clinical trials.30,31,32,33,34 Thus, to investigate the effect of Notch-1 inhibition on DTX-resistant LAD, we introduced a widely used GSI DAPT. Our data revealed that inhibition of Notch-1 by DAPT sensitizes resistant LAD cells to DTX treatment in vitro and in vivo. This indicated a novel strategy to treat DTX-resistant LAD by combination of GSI and DTX.
Recently, we demonstrated the critical roles of miR-451 in invasion, metastasis, and radioresistance of DTX-resistant LAD cells.35,36 We revealed the involvement of miR-451/c-Myc/ERK/GSK-3b signaling axis in the acquisition of EMT phenotype in DTX-resistant LAD cells, and found that the dysregulation of miR-451/c-Myc-survivin/rad-51 signaling is responsible for radioresistance of DTX-resistant LAD cells, suggesting that re-expression of miR-451 or targeting c-Myc will be a potential strategy for the treatment of DTX-resistant LAD patients. In this study, we found that Notch-1 mediates chemoresistance response and proliferation capacity in LAD at least in part through repression of miR-451. Restoration of miR-451 is critical for reversing chemoresistance induced by Notch-1. Moreover, we found that the direct target of miR-451 and MDR-1 is involved. MiR-451 has been previously reported to be up-regulated in MDR cancer cell lines A2780DX5 and KB-V1, comparing with their parental lines A2780 and KB-3-1 (ref. 37) The authors demonstrated that the increased expression of miR-451 contribute to the MDR phenotype in cancer cells, and miR-451 function as activators of MDR1/P-glycoprotein expression. We demonstrated in this study that miR-451 directly target MDR-1, participating into the DTX-resistance induced by Nothch-1 in LAD cells. Then, we investigated how Notch-1 regulates miR-451. We found that the c-jun, an indicator of AP-1 activation, is regulated by Notch-1 in DTX-resistance LAD cells. AP-1 is a complex homodimers composed of Jun family members and Fos proteins.38 Notch-1 inhibition has been reported to restore TRAIL-mediated apoptosis via AP-1-dependent upregulation of DR4 and DR5 TRAIL receptors in MDA-MB-231 breast cancer cells.39 Our data showed the negative regulation in miR-451 and AP-1 by Notch-1, and the transcriptional activation in miR-451 by AP-1. Thus, finally we described a Notch-1/AP-1/miR-451/MDR-1 signaling axis which plays a key role in chemoresistance of LAD to DTX.
In conclusion, our data demonstrated the role of Notch-1/AP-1/miR-451/MDR-1 signaling axis in DTX-resistance of LAD. Notch-1 negatively regulates miR-451 by transcription factor AP-1, and MDR-1 is a direct target of miR-451 which finally induces DTX-resistance in LAD cells. This newly identified Notch-1/AP-1/miR-451/MDR-1 signaling axis suggested a novel therapeutic strategy of combining DTX with GSIs for DTX-resistant LAD.
Materials and Methods
Patients and tissue samples. A number of 101 patients diagnosed with LAD were collected from 2007 to 2009 in Thoracic Surgery Department and Pathological Department of Jinling Hospital. Retrospect of their all medical records, only 39 patients were enrolled because of adjuvant chemotherapy based on DTX was received every 3 weeks at least six cycles before or after surgical resection. The details of clinical records were listed in Table 1. All cases were closely followed up and the overall survival time was identified from surgery date to the latest follow-up or death. Scientific protocol and ethic certification was granted by the Ethics Committee of Jinling Hospital.
Cell culture. Human LDA cell lines SPC-A1 and H1299 and their DTX-resistant cell lines were descried previously.21,22,23,24 All the cells were cultured in Roswell Park Memorial Institute medium (RPMI) 1640 medium supplemented with 10% fetal bovine serum, as well as 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified environment containing 5% CO2.
Cell transfection. The Notch-1 short hair RNA plasmid (pGPU6/sh-Notch-1) and Notch-1 overexpression plasmid (pcDNA3/Notch-1) were purchased from Genechem (Shanghai, China). The miR-451-overexpressing plasmid (pcDNA/miR-451) were constructed and conserved in our lab previously.25 MiR-451 mimics and inhibitors (anti-miR-451) were purchased from Genepharma (Shanghai, China). c-Jun overexpressed plasmid (GV144/c-Jun) was chemically synthesized by Genechem, the target sequences were inserted into the XhoI and BamHI enzyme sites of the GV144-CMVneo vector. All the primers were listed in Supplementary Table S1. All plasmids DNA was extracted using DNA plasmid Mini prep kit II (Omega Bio-tek, Norcross, GA). DNA and RNA oligonucleotides were transfected into cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and siRNA-mate (Genepharma, Shanghai, China) according to the manufacturer's protocol. Stably transfected cells were selected by G418.
RNA extraction and qRT-PCR assay. Total RNA was extracted by TRIzol (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. qRT-PCR assay was described as we described previously.24 The primers used here were all obtained from the Harvard Primer Bank and listed in Supplementary Table S2. MiRNA expression was normalized to U6 snRNA and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as control.
Western blotting assay. The standard procedure of Western blot was described previously.24 All the primary antibodies used in our research including Notch-1, P-gp, phosphor-c-Jun, and GAPDH were obtained from Univ-bio (Shanghai, China).
Cell viability and drug sensitivity assay. The chemotherapeutic sensitivity was adjudged by the cell counting kit-8 according to the manufacturer's instructions (Dojindo Laboratories, Kumamoto, Japan). Briefly, 1 × 104 target cells were transplated into 96-well plates in 100 µl of culture medium. The cells were then treated with DTX, DAPT or combination and cultured at normal incubator as usual for 48 hours. The cell counting kit-8 solution was mixed into each well and incubated at 37°C in a humidified incubator containing 95% air and 5% CO2 for 1 hour. The absorbance was recorded at 450 nm using an enzyme-linked immunosorbent assay plate reader (Bio-Rad, Hercules, CA).
Colony formation assay. The cells were transplanted in a six-well culture plate with maintained in regular medium after stable transfected or directly. DTX was mixed in medium with different concentrations and renewed the mixture medium 48 hours later. Cells were fixed with methanol and then stained with 0.1% crystal violet 2 weeks later. Visible colonies were calculated manually.
Flow cytometric analysis. Apoptosis assays and cell cycle assays by flow cytometric analysis were described previously.23 An annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit and cell cycle detection kit (KeyGEN Biotech, Nanjing, China) was used to inspect apoptosis process and cell cycle distribution in triplicate according to the manufacturer's instructions.
Immunohistochemistry assay. Immunohistochemical staining assay was performed as previously described.26 The 3–4 µm LAD paraffin sections and formalin-fixed tissue were stained with hematoxylin and eosin and immunohistochemical analysis by Maxvision. Notch-1, Ki-67, proliferating cell nuclear antigen and TUNEL were stained according to the instructions. Normal rabbit serum was used as a negative control. All results were independently assessed by two senior pathologists in double-blind way.
Xenograft transplantation assays. All female athymic BALB/c nude mice (4–6 week old) were purchased from the Comparative Medicine Department (Jinling Hospital, Nanjing, China). The animal study has been ethically approved and performed according to the institutional guidelines. Subcutaneous xenografts model were established by subcutaneously injecting 2 × 106 SPC-A1/DTX directly or stable transfected with sh-Notch-1 or sh-control (n = 6 mice/group). Tumor growth was under surveillance and tumor volumes were calculated with equation. All mice were sacrificed about 8 weeks later. Specimens were collected and embedded in paraffin after fixed by 4% paraformaldehyde, and stained with hematoxylin and eosin, as well as immunohistochemistry. All animal experiments were certificated by Jiangsu Province Animal Care and Use Committee.
Luciferase reporter assay. Luciferase reporter containing wild type 3′-UTR of MDR1 (pLUC/MDR1-3′UTR-wt) and mutant reporter (pLUC/MDR1-3′UTR-mut) were established and synthesized from HongLi technology (Shanghai, China). SPC-A1/DTX was seeded in a 24-well plate and transiently cotransfected with 4 μg of either pcDNA/miR-NC or pcDNA/miR-451, and appropriate wild-type or mutant 3′UTR of MDR-1 luciferase reporter plasmid. Cells were collected for luciferase activity assays 48 hours later. The luciferase activities were measured by Dual-Luciferase Reporter Assay kits (Promega, Madison, WI) according the manufacturer's protocol. Renilla luciferase activities were normalized as control. The data of control groups were defined as 1.0. All experiments were repeated in triplicate.
Promoter reported gene analysis. The luciferase reporter vector phRL-SV including -3kb human miR-451 promoter region was cloned and purchased from Promega. The Dual-Luciferase Reporter Assay kit (Promega, Madison, WI) was used to measure promoter activity in according with manufacturer's protocol as previously described.35
ChIP assay. ChIP assay was examined by Immunoprecipitation Assay Kits (Millipore, Billerica, MA) in accordance with the protocols. SPC-A1/DTX cells were cross-linked with 1% formaldehyde for 10 minutes at 37°C when the confluency nearly 80%, then lysed and sonicated. The collected supernatant was incubated with antibodies including anti-c-Jun (1:80) overnight at 4°C with rotation. RNA polII antibody was used as positive control while an isotype IgG was used as negative control. The antibody/DNA complex was collected after rotation, washed by wash buffer and eluted by elution buffer. Crosslinks were reversed by 5 mol/l NaCl mixture under 65°C for 2 hours. At last, DNA sample was purified and identified by qRT-PCR. The putative c-Jun binding sites in miR-451 promoter were amplified by PCR with the input DNA (5%) or DNA isolated from precipitated chromatin as templates. The primers were listed in Supplementary Table S1.
Statistical analysis. All statistical analysis was performed with SPSS version 17.0 (SPSS, Chicago, IL). Data were presented as mean ± SD at least in triple. Student's t-test, chi-square test or Fisher's exact test was determined to value wherever appropriate. Kaplan–Meier method and Cox proportional hazards regression model were employed to estimate survival analysis and prognostic factor. P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. The effects of Notch inhibitor DAPT on cells. Figure S2. The effects of Notch-1 overexpression on cells. Figure S3. DAPT sensitizes DTX-resistant LAD cells to DTX in vivo. Figure S4. The effects of miR-451 on cells. Table S1. Primers sequences used in our research. Table S2. Primers used in Quantitative Real-Time Polymerase Chain Reaction (q-PCR).
Acknowledgments
We appreciate the participation of all patients and the sincere technical assistance from the Pathology Department of Jinling Hospital. This research was supported by the National Science Foundation of China (Grant No. 81402492, 81301913, 81272474, and 81172335) and Natural Science Foundation of Jiangsu Province (Grant No. BK2012371). The authors declare that there are no conflicts of interest.
Supplementary Material
References
- Tan, N, Malek, M, Zha, J, Yue, P, Kassees, R, Berry, L et al. (2011). Navitoclax enhances the efficacy of taxanes in non-small cell lung cancer models. Clin Cancer Res 17: 1394–1404. [DOI] [PubMed] [Google Scholar]
- Avan, A, Maftouh, M, Avan, A, Tibaldi, C, Zucali, PA and Giovannetti, E (2014). SNPs in PI3K-PTEN-mTOR and brain metastases in NSCLC–letter. Clin Cancer Res 20: 3623–3624. [DOI] [PubMed] [Google Scholar]
- Cai, J, Fang, L, Huang, Y, Li, R, Yuan, J, Yang, Y et al. (2013). miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res 73: 5402–5415. [DOI] [PubMed] [Google Scholar]
- Yu, T, Li, J, Yan, M, Liu, L, Lin, H, Zhao, F et al. (2014). MicroRNA-193a-3p and -5p suppress the metastasis of human non-small-cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene 34: 413–423. [DOI] [PubMed] [Google Scholar]
- Wang, LH, Yang, JY, Yang, SN, Li, Y, Ping, GF, Hou, Y et al. (2014). Suppression of NF-κB signaling and P-glycoprotein function by gambogic acid synergistically potentiates adriamycin-induced apoptosis in lung cancer. Curr Cancer Drug Targets 14: 91–103. [DOI] [PubMed] [Google Scholar]
- Nan, J, Du, Y, Chen, X, Bai, Q, Wang, Y, Zhang, X et al. (2014). TPCA-1 is a direct dual inhibitor of STAT3 and NF-κB and regresses mutant EGFR-associated human non-small cell lung cancers. Mol Cancer Ther 13: 617–629. [DOI] [PubMed] [Google Scholar]
- Wynes, MW, Hinz, TK, Gao, D, Martini, M, Marek, LA, Ware, KE et al. (2014). FGFR1 mRNA and protein expression, not gene copy number, predict FGFR TKI sensitivity across all lung cancer histologies. Clin Cancer Res 20: 3299–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, L, Chen, W, Guo, W, Wang, J, Tian, Y, Shi, D et al. (2013). Berberine targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF, and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS One 8: e69240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, BN, Yan, HQ, Wu, X, Pan, ZH, Zhu, Y, Meng, ZW et al. (2013). Apoptosis induced by benzyl isothiocyanate in gefitinib-resistant lung cancer cells is associated with Akt/MAPK pathways and generation of reactive oxygen species. Cell Biochem Biophys 66: 81–92. [DOI] [PubMed] [Google Scholar]
- Gong, Y, Yao, E, Shen, R, Goel, A, Arcila, M, Teruya-Feldstein, J et al. (2009). High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507). PLoS One 4: e7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z, Li, Y, Ahmad, A, Azmi, AS, Banerjee, S, Kong, D et al. (2010). Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta 1806: 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu, P, Chen, KY, Chen, JH, Wang, L, Walters, J, Shin, YJ et al. (2013). A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell 12: 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L, Wu, J, Chen, Q, Hu, X, Li, W and Hu, G (2011). Notch1 expression is upregulated in glioma and is associated with tumor progression. J Clin Neurosci 18: 387–390. [DOI] [PubMed] [Google Scholar]
- Gu, F, Ma, Y, Zhang, Z, Zhao, J, Kobayashi, H, Zhang, L et al. (2010). Expression of Stat3 and Notch1 is associated with cisplatin resistance in head and neck squamous cell carcinoma. Oncology Reports 23: 671–676. [DOI] [PubMed] [Google Scholar]
- Ning, L, Jaskula-Sztul, R, Kunnimalaiyaan, M and Chen, H (2008). Suberoyl bishydroxamic acid activates notch1 signaling and suppresses tumor progression in an animal model of medullary thyroid carcinoma. Ann Surg Oncol 15: 2600–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani, I, Eliasz, S, De Marco, MA, Chen, Y, Pass, HI, De May, RM et al. (2008). Opposite effects of Notch-1 and Notch-2 on mesothelioma cell survival under hypoxia are exerted through the Akt pathway. Cancer Res 68: 9678–9685. [DOI] [PubMed] [Google Scholar]
- Zhang, X, Zhao, X, Shao, S, Zuo, X, Ning, Q, Luo, M et al. (2015). Notch1 induces epithelial-mesenchymal transition and the cancer stem cell phenotype in breast cancer cells and STAT3 plays a key role. Int J Oncol 46: 1141–1148. [DOI] [PubMed] [Google Scholar]
- Baumgart, A, Mazur, PK, Anton, M, Rudelius, M, Schwamborn, K, Feuchtinger, A et al. (2015). Opposing role of Notch1 and Notch2 in a Kras(G12D)-driven murine non-small cell lung cancer model. Oncogene 34: 578–588. [DOI] [PubMed] [Google Scholar]
- Hales, EC, Taub, JW and Matherly, LH (2014). New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia. Cell Signal 26: 149–161. [DOI] [PubMed] [Google Scholar]
- Cui, SY, Wang, R and Chen, LB (2013). MicroRNAs: key players of taxane resistance and their therapeutic potential in human cancers. J Cell Mol Med 17: 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, SY, Huang, JY, Chen, YT, Song, HZ, Feng, B, Huang, GC et al. (2013). Let-7c governs the acquisition of chemo- or radioresistance and epithelial-to-mesenchymal transition phenotypes in docetaxel-resistant lung adenocarcinoma. Mol Cancer Res 11: 699–713. [DOI] [PubMed] [Google Scholar]
- Feng, B, Wang, R, Song, HZ and Chen, LB (2012). MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer 118: 3365–3376. [DOI] [PubMed] [Google Scholar]
- Feng, B, Wang, R and Chen, LB (2012). MiR-100 resensitizes docetaxel-resistant human lung adenocarcinoma cells (SPC-A1) to docetaxel by targeting Plk1. Cancer Lett 317: 184–191. [DOI] [PubMed] [Google Scholar]
- Huang, JY, Cui, SY, Chen, YT, Song, HZ, Huang, GC, Feng, B et al. (2013). MicroRNA-650 was a prognostic factor in human lung adenocarcinoma and confers the docetaxel chemoresistance of lung adenocarcinoma cells via regulating Bcl-2/Bax expression. PLoS One 8: e72615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R, Wang, ZX, Yang, JS, Pan, X, De, W and Chen, LB (2011). MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene 30: 2644–2658. [DOI] [PubMed] [Google Scholar]
- Huang, J, Song, H, Liu, B, Yu, B, Wang, R and Chen, L (2013). Expression of Notch-1 and its clinical significance in different histological subtypes of human lung adenocarcinoma. J Exp Clin Cancer Res 32: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, X, Wang, Z, Sarkar, FH and Gupta, SV (2012). Delta-tocotrienol augments cisplatin-induced suppression of non-small cell lung cancer cells via inhibition of the Notch-1 pathway. Anticancer Res 32: 2647–2655. [PubMed] [Google Scholar]
- Zang, S, Chen, F, Dai, J, Guo, D, Tse, W, Qu, X et al. (2010). RNAi-mediated knockdown of Notch-1 leads to cell growth inhibition and enhanced chemosensitivity in human breast cancer. Oncol Rep 23: 893–899. [DOI] [PubMed] [Google Scholar]
- Groth, C and Fortini, ME (2012). Therapeutic approaches to modulating Notch signaling: current challenges and future prospects. Semin Cell Dev Biol 23: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen, HM, Haapasalo, A, Hiltunen, M, Kataja, V, Kosma, VM and Mannermaa, A (2013). Γ-secretase components as predictors of breast cancer outcome. PLoS One 8: e79249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanjunee, S, Wongchana, W and Palaga, T (2008). Inhibition of gamma-secretase affects proliferation of leukemia and hepatoma cell lines through Notch signaling. Anticancer Drugs 19: 477–486. [DOI] [PubMed] [Google Scholar]
- Hu, J, Zhu, X and Lu, Q (2013). Antiproliferative effects of γ-secretase inhibitor, a Notch signalling inhibitor, in multiple myeloma cells and its molecular mechanism of action. J Int Med Res 41: 1017–1026. [DOI] [PubMed] [Google Scholar]
- De Jesus-Acosta, A, Laheru, D, Maitra, A, Arcaroli, J, Rudek, MA, Dasari, A et al. (2014). A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs 32: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, P, Osipo, C, Foreman, K, Golde, T, Osborne, B and Miele, L (2008). Rational targeting of Notch signaling in cancer. Oncogene 27: 5124–5131. [DOI] [PubMed] [Google Scholar]
- Chen, D, Huang, J, Zhang, K, Pan, B, Chen, J, De, W et al. (2014). MicroRNA-451 induces epithelial-mesenchymal transition in docetaxel-resistant lung adenocarcinoma cells by targeting proto-oncogene c-Myc. Eur J Cancer 50: 3050–3067. [DOI] [PubMed] [Google Scholar]
- Wang, R, Chen, DQ, Huang, JY, Zhang, K, Feng, B, Pan, BZ et al. (2014). Acquisition of radioresistance in docetaxel-resistant human lung adenocarcinoma cells is linked with dysregulation of miR-451/c-Myc-survivin/rad-51 signaling. Oncotarget 5: 6113–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H, Wu, H, Liu, X, Evans, BR, Medina, DJ, Liu, CG et al. (2008). Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol 76: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelmann, M, Bosserhoff, A and Kuphal, S (2014). AP-1/c-Jun transcription factors: regulation and function in malignant melanoma. Eur J Cell Biol 93: 76–81. [DOI] [PubMed] [Google Scholar]
- Portanova, P, Notaro, A, Pellerito, O, Sabella, S, Giuliano, M and Calvaruso, G (2013). Notch inhibition restores TRAIL-mediated apoptosis via AP1-dependent upregulation of DR4 and DR5 TRAIL receptors in MDA-MB-231 breast cancer cells. Int J Oncol 43: 121–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.