Abstract
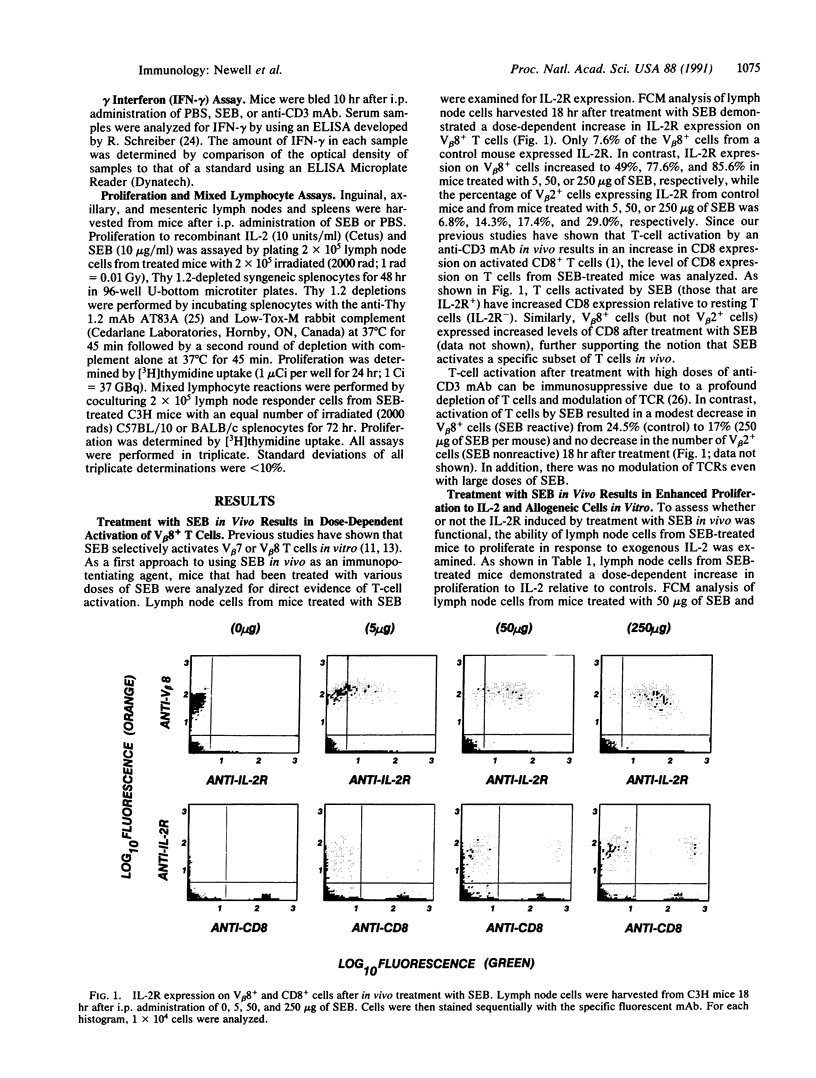
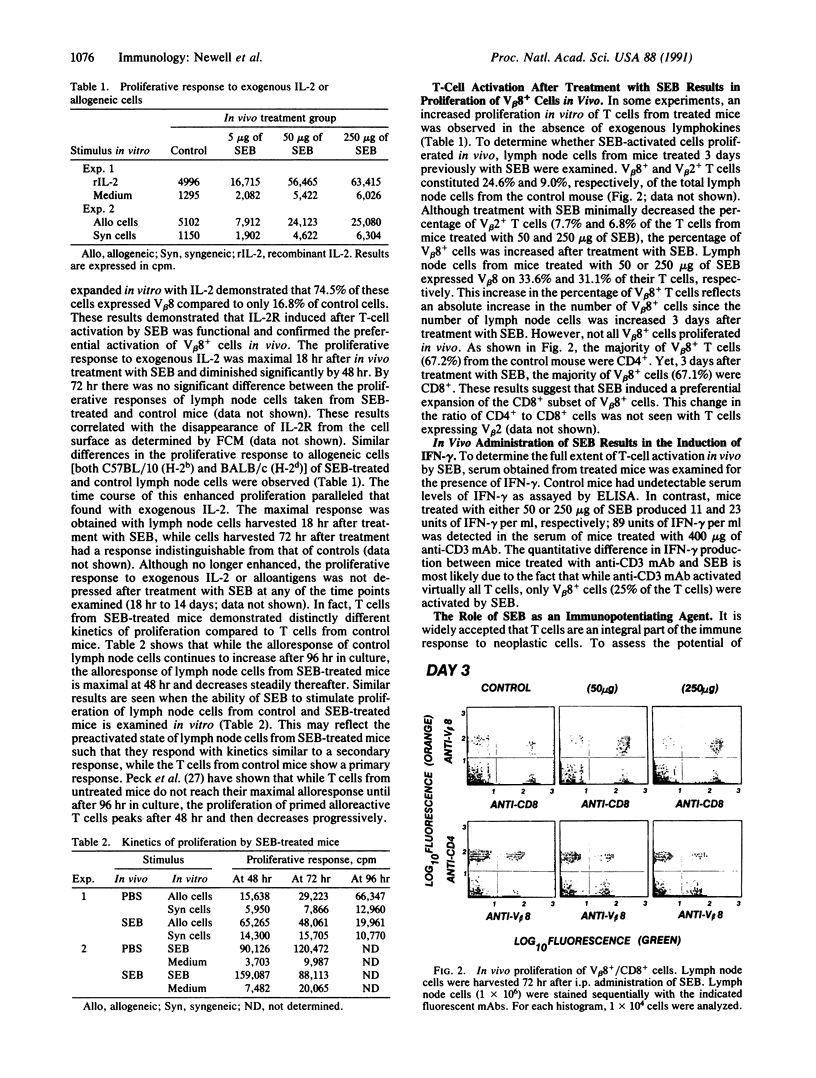
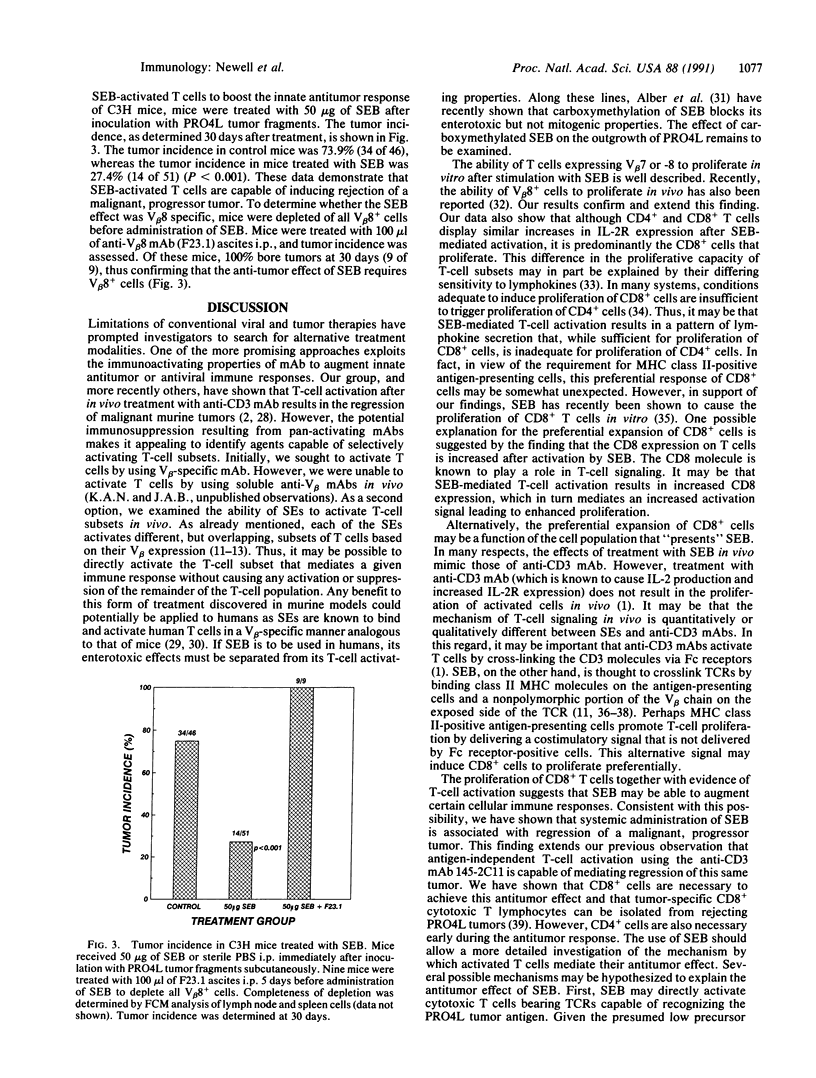
Treatment of T cells with staphylococcal enterotoxins in vitro is known to activate T cells in a subset restricted manner based on beta-chain variable region (V beta) gene expression. In particular, staphylococcal enterotoxin B (SEB) activates T cells bearing V beta 7 or V beta 8. We examined the ability of SEB to activate T cells in vivo. Treatment of C3H mice with doses of SEB ranging from 5 to 250 micrograms resulted in a dose-dependent activation of V beta 8+ T cells as reflected by increased interleukin 2 receptor (IL-2R) expression, proliferation to exogenous IL-2 and allogeneic cells, and production of gamma interferon. SEB also caused proliferation of the CD8+ subset of V beta 8+ cells in vivo. Thus, T-cell activation by SEB in vivo appears to be specific since V beta 2+ cells (non-SEB reactive) did not show increases in IL-2R expression similar to those seen with V beta 8+ cells nor did they proliferate. We then studied the ability of these activated cells to potentiate the immune response to a malignant progressor tumor. Treatment of C3H mice with 50 micrograms of SEB at the time of inoculation with tumor fragments resulted in a statistically significant decrease in the frequency of tumor outgrowth. These data demonstrate that treatment of C3H mice with SEB results in specific activation of V beta 8+ cells in vivo and that these activated cells are capable of preventing the outgrowth of a malignant tumor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber G., Hammer D. K., Fleischer B. Relationship between enterotoxic- and T lymphocyte-stimulating activity of staphylococcal enterotoxin B. J Immunol. 1990 Jun 15;144(12):4501–4506. [PubMed] [Google Scholar]
- Buxser S., Vroegop S. Staphylococcal enterotoxin B stimulation of BALB/c lymphocyte mitogenesis and potential relationship to the Mls response. J Immunogenet. 1988 Feb-Jun;15(1-3):153–159. doi: 10.1111/j.1744-313x.1988.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Callahan J. E., Herman A., Kappler J. W., Marrack P. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990 Apr 1;144(7):2473–2479. [PubMed] [Google Scholar]
- Carlsson R., Sjögren H. O. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985 Nov;96(1):175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe I. N., Bevan M. J., Staerz U. D. Selective activation of Lyt 2+ precursor T cells by ligation of the antigen receptor. Nature. 1985 Oct 17;317(6038):627–629. doi: 10.1038/317627a0. [DOI] [PubMed] [Google Scholar]
- Dellabona P., Peccoud J., Kappler J., Marrack P., Benoist C., Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990 Sep 21;62(6):1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- Ellenhorn J. D., Hirsch R., Schreiber H., Bluestone J. A. In vivo administration of anti-CD3 prevents malignant progressor tumor growth. Science. 1988 Oct 28;242(4878):569–571. doi: 10.1126/science.2902689. [DOI] [PubMed] [Google Scholar]
- Ellenhorn J. D., Schreiber H., Bluestone J. A. Mechanism of tumor rejection in anti-CD3 monoclonal antibody-treated mice. J Immunol. 1990 Apr 1;144(7):2840–2846. [PubMed] [Google Scholar]
- Fischer H., Dohlsten M., Lindvall M., Sjögren H. O., Carlsson R. Binding of staphylococcal enterotoxin A to HLA-DR on B cell lines. J Immunol. 1989 May 1;142(9):3151–3157. [PubMed] [Google Scholar]
- Fleischer B. Bacterial toxins as probes for the T-cell antigen receptor. Immunol Today. 1989 Aug;10(8):262–264. doi: 10.1016/0167-5699(89)90137-0. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989 May 18;339(6221):221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Segal D. M., Hodes R. J. Activation of Lyt-2+ (CD8+) and L3T4+ (CD4+) T cell subsets by anti-receptor antibody. J Immunol. 1989 Apr 1;142(7):2181–2186. [PubMed] [Google Scholar]
- Herrmann T., Accolla R. S., MacDonald H. R. Different staphylococcal enterotoxins bind preferentially to distinct major histocompatibility complex class II isotypes. Eur J Immunol. 1989 Nov;19(11):2171–2174. doi: 10.1002/eji.1830191131. [DOI] [PubMed] [Google Scholar]
- Herrmann T., Maryanski J. L., Romero P., Fleischer B., MacDonald H. R. Activation of MHC class I-restricted CD8+ CTL by microbial T cell mitogens. Dependence upon MHC class II expression of the target cells and V beta usage of the responder T cells. J Immunol. 1990 Feb 15;144(4):1181–1186. [PubMed] [Google Scholar]
- Hirsch R., Eckhaus M., Auchincloss H., Jr, Sachs D. H., Bluestone J. A. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol. 1988 Jun 1;140(11):3766–3772. [PubMed] [Google Scholar]
- Hirsch R., Gress R. E., Pluznik D. H., Eckhaus M., Bluestone J. A. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol. 1989 Feb 1;142(3):737–743. [PubMed] [Google Scholar]
- Hoskin D. W., Stankova J., Anderson S. K., Roder J. C. Amelioration of experimental lung metastasis in mice by therapy with anti-CD3 monoclonal antibodies. Cancer Immunol Immunother. 1989;29(3):226–230. doi: 10.1007/BF00200000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Kappler J., Kotzin B., Herron L., Gelfand E. W., Bigler R. D., Boylston A., Carrel S., Posnett D. N., Choi Y., Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989 May 19;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Bluestone J. A., Heemskerk M. H., Spaargaren J., Voordouw A. C., Ellenhorn J. D., Melief C. J. Treatment with monoclonal anti-CD3 antibody protects against lethal Sendai virus infection by induction of natural killer cells. J Immunol. 1990 Oct 1;145(7):2254–2259. [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med. 1990 Oct 1;172(4):1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Patel M. R., Linna T. J., Rogers T. J. Suppression of cytolytic T-cell activity by staphylococcal enterotoxin B-induced suppressor cells: role of interleukin 2. Cell Immunol. 1986 Nov;103(1):147–159. doi: 10.1016/0008-8749(86)90076-6. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Corthésy P., Tougne C., Lees R., MacDonald H. R., Nabholz M. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J Immunol. 1985 Dec;135(6):3988–3994. [PubMed] [Google Scholar]
- Marrack P., Blackman M., Kushnir E., Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990 Feb 1;171(2):455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Mollick J. A., Cook R. G., Rich R. R. Class II MHC molecules are specific receptors for staphylococcus enterotoxin A. Science. 1989 May 19;244(4906):817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Bluestone J. A., Kruisbeek A., Maloy W. L., Coligan J. E., Schwartz R. H. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987 Mar 5;326(6108):79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Peck A. B., Andersson L. C., Wigzell H. Secondary in vitro responses of T lymphocytes to non-H-2 alloantigens self-H-2-restricted responses induced in heterologous serum are not dependent on primary-stimulating non-H-2 alloantigens. J Exp Med. 1977 Apr 1;145(4):802–818. doi: 10.1084/jem.145.4.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M., Torten M., Birnbaum S. C. Suppression of the in vivo humoral and cellular immune response by staphylococcal enterotoxin B (SEB). Transplantation. 1978 Jun;25(6):320–323. doi: 10.1097/00007890-197806000-00008. [DOI] [PubMed] [Google Scholar]
- Rellahan B. L., Jones L. A., Kruisbeek A. M., Fry A. M., Matis L. A. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J Exp Med. 1990 Oct 1;172(4):1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Loken M. R., Fitch F. W. Structural differences in cell surface T25 polypeptides from thymocytes and cloned T cells. Hybridoma. 1981;1(1):13–26. doi: 10.1089/hyb.1.1981.1.13. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Hicks L. J., Celada A., Buchmeier N. A., Gray P. W. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985 Mar;134(3):1609–1618. [PubMed] [Google Scholar]
- Smith B. G., Johnson H. M. The effect of staphylococcal enterotoxins on the primary in vitro immune response. J Immunol. 1975 Aug;115(2):575–578. [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Urban J. L., Burton R. C., Holland J. M., Kripke M. L., Schreiber H. Mechanisms of syngeneic tumor rejection. Susceptibility of host-selected progressor variants to various immunological effector cells. J Exp Med. 1982 Feb 1;155(2):557–573. doi: 10.1084/jem.155.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. R., Leatherman D. L., Metzger J. F. Evidence for cell-receptor activity in lymphocyte stimulation by staphylococcal enterotoxin. J Immunol. 1975 Jul;115(1):49–53. [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]



