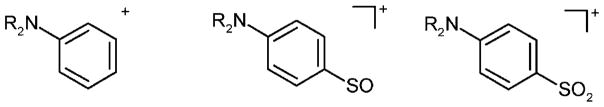
Abstract
Mono-, di- and trialkyl derivatives of ‘sulfabenzamide’ (N-4-aminophenylsulfonylbenzamide) have been prepared and their electron ionization (EI) mass spectra examined. It is found that the fragmentation of N-alkylsulfabenzamides (alkyl =CH3 to n-C5H11) proceeds via a very specific rearrangement process. The proposed mechanism involves an intermediate formation of distonic molecular ions, and the driving force for this process is the formation of stable N-alkylphenylcyanide cations [R-N+ ≡ CC6H5]. The findings are confirmed by exact mass measurements, tandem mass spectrometry (MS/MS) experiments and deuterium labeling.
Two major activities of the NIST (Gaithersburg, MD, USA) Mass Spectrometry Data Center (MSDC) include measurement of electron ionization (EI) mass spectra and gas chromatographic indices for compounds (a) with low quality EI spectra present in the 2008 edition of the NIST/NIH/EPA mass spectral library;[1,2] (b) with poor gas chromatographic properties; and (c) that are labile chemicals under gas chromatography/mass spectrometry (GC/MS) conditions. Metabolites, drugs and environmentally important compounds are on the priority list of the NIST MSDC. In this study the derivatization of an antimicrobial drug, sulfabenzamide, is conducted to determine which derivatives are the best for use in GC/MS analysis.
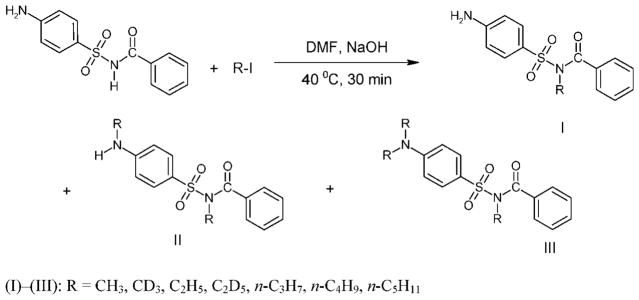
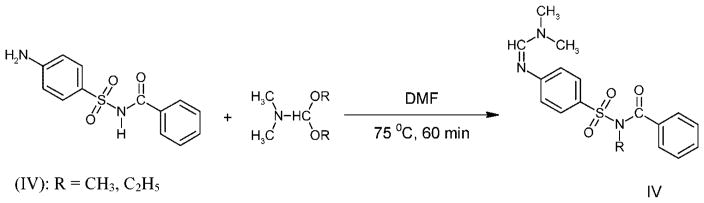
Common derivatization reactions,[3] such as trialkylsilylation and perfluoroacylation, appear to be unsuitable for GC/MS analysis of sulfabenzamide as the compound is labile under the reaction conditions and mainly derivatives of degradation products are formed. Alkylation with alkyl iodides and N,N-dimethylformamide dialkyl acetals is more successful, and the derivatives (I)–(III) and (IV) are obtained.
EXPERIMENTAL
Chemicals
N-(4-Aminophenylsulfonyl)benzamide (‘sulfabenzamide’), methyl, trideuteromethyl, ethyl, pentadeuteroethyl, n-prop-yl, n-butyl, n-pentyl iodides, dimethyl and diethyl acetals of N,N-dimethylformamide are commercially available (Sigma-Aldrich, St. Louis, MO, USA).
Synthesis of derivatives
Alkylation of ‘sulfabenzamide’ is accomplished by heating (40 °C, 30 min) with alkyl iodides in N,N-dimethylformamide (DMF) in the presence of NaOH. As a result, the mono-, di-and trialkyl derivatives (I–III) are obtained (Scheme 1).
Scheme 1.
The mixed derivatives (IV) were obtained by alkylation of ‘sulfabenzamide’ with N,N-dimethylformamide dialkyl acetals (DMF, 75 °C, 60 min) (Scheme 2).
Scheme 2.
Instrumentation
The EI mass spectra were recorded on GC/MS systems with quadrupole (Agilent 6890/5973: Agilent Technologies, Santa Clara, CA, USA; ionization energy 70 eV; ion source temperature 230 °C) and magnetic sector analyzers (Finnigan MAT 95XL: Thermo Scientific, Bremen, Germany; ionization energy 70 eV, temperature of ionization chamber 220 °C). A fused quartz capillary column (30 m × 0.25 mm i.d.) with a non-polar stationary liquid phase (polydimethylsiloxane, containing 5% of phenyl groups) was used. The oven temperature was increased from 60 °C to 200–270 °C at a rate of 5 °C/min (injection temperature −250 to 270 °C). Exact mass determinations and linked-scan experiments (collision-induced dissociation (CID), helium as a collision gas) were performed on a GCmate II magnetic sector mass spectrometer (JEOL, Tokyo, Japan).
RESULTS AND DISCUSSION
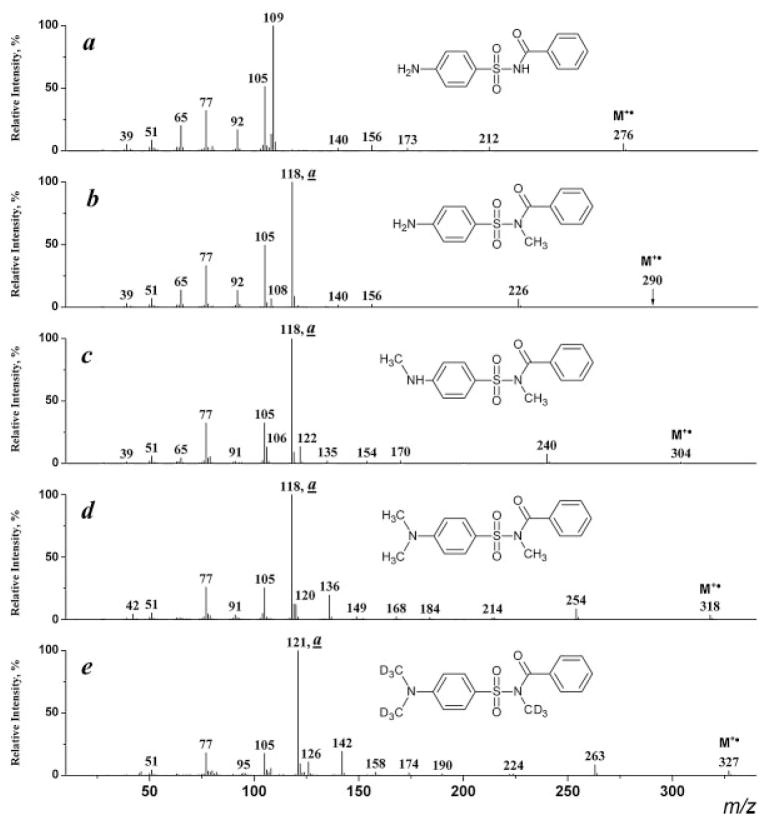
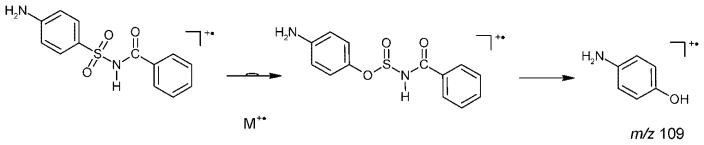
The EI mass spectrum of the initial unsubstituted sulfabenzamide (Fig. 1(a)) can be easily interpreted on the basis of known fragmentation rules. It reveals rather weak peaks for the M+• and [M–SO2]+• ions and an expected strong peak for the benzoyl cation at m/z 105. However, the base peak in the spectrum at m/z 109 is a product of an expected rearrangement process[4] of the M+• followed by a hydrogen migration (Scheme 3). Analogs of this radical cation are not present in the spectra of the alkyl derivatives.
Figure 1.
EI mass spectra of sulfabenzamide (a), N-methyl-N-(4-aminophenylsulfonyl)- (b), N-methyl-N-(4-methyl-aminophenylsulfonyl)- (c), N-methyl-N-(4-dimethylaminophenylsulfonyl)- (d), and N-trideuteromethyl-N-(4-di(trideuteromethyl) aminophenylsulfonyl)benzamides (e).
Scheme 3.
Other peaks in the high-mass region of the spectrum correspond to ions formed by simple bond cleavages (Scheme 4). The same set of ions is present in the spectra of the alkyl derivatives (I)–(IV).
Scheme 4.
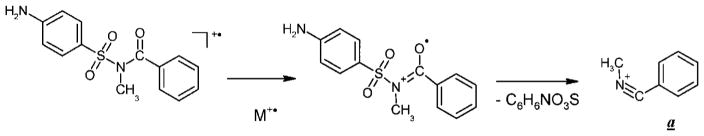
The mass spectra of the mono-, di- and trimethylsulfa-benzamides (I–III, R =CH3) are depicted in Figs. 1(b)–1(d). The base peaks at m/z 118 cannot be explained on the basis of any simple bond cleavage or known fragmentation pathways for similar compounds. The same mass value (m/z 118) in all three spectra may point to the benzamide moiety being the source for the formation of this ion (a). The mass of this peak in the spectra of the tri-, hexa- and nonadeutero analogs (Fig. 1(e)) is shifted by 3 m/z units from m/z 118 to 121. All of the above indicate the presence in this ion of the methyl (trideuteromethyl) group attached to the amide N-atom, and the absence of the carbonyl oxygen atom. As a result, C8H8N is the only possible elemental composition that can be suggested for this ion, and this assignment is corroborated by exact mass measurements of the ion: experimental value of 118.0656, and calculated value of 118.0657 (error: ±0.8 ppm).
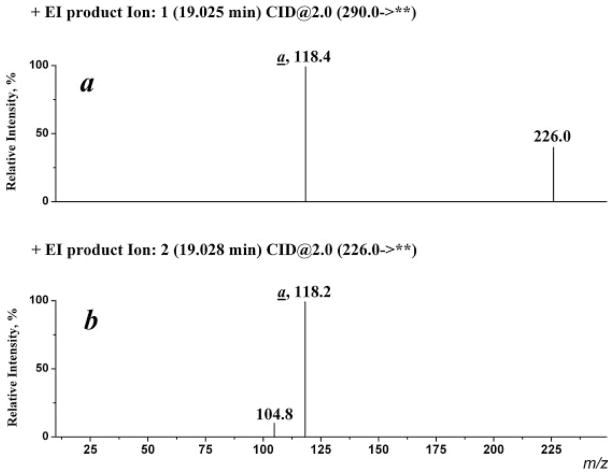
CID experiments indicate that the ion (a) can be derived from both the molecular and the [M–SO2]+ ion (Fig. 2). The driving force for the rearrangement process is probably the formation of a stable immonium cation via a distonic molecular ion (Scheme 5). The mechanism for the formation of ion (a) involves a skeletal rearrangement accompanied with a migration of either the arylsulfonyl group to the carbonyl oxygen atom or the carbonyl O-atom to the arylsulfonyl group. It should be emphasized that the migration of a carbonyl oxygen atom under EI conditions has not previously been reported.[5,6]
Figure 2.
Product ion spectra recorded for (a) molecular ion (m/z 290) and (b) ion [M–SO2]+• (m/z 226) derived from N-methyl-N-(4-aminophenylsulfonyl)benzamide (I, R =CH3).
Scheme 5.
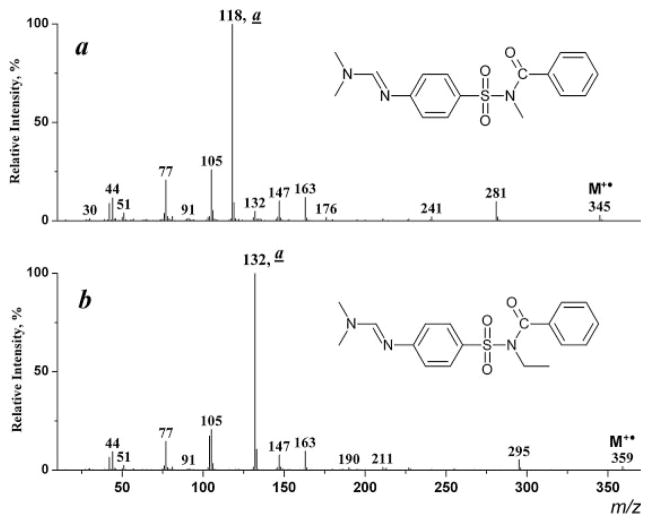
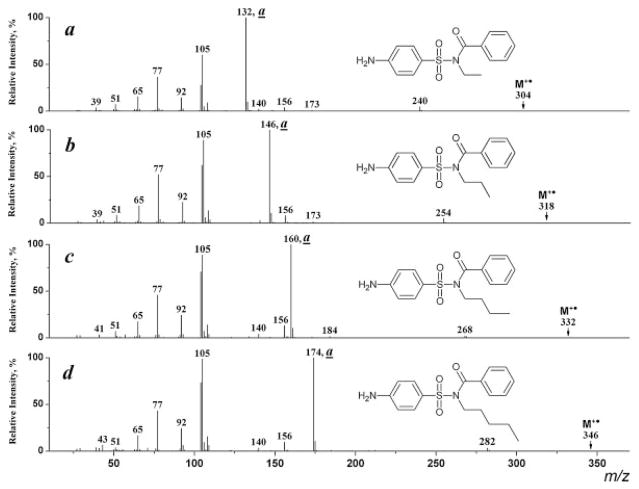
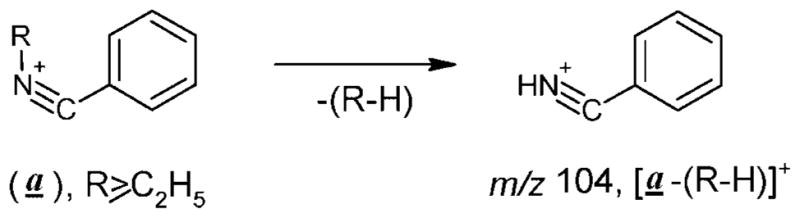
The homologous type (a) ions exhibit maximum intensity in the spectra of the derivatization products with higher N-alkyl groups (I–IV, R =C2H5, C3H7, C4H9, C5H11; Figs. 3(b) and 4). As expected[6] these ions further eliminate olefin molecules (R-H) giving rise to an ion of m/z 104 (Scheme 6).
Figure 3.
EI mass spectra of N-methyl- (a) and N-ethyl-(4-dimethylaminomethylenaminophenylsulfonyl)benzamides (b).
Figure 4.
EI mass spectra of N-ethyl- (a), N-propyl- (b), N-butyl- (c), and N-pentyl-N-(4-aminophenylsulfonyl)benzamides (d).
Scheme 6.
A competing reaction leading to formation of the benzoyl cation (m/z 105) from the benzamide moiety becomes significant for compounds with an alkyl group larger than CH3 at the amide N-atom (Figs. 3(b) and 4); the ions characterizing the arylsufonyl part of the molecule are also present: [NH2C6H4]+ (m/z 92), [NH2C6H4SO]+ (m/z 140), [NH2C6H4SO2]+ (m/z 156) and [NH2C6H4O]+ (m/z 108). The latter ion is the product of a rearrangement process similar to that given in Scheme 3. However, this rearrangement proceeds without a hydrogen migration since the amide hydrogen atom is substituted by an alkyl group.
CONCLUSIONS
The possibility of obtaining stable and convenient derivatives of sulfabenzamide for GC/MS analysis has been studied. A skeletal rearrangement of alkylated sulfabenzamides leading to the formation of N-alkylphenylcyanide cations via the intermediate formation of distonic molecular ions is reported. A loss of a neutral R2NC6H4SO3 fragment can be used for screening/identification and for quantification of modified sulfabenzamides.
Footnotes
This article is a U.S. Government work and is in the public domain in the U.S.A.
Disclaimer
Certain commercial materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the identified materials are necessarily the best available for the purpose.
References
- 1.NIST/NIH/EPA Mass Spectral Library, Standard Reference Database 1, NIST 08. Standard Reference Data Program, National Institute of Standards and Technology; Gaithersburg, MD, USA: [Google Scholar]
- 2.Ausloos P, Clifton CL, Lias SG, Mikaya AI, Stein SE, Tchekhovskoi DV, Sparkman OD, Zaikin V, Zhu D. J Am Soc Mass Spectrom. 1999;10:287. doi: 10.1016/S1044-0305(98)00159-7. [DOI] [PubMed] [Google Scholar]
- 3.Zaikin V, Halket J. A Handbook of Derivatives for Mass Spectrometry. IM Publications; Chichester: 2009. p. 517. [Google Scholar]
- 4.Budzikiewicz H, Djerassi C, Williams DH. Mass Spectrometry of Organic Compounds. Holden-Day Inc; San Francisco: 1967. p. 690. [Google Scholar]
- 5.MacLafferty FW, Turecek F. Interpretation of Mass Spectra. University Science Books; Mill Valley: 1993. p. 371. [Google Scholar]
- 6.Wulfson NS, Zaikin VG, Mikaia AI. Mass Spectrometry of Organic Compounds. Khimia; Moscow: 1986. p. 312. [Google Scholar]