Abstract
Aims
Bicuspid aortic valve (BAV) is known to exhibit familial inheritance and is associated with aortopathy and altered aortic haemodynamics. However, it remains unclear whether BAV-related aortopathy can be inherited independently of valve morphology.
Methods and results
Four-dimensional flow magnetic resonance imaging for the in vivo assessment of thoracic aortic 3D blood flow was performed in 24 BAV relatives with trileaflet aortic valves (age = 40 ± 14 years) and 15 healthy controls (age = 37 ± 10 years). Data analysis included aortic dimensions, shape (round/gothic/cubic), and 3D blood flow characteristics (semi-quantitative vortex/helix grading and peak velocities). Cubic and gothic aortic shapes were markedly more prevalent in BAV relatives compared with controls (38 vs. 7%). Ascending aorta (AAo) vortex flow in BAV relatives was significantly increased compared with controls (grading = 1.5 ± 1.0 vs. 0.6 ± 0.9, P = 0.015). Aortic haemodynamics were influenced by aortic shape: peak velocities were reduced for gothic aortas vs. round aortas (P = 0.003); vortex flow was increased for cubic aortas in the AAo (P < 0.001) and aortic arch (P = 0.004); vortex and helix flows were elevated for gothic aortas in the AAo and descending aorta (P = 0.003, P = 0.029). Logistic regression demonstrated significant associations of shape with severity of vortex flow in AAo (P < 0.001) and aortic arch (P = 0.016) in BAV relatives.
Conclusion
BAV relatives expressed altered aortic shape and increased vortex flow despite the absence of valvular disease or aortic dilatation. These data suggest a heritable component of BAV-related aortopathy affecting aortic shape and aberrant blood flow, independent of valve morphology.
Keywords: bicuspid aortic valve, haemodynamics, relatives, 4D flow MRI
Introduction
With an incidence of 1–2%, bicuspid aortic valve (BAV) is the most common congenital anomaly in the general population.1–3 BAV complications, which include aortic stenosis, aortic insufficiency, aneurysm, and dissection, can cause significant morbidity and mortality.4–9 There is increasing evidence supporting a genetic basis for BAV10 as previous studies have reported a 9–21%8,10–12 prevalence of BAV in first-degree relatives (FDRs). Based on the complications associated with BAV, the American Heart Association has recommended echocardiographic screening in FDRs of patients with BAV.13
An embryonic relationship between the aortic arch and the semilunar valves may explain the association between BAV and an abnormal aortic arch. Interestingly, neural crest cells are both essential in arch development and present at the junctures of the leaflet commissures as the semilunar valves develop.14 A number of recent studies suggest that alterations in aortic haemodynamics may play an important role in BAV-related aortopathy.15–19 While the familial association of BAV with aortopathy has been well described, few studies have addressed aortopathy in BAV relatives with normal, trileaflet aortic valves.8,10,20,21 Biner et al. demonstrated that the aortic root is functionally abnormal and dilated in up to 32% of FDRs.21 However, other studies found a lower prevalence of aortic dilatation among BAV relatives.8,10
Previous studies investigating BAV-related aortic geometry and blood flow were based on echocardiography as the main imaging modality. Employing 4D flow magnetic resonance imaging (MRI) (time-resolved 3D phase-contrast MRI with three-directional velocity encoding), we systematically evaluated differences in haemodynamics and aortic geometry in BAV relatives with trileaflet aortic valves.
Methods
Study population
Fifteen age-matched healthy controls with no history of cardiovascular disease and 24 subjects with normal, trileaflet aortic valve morphology from 7 families were enrolled in this Institutional Review Board-approved study (details in Table 1). One or more members of each family were diagnosed with BAV. Exclusion criteria for controls and BAV relatives included: previous cardiac surgery, aortic intervention, or history of congenital heart disease. Two BAVs and one unicuspid aortic valve were discovered during recruitment and were excluded from the study.
Table 1.
Demographics, aortic dimensions, flow quantification, and flow pattern grading for all 24 BAV relatives (FAll), n = 22 FDRs and 15 controls (CON).
| BAV relatives |
CON | P-value (FAll vs. CON) | P-value (FDR vs. CON) | ||
|---|---|---|---|---|---|
| FAll | FDR | ||||
| N (female) | 24 (9) | 22 (8) | 15 (4) | ||
| Age | 40.2 ± 14.4 | 39.0 ± 14.6 | 37.2 ± 10.4 | 0.48a | 0.66a |
| SOV diameter (mm) | 31.8 ± 3.7 | 31.8 ± 3.9 | 29.6 ± 2.3 | 0.06b | 0.08b |
| MAA diameter (mm) | 28.3 ± 3.7 | 27.9 ± 3.7 | 27.7 ± 3.6 | 0.21b | 0.36b |
| PROX diameter (mm) | 26.5 ± 2.4 | 26.5 ± 2.5 | 25.0 ± 2.7 | 0.09b | 0.13b |
| ARCH diameter (mm) | 23.4 ± 2.9 | 23.1 ± 3.0 | 22.7 ± 2.7 | 0.51b | 0.66b |
| DIST diameter (mm) | 21.6 ± 2.4 | 21.5 ± 2.6 | 21.0 ± 2.5 | 0.65b | 0.65b |
| DESC diameter (mm) | 21.0 ± 2.3 | 21.0 ± 2.5 | 20.8 ± 3.2 | 0.83b | 0.82b |
| Width–height ratio | 1.9 ± 0.3 | 1.9 ± 0.3 | 1.7 ± 0.4 | 0.47b | 0.48b |
| Peak velocity (m/s) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.22b | 0.34b |
| Net flow (mL/cycle) | 57.2 ± 20.3 | 57.1 ± 20.4 | 60.4 ± 22.6 | 0.49b | 0.50b |
| Aortic shape | |||||
| Round | 15 | 15 | 14 | ||
| Cubic | 6 | 5 | |||
| Gothic | 3 | 2 | 1 | ||
| Vortex AAo | 1.5± 1.0 | 1.3± 1.0 | 0.6 ± 0.9 | 0.014b | 0.027b |
| Vortex Arch | 0.5 ± 0.9 | 0.4 ± 0.9 | 0.1 ± 0.3 | 0.50b | 0.63b |
| Vortex DAo | 0.3 ± 0.7 | 0.2 ± 0.6 | 0.0 ± 0.0 | 0.11b | 0.15b |
| Helix AAo | 0.6 ± 0.8 | 0.4 ± 0.6 | 0.3 ± 0.6 | 0.15b | 0.29b |
| Helix Arch | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.5 ± 0.9 | 0.66b | 0.77b |
| Helix DAo | 0.4 ± 0.7 | 0.3 ± 0.5 | 0.6 ± 1.0 | 0.80b | 0.70b |
aUnpaired t-test.
bMann–Whitney U-test.
Within the BAV relatives cohort, 92% of the cases were FDRs (Table 1). The seven families (F) consisted of: F1 (n = 12, FDR = 11, age: 38 ± 12 years, 4 females), F2 (n = 2, FDR = 1, age: 42 ± 13 years, 1 female), F3 (n = 3, FDR = 3, age: 51 ± 26 years, 1 female), F4 (n = 2, FDR = 2, age: 39 ± 1 years, 1 female), F5 (n = 1, FDR = 1, age: 25 years, 1 female), F6 (n = 3, FDR = 3, age: 47 ± 21 years, 1 female), and F7 (n = 1, FDR = 1, age: 30 years, no females). Family members of BAV patients were prospectively recruited for research MRI exams. All subjects underwent cardiac MRI and thoracic contrast-enhanced magnetic resonance angiography (MRA) between December 2012 and May 2013. Informed consent was obtained from all study participants.
MR imaging
Cardiac MRI was performed using 1.5 T or 3 T magnetic resonance (MR) systems (MAGNETOM Espree, Aera or Skyra, Siemens Medical Systems, Germany). To assess valve morphology, breath-held, electrocardiogram (ECG)-gated time-resolved (Cine) two-dimensional balanced SSFP (2D Cine bSSFP) images were acquired in all patients.22 The 2D imaging plane was carefully positioned orthogonal to the aorta at the level of the aortic valve. The pulse sequence parameters were as follows: TE = 1.07 − 1.19 ms, TR = 2.14 − 2.38 ms, spatial resolution = 1.00 − 2.03 × 1.00 − 2.03 mm2, slice thickness = 6 mm, temporal resolution = 11.3 − 35.5 ms, and a flip angle of 28–80°. Aortic dimensions were determined based on a 3D electrocardiography ECG-gated contrast-enhanced MR angiogram (CE-MRA) scan using a Fast Low Angle SHot MRI (FLASH) sequence with the following parameters: TR = 2.76–3.21 ms, TE = 1.00–1.13 ms, spatial resolution = 0.91–1.17 × 0.91–1.17 mm2, slice thickness = 1.2–1.7 mm, and flip angle = 17–40°.
For the assessment of aortic blood flow, time-resolved 3D phase-contrast MRI with three-directional velocity encoding (4D flow MRI) was employed to measure 3D blood flow velocities with full volumetric coverage of the thoracic aorta.23 4D flow MRI was acquired during free breathing using respiratory and prospective ECG gating in a sagittal oblique 3D volume of the thoracic aorta. Scan parameters: velocity sensitivity (venc) = 150–250 cm/s, flip angle = 7–15°, spatial resolution = 1.67–2.5 × 1.67–2.5 × 2.2–3.0 mm3, TE = 2.34–2.85 ms, TR = 4.8–5.4 ms, and temporal resolution = 38.4–43.2 ms.
All BAV relatives received a single dose of MultiHance contrast agent (gadobenic acid, Bracco Diagnostics, Inc., Milan, Italy) for improved MRI contrast. All controls received Ablavar contrast agent (gadofosveset trisodium, Lantheus Medical Imaging, Inc., N. Billerica, MA).
Aortic dimensions and valve morphology
Aortic valve morphology was visually assessed. A 2D Cine bSSFP slice was positioned at the valve plane by an experienced radiologist to make sure that all subjects had tricuspid aortic valves and no partially fused raphes. For the measurement of the aortic dimensions, the axial source images of the 3D CE-MRA data were transferred to an external post-processing workstation (Vitrea version 6.0.0.1 software, Vital Images, Minnetonka, MN). The diameter measurements were performed as previously reported by Entezari et al.24 using a semi-automated tool (Vitrea version 6.0.0.1 software). A thoracic aorta centreline was created by the software, and automatic segmentation was initiated upon loading of a dataset. Three-dimensional (3D) aorta segmentation was based on region growing around the existing centreline. Both centreline and segmented aortic lumen could be manually refined by editing tools. Next, maximum outer wall to outer wall diameters using 2D planes perpendicular to the aortic lumen were measured at seven anatomic locations according to the guideline by Hiratzka et al.25 and shown in Figure 1A.
Figure 1.
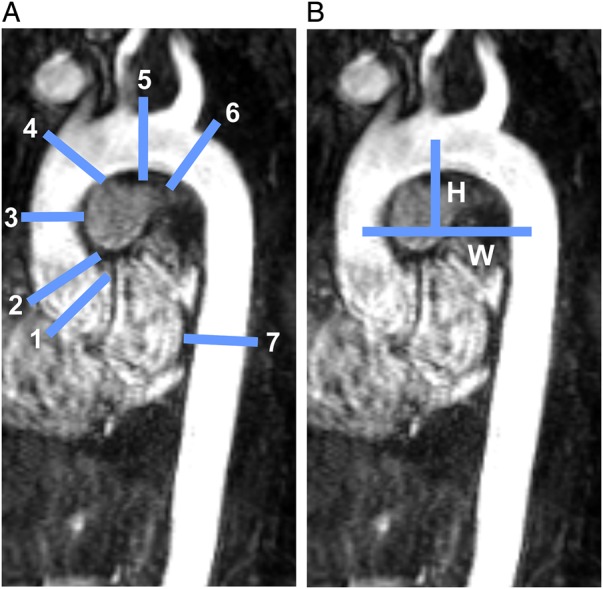
(A) Locations for diameter measurements along the aorta (Subject 3, Family 1): (1) SOV, (2) sinotubular junction (STJ), (3) mid-AAo at the level of right pulmonary artery (MAA), (4) proximal aortic arch immediately proximal to brachiocephalic trunk (PROX), (5) aortic arch between the left common carotid and subclavian arteries (ARCH), (6) distal aortic arch immediately distal to left subclavian artery (DIST), and (7) DAo at the level of left atrium (DESC). (B) Measurement of aorta width and height for calculating the width-to-height ratio.
Aortic arch shape and geometry
The shape of the aortic arch was assessed using 3D CE-MRA data, viewed as a maximum intensity projection (MIP) in a sagittal oblique orientation. Similar to previous studies by Ou et al. and Frydychowicz et al.26,27, aortic shapes were categorized as gothic, cubic, or round (Figure 2). Gothic shape was characterized by an acute angulation between the ascending (AAo) and descending aortas (DAo). Cubic shape was defined by a rectangular form of the aortic arch with a reduced length of the inner compared with the outer curvature of aortic arch.27 Round-shaped aortas were considered normal. Aortic arch width-to-height ratio was determined as an estimate of aortic geometry (Figure 1B). Arch width (W) was defined as the maximum horizontal distance between the midpoint of the AAo and DAo. Height was measured as the maximal vertical distance between W and the highest midpoint of the aortic arch.26
Figure 2.
Velocity MIP for BAV relatives with round (normal), cubic, and gothic aortas. The white arrow in the gothic aorta points the acute angulation between AAo and DAo with the horizontal part of the aorta being shortened. Differences in blood flow velocities for different shapes are clearly evident.
4D flow data analysis and quantification
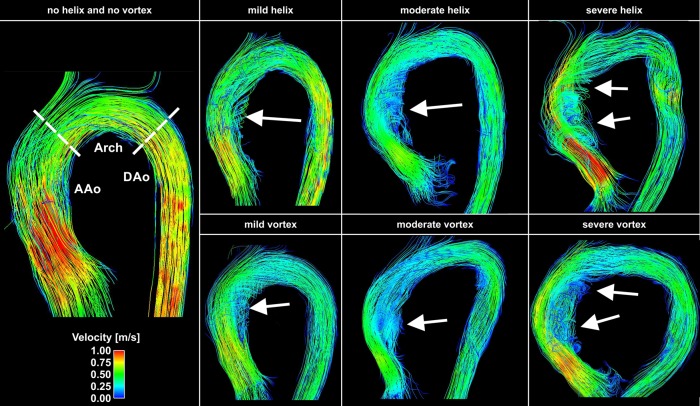
4D flow data were pre-processed to correct for noise, eddy currents, Maxwell terms, and velocity aliasing, as previously described.28,29 A 3D phase-contrast angiogram (PC-MRA) was calculated from the velocity data by combining magnitude and filtered velocity images. The 4D flow data and 3D PC-MRA were loaded in 3D visualization software (Ensight, CEI, Inc., Apex, NC). Three-dimensional blood flow characteristics were visualized by 3D streamlines (i.e. lines tangent to the velocity field) within the segmented aortic lumen at peak flow systole. 3D streamlines were colour-coded to reflect the local systolic blood flow velocity (Figure 3).
Figure 3.
Examples of helix (top row) and vortex (bottom row) flows visualized using velocity-colour-coded 3D streamlines (red = fast velocities and blue = slow, see legend on the left) at peak systole. From left to right, the degree of vortex and helix flows increases from mild to severe. The left panel shows an example without helix or vortex flow. The white arrows point to the particular area of vortex or helix flow. Both the mild and severe helix examples had similar degrees of vortex flow.
Fort quantification of net flow and peak velocity, 2D analysis plane was positioned in the proximal AAo ∼2 cm above the aortic valve [sinus of Valsalva (SOV)], in the mid-ascending aorta (MAA), mid-arch (ARCH), and proximal DAo (DIST).30
Flow pattern assessment
For 3D flow pattern assessment, helix and vortex flows were graded in all subjects on a four-point scale by two investigators in a blinded manner based on strategies that have previously been reported to visually grade helix and vortex flows.31–34 Vortex flow was defined as the rotation of fluid elements around an axis orthogonal to the vessel centreline. Helix flow was considered the rotation of fluid elements around the longitudinal axis of the vessel centreline (i.e. the physiologic flow direction) creating a corkscrew-like flow pattern. Figure 2 illustrates the four different flow pattern grades with Grade 0: linear flow or flow rotation <90°, Grade 1: rotation of 90–180°, Grade 2: vortex/ helix flow 180–360°, and Grade 3: flow rotation >360°.35 Flow pattern grading was performed at three segments of the aorta (Figure 3, left panel): (1) SOV to PROX (AAo), (2) PROX to DIST (Arch), and (3) DIST to DESC (DAo).
Statistical analysis
All continuous variables are expressed as mean ± standard deviation (SD). Inter-observer agreement between both readers in assessment of aortic vortex and helix flows was measured using the Cohen's kappa statistics. T-values of >0.8, >0.6–0.8, >0.4–0.6, >0.2–0.4, and ≤0.2 were considered to indicate excellent, good, moderate, fair, and poor agreements, respectively.
A Lilliefors test revealed non-normal distribution of the peak velocity and net flow. Non-parametric one-way ANOVA using χ2 statistics (Kruskal–Wallis test) and a Mann–Whitney U-test were thus used to compare medians.
Non-parametric one-way ANOVA was also employed to test for differences between aortic shapes. If the Kruskal–Wallis test revealed significant differences (P < 0.05) in aortic shapes, they were then assessed using Fisher's exact test.
In order to understand the driving factor of haemodynamic changes, we performed logistic regression (LR) analysis. The binary regression model is described as follows: , where P is the probability of the presence of either cubic/gothic aortic shape (round aorta = 0 and cubic/gothic aorta = 1) or the BAV relation (control = 0 and BAV relative = 1), α represents the intercept, X is the haemodynamic parameter to be tested, and β is the regression coefficient. For testing the significance of the coefficient or the ‘goodness of fit’, we calculated the likelihood ratio and its P-value using the right-tailed probability of the χ2 distribution with degree of freedom = 1 (i.e. one coefficient in question). The likelihood ratio statistic is: , with L being the maximum likelihood of either the reduced model (for β = 0) or the full model. For all statistical tests, a P-value of <0.05 was considered significant.
Results
Study cohort
Subject demographics, aortic dimensions and shape, as well as the results of flow quantification and pattern grading are summarized in Table 1. The family relation to the BAV patient is illustrated for each family in Figure 4. All subjects included in the study had normally functioning trileaflet aortic valves, and no partial fusion of the raphe was detected. Aortic dimensions were normal, with no dilatation, in all healthy controls and BAV family members. The demographics, BAV fusion pattern as well as aortic measurements and related abnormalities are listed in Supplementary data online, Table S1.
Figure 4.
Family trees of all seven BAV families showing all relatives with a tricuspid aortic valve without colour filling, all known kindred, but with unknown valve morphology with a grey filling and all BAV cases with black filling. According to the standardized human pedigree nomenclature,45 females are represented with a circle, males with a square, and unknown gender with a diamond.
Aortic arch shape and geometry
Abnormal cubic and gothic aortic shapes were found in 38% of BAV family members and 32% of the subset of FDRs, while the control group predominantly exhibited a normal round shape (14 out of 15 subjects, 93%, exhibited round-shaped aortas). Gothic aortas expressed a significantly smaller width-to-height ratio than round aortas (1.52 ± 0.19 vs. 1.8 ± 0.2, P = 0.03).
3D flow visualization
Three-dimensional visualization of complex aortic flow patterns was successfully performed in all subjects. 3D visualization of aortic flow from a F1 FDR is provided in the Supplementary data online, Video (male, age: 56, cubic aorta, SOV diameter 3.5 cm, severe vortex in AAo and arch, moderate vortex in DAo, moderate helix in the AAo).
As summarized in Table 1 and Figure 5A and B, vortex flow in the AAo was significantly more frequent in all BAV relatives (P = 0.015) and FDRs (P = 0.027) compared with controls. Helix flow was similar for all groups. As summarized in Table 2 and Figure 5C and D, significantly elevated vortex flow was found in cubic aortas in the AAo (P < 0.001) and Arch (P = 0.004), and in gothic aortas at the AAo (P = 0.003). Gothic aortas also expressed higher incidence of helical flow in the DAo (P = 0.029). Aortic vortex (unweighted kappa = 0.56) and helix flow (unweighted kappa = 0.46) grading was performed with moderate inter-observer agreement.
Figure 5.
Results of semi-quantitative flow pattern grading. Significant differences (P < 0.05) between controls, BAV relatives, and FDRs as well as between round and cubic and round and gothic are indicated with an asterisk. (A) Vortex flow for AAo, aortic arch, and DAo. (B) Helix flow for AAo, arch, and Dao. (C) Occurrence of vortex flow for round, cubic, and gothic aortas. (D) Occurrence of helix flow in round, cubic, and gothic aortas.
Table 2.
Segmental peak velocity and flow pattern grading results for different aortic shapes.
| Parameter | Aortic shape | SOV | AAo/MAA | ARCH | DAo/DIST |
|---|---|---|---|---|---|
| Peak velocity (m/s) | Round | 1.34 ± 0.4 | 0.96 ± 0.2 | 1.13 ± 0.5 | 1.08 ± 0.3 |
| Cubic | 1.25 ± 0.4 | 0.82 ± 0.2 | 0.91 ± 0.24 | 0.88 ± 0.3 | |
| Gothic | 1.37 ± 0.6 | 0.65 ± 0.2 | 0.81 ± 0.1 | 0.87 ± 0.1 | |
| P-value (cubic vs. round) | 0.83a | 0.17a | 0.22a | 0.08a | |
| P-value (gothic vs. round) | 0.68a | 0.007a | 0.031a | 0.12a | |
| Vortex flow (grading) | Round | 0.69 ± 0.84 | 0.17 ± 0.43 | 0.09 ± 0.38 | |
| Cubic | 2.25 ± 0.61 | 1.50 ± 1.34 | 0.33 ± 0.82 | ||
| Gothic | 2.38 ± 0.48 | 0.00 ± 0.00 | 0.63 ± 1.25 | ||
| P-value (cubic vs. round) | 0.001a | 0.004a | 0.44a | ||
| P-value (gothic vs. round) | 0.003a | 0.40a | 0.22a | ||
| Helix flow (grading) | Round | 0.33 ± 0.52 | 0.47 ± 0.74 | 0.34 ± 0.72 | |
| Cubic | 0.92 ± 1.28 | 0.00 ± 0.00 | 0.5 ± 1.00 | ||
| Gothic | 0.75 ± 0.96 | 0.00 ± 0.00 | 1.38 ± 1.03 | ||
| P-value (cubic vs. round) | 0.37a | 0.10a | 0.58a | ||
| P-value (gothic vs. round) | 0.39a | 0.18a | 0.030a | ||
aMann–Whitney U-test.
Flow quantification
No significant differences were found for net flow for all BAV relatives or FDRs compared with the control group. Net flow was also similar for different aortic shapes. Peak velocity was similar in all three groups at all locations (Table 1). As shown in Table 2, peak velocity was reduced for gothic aortas at the AAo (pveloround@AAO= 1.34 ± 0.39 m/s, pvelocubic@AAO= 1.25 ± 0.42 m/s, pvelogothic@AAO= 1.37 ± 0.58 m/s, P = 0.004) and aortic arch (pveloround@ARCH= 1.13 ± 0.47 m/s, pvelocubic@ARCH= 0.91 ± 0.24 m/s, pvelogothic@ARCH= 0.81 ± 0.06 m/s, P = 0.031).
Relationships between aortic shape and haemodynamics
LR revealed significant relationships between peak velocity and aortic shape for the MAA (D(likelihood ratio)= 7.56, P = 0.006), the ARCH (D= 4.29, P = 0.038), and the DIST (D= 4.21, P = 0.04). In addition, there were significant associations between vortex grading results and aortic shapes in the AAo (D= 22.71, P < 0.001) and Arch (D= 5.78, P = 0.016). In addition, there was a relationship between helix flow and aortic shape in the aortic arch (D= 7.04, P = 0.008).
Discussion
The major findings of our study demonstrate significant differences in aortic shape and altered haemodynamics for BAV relatives despite normal aortic diameter and trileaflet aortic valves. BAV relatives demonstrated a higher prevalence of non-round (i.e. cubic- and gothic-shaped) aortas than the control group, which may explain the observed differences in aortic blood flow. The incidence of vortices in the AAos of BAV relatives was significantly higher than controls. Interestingly, gothic and cubic aortic shapes were also associated with increased vortex flow in the AAo.
This correlation suggests a relationship between aortic shape and potentially aortopathic vortices as a secondary effect, which are both found more commonly in BAV relatives. We speculate that altered aortic geometry can result in redirection of flow through the aortic valve along the differently shaped and curved aorta and thus result in the development of vortex and helix flows. These assumptions were supported by LR analysis, which demonstrated significant associations of aortic shape with the severity of vortex flow in the AAo. While our cohort of BAV relatives did not demonstrate aneurysmal dilatation, the association between aortic shape and haemodynamics is in agreement with recent 4D flow studies of patients with trileaflet aortic valve morphology and altered aortic shape or diameter.27,36–38 Future studies with larger cohorts are warranted to systematically investigate and clarify the impact of abnormal aortic shape on flow patterns in the absence of valvular disease or aortic dilatation.
In a recent 4D flow study, age and aortic diameter were both correlated with increased vortex flow aneurysmal dilatation or abnormal aortic valve morphology.27 Despite increased vorticity in BAV relatives' aortas, we did not find a difference in aortic diameters of age-matched BAV relatives and controls. This may be explained by the small number of non-round aortic shapes in the BAV relatives cohort (cubic = 6, gothic = 3).
There is increasing evidence that changes in aortic 3D flow patterns are associated with aneurysmal dilatation. Buerk et al.36 showed that the incidence and severity of helix and vortex flows was significantly higher in patients with ascending aortic dilatation (≥40 mm), compared with age-matched controls and younger healthy volunteers. Early work by Bogren et al.37 described abnormal vortex flow in patients with aortic aneurysms of varying aetiologies. A more recent study by Hope et al.,38 using 4D flow MRI, presented evidence of increased helix flow as well as earlier and prolonged retrograde flow in patients with ascending aneurysm. Demonstration of similar haemodynamic aberrations in otherwise healthy-appearing aortas suggests that these flow patterns may be a harbinger of developing aortopathy in a genetically predisposed BAV relative. How and when to use 4D flow MRI to predict future, clinically significant aortopathy in BAV relatives is yet to be determined.
The current literature demonstrates that BAV patients often show elevated peak velocity in the AAo and DAo.15,38 In our current study, we could not confirm this relationship in BAV relatives, nor in the subgroup of FDRs, who demonstrate normal peak velocity. Nevertheless, increased vortex flow provides the potential haemodynamic basis for the development of aortopathy.18,39,40 Our findings suggest that BAV relatives are more likely to have altered aortic shapes associated with abnormal aortic blood flow patterns, which have been associated with pathologic aneurysmal dilatation. Additional studies in larger cohorts are warranted to investigate the impact of aortic shape on flow patterns in the absence of valvular disease.
Study limitations
Limitations include the small sample size, which underlines the feasibility character of this study and limits the conclusions that can be drawn with respect to the diagnostic value of the presented findings. A further drawback is related to the heterogeneity of familial relationship to the BAV patient and thus limited comparison of 3D blood flow characteristics. Some families contributed just one FDR while others more, which limits the conclusions that can be drawn with respect to the degree of familial relationship. This especially accounts for Family 1, who contributed half of the study's subjects; however, when looking at the family tree, it can be seen that 4 relatives are second degree to 9 other family members and one subject was distantly related (third degree and less) to all but one.
The acquired MRI data included a 2D Cine bSSFP at the aortic valve to assess valve morphology and a CE-MRA in addition to the 4D flow MRI. However, a full stack of short-axis CINE SSFP scans for the assessment of global cardiac function was not included in the scan protocol. Due to the normal net flow (around 60 mL per cycle) as well as the normal aortic diameters in the healthy volunteers compared with the relatives, we are certain that all recruited BAV relatives had normal cardiac structure and function, except for those in which we detected the BAV. Net flow was derived from the 4D flow MRIs and corresponds to stroke volume.
A further drawback of our study is related to subject recruitment. BAV relatives were prospectively recruited if a known BAV patient had an appointment for routine screening. As a result, not all FDR's underwent research scans, and thus it is likely that the full spectrum of potential aortic abnormalities may not have been sampled. Ideally, all FDRs of a BAV patient should be systematically recruited and screened for the 4D flow study. Despite these limitations, our study is the first to assess aortic shape and secondary alterations in haemodynamics and found a substantial fraction of subjects presented with abnormalities.
Due to the low occurrence of cubic-shaped aortas in the normal population, we could not provide control examples for this cohort. In future studies with more subjects, the control cohorts should represent all aortic shapes to reflect expected natural occurrence.
A further limitation of the study includes the semi-quantitative data analysis (e.g. visual grading of helix and vortex flows) and thus lack of parametric analysis for a less user-dependent quantification of altered aortic flow. Future work should include automated detection systems for vorticity and helicity and quantitative evaluation of flow patterns at the vessel wall. However, flow pattern grading was based on strategies that have previously been reported and successfully employed to detect and characterize abnormal carotid,31 pulmonary,32 and aortic flows.33,34
Care was taken to screen all study subjects for abnormal valve morphology, such that three valves of recruited family members were discovered to have bicuspid or unicuspid morphology. While it may be possible that partially fused raphes could be missed, we are confident that the attending radiologist (JeC, 10 years of training and expertise), who screened the bSSFP valve images, detected all BAV patterns within the previously reported sensitivity of MRI.22
A number of recent studies provide strong evidence that BAV is associated changes in aortic wall shear stress (WSS) even in the absence of valve stenosis or aortic dilatation. In this context, useful quantitative data could be gleaned by quantifying regional aortic WSS or oscillatory shear index, which have been shown to be associated with endothelial cell function and thus vascular remodelling.41 These WSS changes are suspected to indicate heightened risk for vascular remodelling and thus development of aortic complications. The analysis of aortic WSS could thus be a potentially interesting metric to evaluate for risk of aortopathy in BAV relatives. In our study, the analysis of WSS would have required a detailed segmentation of the aorta, which was beyond the scope of the data analysis of this feasibility study. Aortic distensibility is another factor that could contribute to changes in aortic haemodynamics. Unfortunately, data on aortic compliance (i.e. changes in aortic diameters over the cardiac cycle) could not be derived from the 4D flow data due to limited spatial resolution for this type of analysis.
Previous studies have shown that aortic haemodynamics and geometry can significantly change with age.27,42 BAV relatives and healthy control subjects in this study were similar in age (Table 1), and we therefore do not expect any effect of age in our study population.
The two different contrast agents used were chosen based on availability at the time of scan. Both contrast agents used (Multihance and Ablavar) are well suited for contrast-enhanced MRA and 4D flow MRI as previously shown,43,44 with no difference in quantification results between the two agents.
Finally, 4D flow MRI and detailed valve imaging was not performed for all BAV patients related to the patients in our study. Therefore, information on the BAV fusion pattern in those patients is not available; however, Supplementary data online, Table S1 provides all the available information of the BAV patients for each family. Future studies should investigate the relationship between valve fusion pattern in the BAV patient and aortic shape and flow patterns in BAV relatives.
Conclusions
In this pilot feasibility study, we demonstrate an altered aortic shape and aberrant blood flow characteristics in BAV relatives despite normal aortic diameter and valve morphology. Given the large body of literature on BAV-related aortopathy, known familial inheritance of BAV, and the evidence for altered haemodynamics in aneurysmal dilatation, further investigation into the predictive value of 4D flow MRI in BAV relatives is warranted.
Supplementary data
Supplementary data are available at European Heart Journal—Cardiovascular Imaging online.
Funding
The work was supported by the Deutsche Forschungsgemeinschaft (DFG SCHN 1170/1-1), the SIR Foundation pilot study grant, and the National Institutes of Health (grant numbers R01HL115828 and K25HL119608). Additional support was provided by the Martha and Richard Melman Family Bicuspid Aortic Valve Program at the Bluhm Cardiovascular Institute at Northwestern University.
Conflict of interest
None declared.
References
- 1.Gray GW, Salisbury DA, Gulino AM. Echocardiographic and color flow Doppler findings in military pilot applicants. Aviat Space Environ Med 1995;66:32–4. [PubMed] [Google Scholar]
- 2.Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000;83:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nistri S, Basso C, Marzari C, Mormino P, Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol 2005;96:718–21. [DOI] [PubMed] [Google Scholar]
- 4.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104–12. [DOI] [PubMed] [Google Scholar]
- 5.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317–25. [DOI] [PubMed] [Google Scholar]
- 6.Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation 2000;102(19 Suppl 3):III35–9. [DOI] [PubMed] [Google Scholar]
- 7.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005;111:920–5. [DOI] [PubMed] [Google Scholar]
- 8.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004;44:138–43. [DOI] [PubMed] [Google Scholar]
- 9.Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart 1999;82:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol 1997;30:1809–12. [DOI] [PubMed] [Google Scholar]
- 11.McDonald K, Maurer BJ. Familial aortic valve disease: evidence for a genetic influence? Eur Heart J 1989;10:676–7. [DOI] [PubMed] [Google Scholar]
- 12.Glick BN, Roberts WC. Congenitally bicuspid aortic valve in multiple family members. Am J Cardiol 1994;73:400–4. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521–643. [DOI] [PubMed] [Google Scholar]
- 14.Gruber PJ, Wessels A, Kubalak SW. Development of the Heart and Great Vessels, in Pediatric Cardiac Surgery. Blackwell Publishing Ltd.; 2012. pp. 1–26. [Google Scholar]
- 15.Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 2014;129:673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bissell MM, Hess AT, Biasiolli L, Glaze SJ, Loudon M, Pitcher A et al. Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging 2013;6:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker AJ, Markl M, Burk J, Lorenz R, Bock J, Bauer S et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012;5:457–66. [DOI] [PubMed] [Google Scholar]
- 18.Hope MD, Hope TA, Meadows AK, Ordovas KG, Urbania TH, Alley MT et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010;255:53–61. [DOI] [PubMed] [Google Scholar]
- 19.Hope MD, Wrenn J, Sigovan M, Foster E, Tseng EE, Saloner D. Imaging biomarkers of aortic disease: increased growth rates with eccentric systolic flow. J Am Coll Cardiol 2012;60:356–7. [DOI] [PubMed] [Google Scholar]
- 20.Loscalzo ML, Goh DL, Loeys B, Kent KC, Spevak PJ, Dietz HC. Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A 2007;143A:1960–7. [DOI] [PubMed] [Google Scholar]
- 21.Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009;53:2288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaisrie SC, Carr J, Mikati I, Rigolin V, Yip BK, Lapin B et al. Cardiac magnetic resonance imaging is more diagnostic than 2-dimensional echocardiography in determining the presence of bicuspid aortic valve. J Thorac Cardiovasc Surg 2012;144:370–6. [DOI] [PubMed] [Google Scholar]
- 23.Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 2007;25:824–31. [DOI] [PubMed] [Google Scholar]
- 24.Entezari P, Kino A, Honarmand AR, Galizia MS, Yang Y, Collins J et al. Analysis of the thoracic aorta using a semi-automated post processing tool. Eur J Radiol 2013;82:1558–64. [DOI] [PubMed] [Google Scholar]
- 25.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr. et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27–e129. [DOI] [PubMed] [Google Scholar]
- 26.Ou P, Bonnet D, Auriacombe L, Pedroni E, Balleux F, Sidi D et al. Late systemic hypertension and aortic arch geometry after successful repair of coarctation of the aorta. Eur Heart J 2004;25:1853–9. [DOI] [PubMed] [Google Scholar]
- 27.Frydrychowicz A, Berger A, Munoz Del Rio A, Russe MF, Bock J, Harloff A et al. Interdependencies of aortic arch secondary flow patterns, geometry, and age analysed by 4-dimensional phase contrast magnetic resonance imaging at 3 Tesla. Eur Radiol 2012;22:1122–30. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein MA, Zhou XJ, Polzin JA, King KF, Ganin A, Pelc NJ et al. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med 1998;39:300–8. [DOI] [PubMed] [Google Scholar]
- 29.Walker PG, Cranney GB, Scheidegger MB, Waseleski G, Pohost GM, Yoganathan AP. Semiautomated method for noise reduction and background phase error correction in MR phase velocity data. J Magn Reson Imaging 1993;3:521–30. [DOI] [PubMed] [Google Scholar]
- 30.Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magn Reson Med 2008;60:1218–31. [DOI] [PubMed] [Google Scholar]
- 31.Harloff A, Albrecht F, Spreer J, Stalder AF, Bock J, Frydrychowicz A et al. 3D Blood flow characteristics in the carotid artery bifurcation assessed by flow-sensitive 4D MRI at 3T. Magn Reson Med 2009;61:65–74. [DOI] [PubMed] [Google Scholar]
- 32.Geiger J, Markl M, Jung B, Grohmann J, Stiller B, Langer M et al. 4D-MR flow analysis in patients after repair for tetralogy of Fallot. Eur Radiol 2011;21:1651–7. [DOI] [PubMed] [Google Scholar]
- 33.Semaan E, Markl M, Chris Malaisrie S, Barker A, Allen B, McCarthy P et al. Haemodynamic outcome at four-dimensional flow magnetic resonance imaging following valve-sparing aortic root replacement with tricuspid and bicuspid valve morphology. Eur J Cardiothorac Surg 2014;45:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiger J, Markl M, Herzer L, Hirtler D, Loeffelbein F, Stiller B et al. Aortic flow patterns in patients with Marfan syndrome assessed by flow-sensitive four-dimensional MRI. J Magn Reson Imaging 2012;35:594–600. [DOI] [PubMed] [Google Scholar]
- 35.Meierhofer C, Schneider EP, Lyko C, Hutter A, Martinoff S, Markl M et al. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur Heart J Cardiovasc Imaging 2013;14:797–804. [DOI] [PubMed] [Google Scholar]
- 36.Burk J, Blanke P, Stankovic Z, Barker A, Russe M, Geiger J et al. Evaluation of 3D blood flow patterns and wall shear stress in the normal and dilated thoracic aorta using flow-sensitive 4D CMR. J Cardiovasc Magn Reson 2012;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogren HG, Mohiaddin RH, Yang GZ, Kilner PJ, Firmin DN. Magnetic resonance velocity vector mapping of blood flow in thoracic aortic aneurysms and grafts. J Thorac Cardiovasc Surg 1995;110:704–14. [DOI] [PubMed] [Google Scholar]
- 38.Hope TA, Markl M, Wigstrom L, Alley MT, Miller DC, Herfkens RJ. Comparison of flow patterns in ascending aortic aneurysms and volunteers using four-dimensional magnetic resonance velocity mapping. J Magn Reson Imaging 2007;26:1471–9. [DOI] [PubMed] [Google Scholar]
- 39.Girdauskas E, Borger MA, Secknus MA, Girdauskas G, Kuntze T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg 2011;39:809–14. [DOI] [PubMed] [Google Scholar]
- 40.Bauer M, Siniawski H, Pasic M, Schaumann B, Hetzer R. Different hemodynamic stress of the ascending aorta wall in patients with bicuspid and tricuspid aortic valve. J Card Surg 2006;21:218–20. [DOI] [PubMed] [Google Scholar]
- 41.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999;282:2035–42. [DOI] [PubMed] [Google Scholar]
- 42.van Ooij P, Garcia J, Potters WV, Malaisrie SC, Collins JD, Carr JC et al. Age-related changes in aortic 3D blood flow velocities and wall shear stress: Implications for the identification of altered hemodynamics in patients with aortic valve disease. J Magn Reson Imaging 2016;43:1239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bock J, Frydrychowicz A, Stalder AF, Bley TA, Burkhardt H, Hennig J et al. 4D phase contrast MRI at 3 T: effect of standard and blood-pool contrast agents on SNR, PC-MRA, and blood flow visualization. Magn Reson Med 2010;63:330–8. [DOI] [PubMed] [Google Scholar]
- 44.Frydrychowicz A, Russe MF, Bock J, Stalder AF, Bley TA, Harloff A et al. Comparison of gadofosveset trisodium and gadobenate dimeglumine during time-resolved thoracic MR angiography at 3T. Acad Radiol 2010;17:1394–400. [DOI] [PubMed] [Google Scholar]
- 45.Bennett RL, French KS, Resta RG, Doyle DL. Standardized human pedigree nomenclature: update and assessment of the recommendations of the National Society of Genetic Counselors. J Genet Couns 2008;17:424–33. [DOI] [PubMed] [Google Scholar]