Abstract
Recently introduced microincisional glaucoma surgeries that enhance conventional outflow offer a favorable risk profile over traditional surgeries, but can be unpredictable. Two paramount challenges are the lack of an adequate training model for angle surgeries and the absence of an intraoperative quantification of surgical success. To address both, we developed an ex vivo training system and a differential, quantitative canalography method that uses slope-adjusted fluorescence intensities of two different chromophores to avoid quenching. We assessed outflow enhancement by trabecular micro-bypass (TMB) implantation or by ab interno trabeculectomy (AIT). In this porcine model, TMB resulted in an insignificant (p > 0.05) outflow increase of 13 ± 5%, 14 ± 8%, 9 ± 3%, and 24 ± 9% in the inferonasal, superonasal, superotemporal, and inferotemporal quadrant, respectively. AIT caused a 100 ± 50% (p = 0.002), 75 ± 28% (p = 0.002), 19 ± 8%, and 40 ± 21% increase in those quadrants. The direct gonioscopy and tactile feedback provided a surgical experience that was very similar to that in human patients. Despite the more narrow and discontinuous circumferential drainage elements in the pig with potential for underperformance or partial stent obstruction, unequivocal patterns of focal outflow enhancement by TMB were seen in this training model. AIT achieved extensive access to outflow pathways beyond the surgical site itself.
Advances in glaucoma surgery device engineering in the sub-millimeter range and improved surgical techniques have caused a rapid evolution of microincisional glaucoma surgeries (MIGS)1. Compared to traditional trabeculectomies and tube shunts2, these procedures are significantly faster and have a favorable risk profile1,3. This also allows combining procedures3 even in complex scenarios4,5. The procedures described here, a trabecular meshwork micro-bypass stent (TMB, iStent® G1, Glaukos Corporation, Laguna Hills, CA, USA) and trabectome-mediated ab interno trabeculectomy (AIT, trabectome, Neomedix, Tustin, California, USA) are the only MIGS approved by the Food and Drug Administration of the United States (USFDA). The TMB is a heparin-coated titanium stent measuring 1 × 0.3 mm that is inserted through the trabecular meshwork (TM) into Schlemm’s canal6. AIT is a plasma surgery ablation technique that uses a 550 kHz bipolar electrode tip to remove the TM1. Ablation is performed over 90 to 180 degrees, allowing to tap into several drainage segments in comparison to single-access MIGS procedures7.
The main shortcoming of both procedures is that they are relatively difficult to master and that surgical success of bypassing or removing the TM depends on a functioning downstream collector channel system. There is currently no method to choose the surgical site based on where reduced flow areas are. Similarly, there is no way to precisely locate where the remaining outflow resistance resides in patients where outflow enhancement fails3 despite an otherwise correct surgical technique that should have eliminated the primary resistance of the TM8,9. Fellman et al. described that upon careful observation a successful TM ablation correlates with change of the episcleral fluid wave and outcomes of AIT10.
Past studies have quantified the bulk outflow of aqueous humor11 or modeled focal outflow mathematically12. Tracers, such as cationized ferritin13 and fluorescent beads14,15, allow to highlight areas of high flow through the TM, but either have cytotoxic effects16 or do not easily permit the examination of elements downstream of the TM.
The purpose of this study was to develop a well calibrated, quantitative, differential canalography technique to assess conventional outflow enhancement. We hypothesized that this method could highlight outflow enhancement obtained by TMB and AIT. We developed a MIGS training model using enucleated pig eyes.
Results
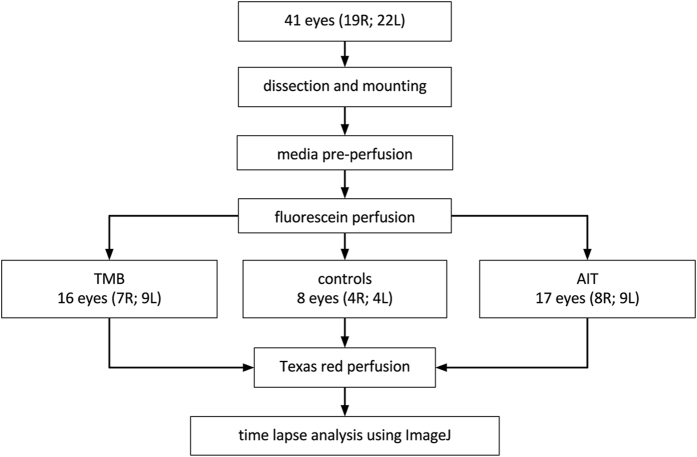
Canalograms could be obtained in 41 out of 42 eyes (Fig. 1). Collector channels of the outflow network could be readily visualized using a new two-dye reperfusion technique. The initial filling times for fluorescein (FU) and Texas red (TR) were measured in pilot eyes to determine the proper dye sequence. These times were not significantly different (p = 0.06, n = 12). A normalization coefficient corrected TR values to match FU at select time points, thereby allowing the comparison of flow rates before and after each intervention. FU demonstrated an average increase of 56 ± 8% fluorescence units compared to TR over 4 quadrants (Supplementary Fig. S1). Eight eyes were used here to achieve >80% power (α = 0.05, two-tailed). Significant differences existed in the inferonasal (IN; p = 0.028), superonasal (SN; p = 0.048), and superotemporal (ST) quadrants (p = 0.040). The chromophore fluorescence intensities in the perilimbal region graphed over 15 minutes for each dye showed relatively linear increases in intensity over time as the dyes crossed the TM and the downstream outflow tract (Fig. 2A). The intensity slopes of TR and FU were characteristic for each dye. TR had a lower peak intensity than FU. Dyes did not exhibit chromophore quenching at the concentrations used in our experiments (Fig. 2B).
Figure 1. Group assignment.
Flowchart detailing assignment to controls, trabecular micro-bypass (TMB) implantation or trabectome-mediated ab interno trabeculectomy (AIT).
Figure 2. Fluorescein and Texas red fluorescence intensity and concentrations.
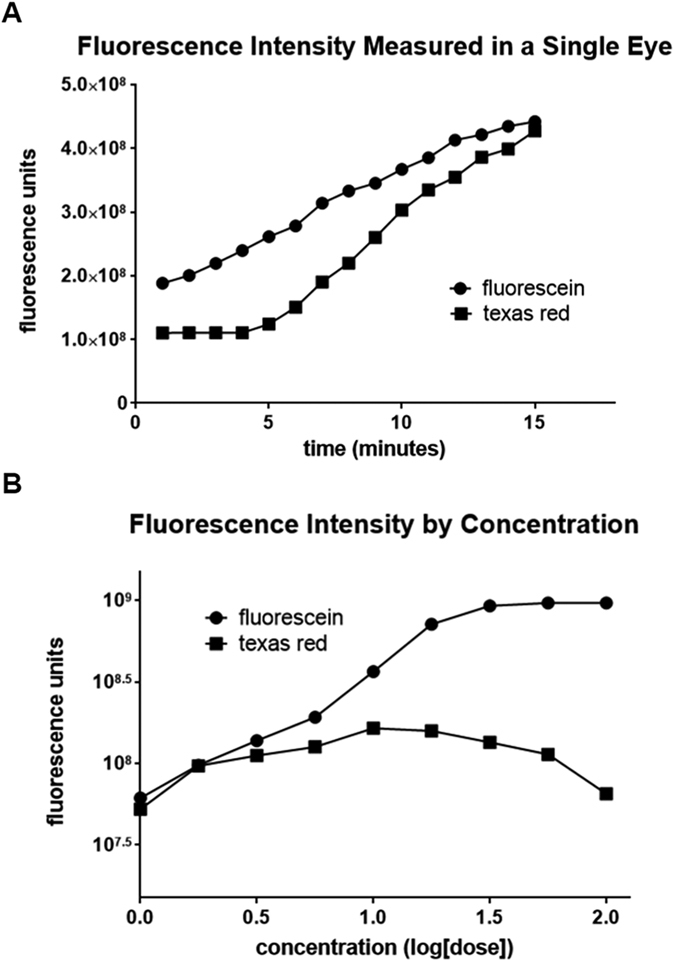
(A) Texas red demonstrated a steeper increase of fluorescence intensity than fluoroscein. (B) Testing of logarithmic concentrations of fluorescein and Texas red indicated that the concentrations of dyes used for the experiments (concentration = 1.0) did not fall under the range of dynamic quenching.
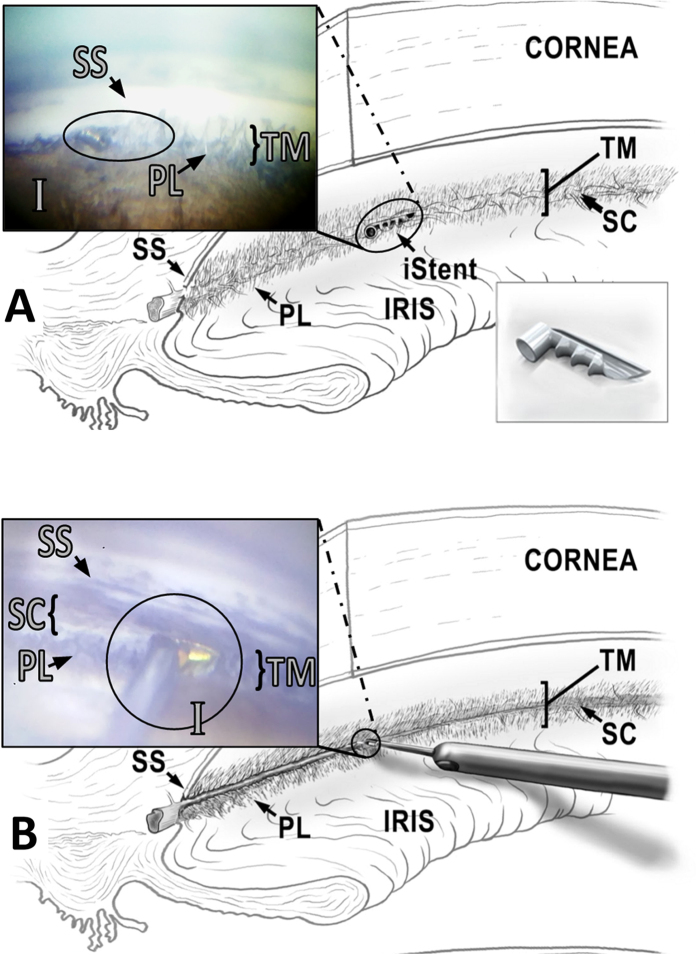
The angle of porcine eyes could be readily visualized by gonioscopy as done in human patients (Fig. 3). TMB implantation proceeded under gonioscopic view using the standard inserter but without viscoelastic (Fig. 3A). AIT could be performed in similar fashion and with tactile feedback that matches human eyes (Fig. 3B).
Figure 3. Microincisional glaucoma surgeries in pig eyes.
(A) Intraoperative view of trabecular micro-bypass (iStent, oval) inserted through the trabecular meshwork and seated within the porcine angular aqueous plexus (distal end) with the snorkel tip parallel to the iris plane (proximal end). (B) Intraoperative view of ab interno trabeculectomy (Trabectome, circle). The footplate is visible immediately before trabecular meshwork engagement on the right of the tip. Meshwork has already been ablated on the left of the tip. (SS: scleral spur, TM: trabecular meshwork, I: iris, PL: pectinate ligaments, SC: Schlemm’s canal).
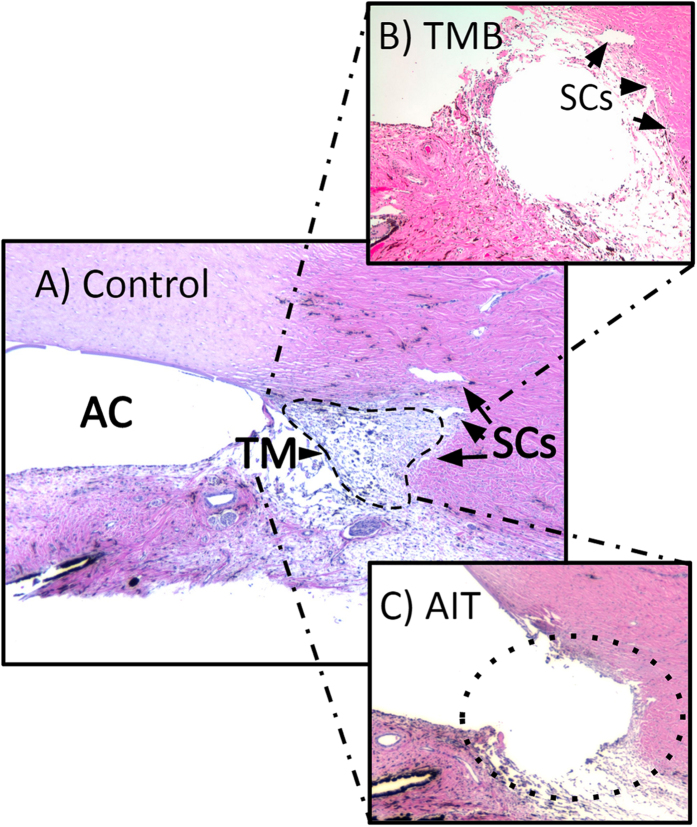
Histology of the angle showed the characteristic pectinate ligaments and the large, wedge-shaped TM of porcine eyes as well as multiple Schlemm’s canal-like structures in variable locations (Fig. 4A). TMB implantation presented as a single lumen that bypassed and displaced the TM and created connections Schlemm’s canal-like structures (Fig. 4B). In contrast, AIT caused a near complete removal of the trabecular meshwork and direct connection to Schlemm’s canal-like structures (Fig. 4C). Thermal, coagulative damage to surrounding structures was mostly absent.
Figure 4. Comparison of sagittal sections of anterior chamber angle.
(A) Control eye showing anterior chamber with trabecular meshwork (TM) and two Schlemm’s canal-like structures (SC). (B) Trabecular micro-bypass (TMB) implantation site is seen as a void. (C) Ab interno trabeculectomy (AIT) results in ablation of TM (dotted line).
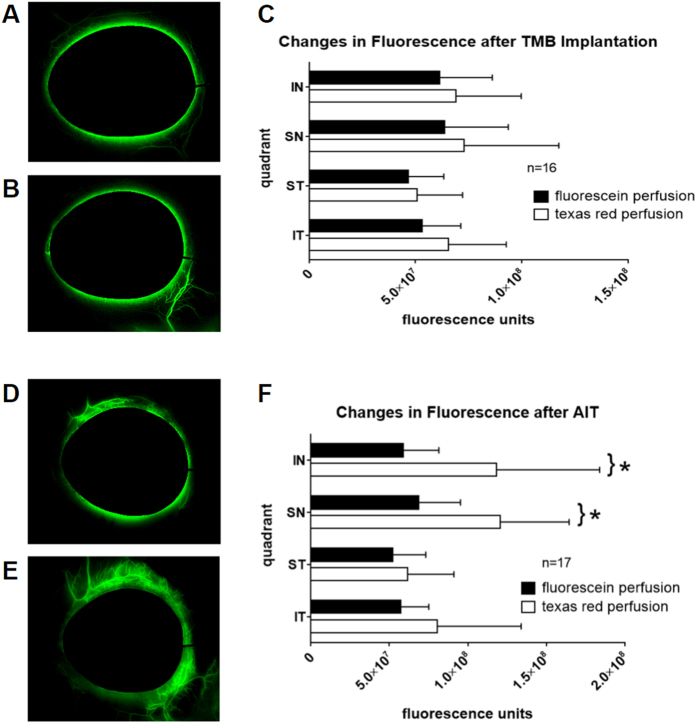
Time lapses of differential canalograms revealed two different outflow patterns. TMB implantation (Fig. 5A) showed a more focal outflow pattern that extended primarily radially from the location of insertion. The stented quadrant was also usually the first quadrant to show dye filling. AIT eyes (Fig. 5D) had increased flow most commonly beginning in the IN or SN, which then extended circumferentially toward centrifugal collector channels.
Figure 5. Canalograms and fluorescence intensities after TMB and AIT.
Matching canalogram time points before (A) and after TMB (B), which often resulted in a confined, focal outflow enhancement near the implantation site. (C) Average fluorescence increases of 9–24% by quadrant (p > 0.05; IN: inferonasal, SN: superonasal, ST: superotemporal, IT: inferotemporal). Canalograms before (D) and after AIT (E), with extensive outflow enhancement beyond ablation ends. (F) Average fluorescence increases of 19–100% by quadrant following nasal AIT (*p < 0.05).
Comparison of canalograms before (Fig. 5A) and after TMB implantation (Fig. 5B) in 16 eyes resulted in a statistically insignificant increase of fluorescence averages by 13 ± 5%, 14 ± 8%, 9 ± 3%, and 24 ± 9% in the IN (p = 0.25), SN (p = 0.44), ST (p = 0.51), and IT (p = 0.06) quadrants, respectively (Fig. 5C). Outflow rates in these respective quadrants was calculated to be equivalent to 0.82, 0.85, 0.62, and 0.71 microliters per minute before and 0.92, 0.97, 0.68, and 0.88 microliters per minute after that procedure. AIT in 17 eyes (Fig. 5D,E) caused fluorescence intensity increases of 100 ± 50% (p = 0.0016), 75 ± 28% (p = 0.0017), 19 ± 8% (p = 0.32), and 40 ± 21% (p = 0.13) in the IN, SN, ST, and IT quadrants (Fig. 5F), respectively. Corresponding outflow rates in these quadrants increased from 0.75, 0.87, 0.66, and 0.73 to 1.49, 1.52, 0.78, and 1.02 microliters per minute, respectively.
Discussion
The trabecular meshwork, a complex sieve-like tissue that permits fluid passage by giant vacuoles, variable pores, and transcytosis17, has long been considered to be the principal cause of decreased outflow in primary open angle glaucoma9 with most of the resistance thought to be residing in the juxtacanalicular tissue or the inner wall of Schlemm’s canal18,19. However, more recent experimental20 and clinical21 evidence suggests that a large portion of this resistance is located further downstream. Disruption22 or ablation of TM4,21 would be expected to achieve an intraocular pressure close to that of episcleral venous pressure around 8 mmHg23 but this is rarely the case3 and failure rates vary considerably from study to study1,3,21,24.
Although the procedures discussed here are considered minimally invasive, they are difficult to learn because they are performed on a scale that is approximately 200-fold smaller than that of traditional glaucoma surgery. Maintaining visualization during these procedures is difficult as they require concurrent movement of a surgical goniolens in one hand and MIGS applicators or ablation hand pieces in the other. Because the target tissue, the trabecular meshwork, is in very close proximity to highly vulnerable and well-vascularized structures such as the ciliary body band and iris root, novice surgeons can produce serious complications without adequate practice. To address the absence of a microincisional glaucoma surgery model, we created an ex vivo porcine eye system25 that can also be used to quantify how much outflow improvement was achieved by the trainee using a dye infusion technique25,26. We found that pig eyes provide a highly realistic and inexpensive practice environment that can serve to hone skills before first surgeries in patients. In our experience, human donor eyes rejected for use in corneal transplantation do not provide sufficient corneal clarity for learning or mastering angle surgery, and are too expensive to allow large numbers. This training model is a powerful preparation tool and does not have to be limited to the procedures discussed here but can also help to master scaffold devices27, ab interno sub-Tenon stents28, or suprachoroidal shunts29, as we can confirm.
Porcine eyes are well suited for this model because they share many features that are similar to human eyes but are lacking in other non-primate eyes30: overall size and shape are comparable; they have a large, wedge-shaped TM that allows TMB and AIT to be performed under the required, direct gonioscopic visualization1; possess circumferential drainage segments within the angular aqueous plexus31 that are considered analogous to Schlemm’s canal30,32,33. A quantitative morphometric analysis of the human versus the porcine outflow tract highlighted the anatomic similarities: human eyes have a Schlemm’s canal with depths varying from 10–25 microns and widths of 200 to 400 microns, while the angular aqueous plexus segments of porcine eyes measure 5 to 30 microns deep and 15–150 microns wide30. As we recently described, the Schlemm’s canal like segments in the pig are typically functionally connected and allow circumferential flow25. The porcine outflow34,35 tract displays biochemical glaucoma markers32 and giant vacuoles36 as seen in human eyes.
In order to provide a technique to compare local outflow enhancement from TMB and AIT, we developed a two dye perfusion technique that allows to compare pre- and postsurgical function. The trabecular meshwork and the outer wall of Schlemm’s canal are impermeable to many larger molecules or particles but can be easily passed by water-soluble fluorescent dyes. We selected the organic fluorophores TR and FU because they are readily available and have a very favorable toxicity profile37. Spectral domain optical coherence tomography has also recently been used to visualize the aqueous spaces of Schlemm’s canal, the collector channels38, and the intrascleral venous network15 yet this method does not allow to determine actual flow. Gold nanorods can be used as a Doppler contrast agent to estimate flow16 but this method is limited by inflammation39.
We had to use a second dye that is different from the first one in postsurgical canalograms because molecules that can flow through the TM may also eventually diffuse into the interstitial space after some time and wash out incompletely. To ensure a valid comparison of pre- and postprocedural canalograms, we thoroughly tested both dyes to account for their characteristic properties. A 19% delay in TR initial filling time in pilot experiments was sufficient evidence for using FU followed by TR for all of the eyes. Choosing this order avoided false positive flow enhancement after the surgical procedures. TR has a molecular weight of 625 g·mol−1, almost twice of the molecular weight of FU (332 g·mol−1). The two dyes revealed different baseline fluorescence intensities and slope magnitudes, thus resulting in variations in fluorescent intensities at similar time points within both time lapses. Although the relationship between dye concentration and fluorescence intensity is initially linear40, very high concentrations of these dyes can result in a decrease of fluorescence intensity as a result of dynamic quenching, an effect described by the Stern-Volmer equation in which excited chromophore molecules will interact with each other and lose energy through processes other than fluorescent emission41. Our testing of logarithmic concentrations of these dyes and measuring their emitted fluorescence through ImageJ ensured that the concentrations used for FU and TR here did not exhibit dynamic quenching. A normalization coefficient corrected TR values to match FU thereby allowing a direct comparison of flow rates before and after each intervention42,43.
The purpose of this study was to developed an ex vivo training system that allows new surgeons to practice in a safe and highly realistic environment. We also wanted to create a differential, quantitative canalography method that uses slope-adjusted fluorescence intensities (to avoid quenching seen at high intensities) of two different chromophores to better highlight outflow enhancements obtained by TMB and AIT. Although outflow patterns of the two MIGS modalities examined here are different, it is important to recall that circumferential flow is more restricted in this pig eye model due to segmentation of the perilimbal outflow tract and that these results do not represent the performance in human eyes. The TMB is designed for insertion into a single lumen human Schlemm’s canal while in the pig placements may be more commonly imperfect and only partially into Schlemm’s canal like segments. Another limitation concerns the size of the aqueous plexus segments themselves. These segments can have a smaller width than the lumen of a singular human Schlemm’s canal30. It is possible that the intracanalicular portion of the TMB stents may at least be partially obstructed by tissue between segments of the aqueous plexus or the outer wall itself, a challenge that can be observed in histological sections of human eyes as well1. Similarly, although TMBs were implanted under direct visualization with confirmed placement, small, unintended variations in technique may impact placement and performance in this porcine model more than in human eyes. Therefore, conclusions about the clinical performance of either TMB or AIT in human eyes based on the results presented here cannot be extrapolated.
The amount of circumferential flow likely correlates with the number of drainage segments that a procedure can access effectively. A single point of access to the outflow tract, as delivered by TMB, is thought to enable flow over approximately 60 degrees in human eyes7 and might be considerably less here. In contrast, AIT can ablate TM over up to 180 degrees in experienced hands thereby providing access to 180 plus 60 degrees, totaling to 240 degrees of outflow segments1. We limited ablation in this study to 90 degrees of the nasal angle because this amount is achievable by most surgeons with ease. It is important to keep in mind that despite differences in amount of angle access and high versus low immediately achieved outflow enhancement, little is known about long-term changes of the outflow system or wound healing and foreign body reaction to microimplants.
In conclusion, we present an ex vivo training model for microincisional glaucoma surgery in pig eyes. We introduce a new differential canalogram technique that controls for dynamic quenching of two different chromophores, and allows for quantification of outflow enhancement after trabectome-mediated ab interno trabeculectomy and trabecular micro-bypass in this species.
Methods
Preparation and Pre-Perfusion of the Eyes
Correctly paired, enucleated porcine eyes from a local abattoir were processed within two hours of sacrifice. Each eye was identified as left or right and copiously irrigated with phosphate buffered saline (PBS, Thermo Fisher Scientific, Waltham, MA). Extraocular tissues were trimmed to the length of the globe. Eyes were placed on cryogenic vial cups (CryoElite Cryogenic Vial #W985100, Wheaton Science Products, Millville, NJ) to encompass the optic nerve in a compression-free mount. A 30-gauge needle was inserted through the nasal cornea approximately 2 mm anterior to the limbus parallel to the iris and advanced to the center of the anterior chamber with the bevel up. Eyes were gravity perfused with 37 °C clear Dulbecco’s Modified Eagle’s Media (DMEM, Hyclone, GE Healthcare Life Sciences, Piscataway, NJ, USA) for 15 minutes with the pressure set to the regular porcine intraocular pressure of 15 mmHg30 as used in anterior chamber perfusion systems42,43,44.
Chromophore Analysis
When incomplete dye washout was observed in single dye reperfusion pilot experiments, two chromophores with different peak excitation wavelengths (fluorescein (FU): 494 nm, Texas red (TR): 589 nm) were used. The proper detection sensitivities for those were determined using a hemocytometer chamber filled with 10 μL of each dye in clear DMEM at concentrations of 0.0332 mg/mL and 0.28 mg/mL, respectively. Identical average gray values on a 14 bit image were obtained at exposures of 15 and 10 milliseconds, respectively.
Six eyes were first perfused with FU (AK-FLUOR 10%, Fluorescein injection, USP, 100 mg/ml, NDC 17478-253-10, Akorn, Lake Forest, IL) followed by TR (sulforhodamine 101 acid chloride, 10 mg, Alfa Aesar, Ward Hill, MA) to establish whether the order of the dyes would affect the perfusion rate or the intensity of fluorescence; another six eyes underwent the reverse order. Initial filling time, recorded as the time at which the dyes could first be observed entering the proximal outflow tract structures (the perilimbal regions), were recorded for each eye quadrant using ImageJ (ImageJ 1.50b, http://imagej.nih.gov/ij, Wayne Rasband, National Institutes of Health, Bethesda, MD). Eight further control eyes (4 left, 4 right) were run with FU and TR with no intervention in between. After FU and TR time lapse analyses were performed at each eye’s respective half-maximum FU intensity frame, fluorescence units were compared in all four quadrants.
Next, FU and TR were sequentially perfused in a single eye with time lapses (CellSens, Olympus Life Science, Tokyo, Japan) taken as described below. Raw fluorescent intensities of the perilimbal flow patterns were collected every minute for a total of 15 minutes for each dye. When the canalograms of the control eyes revealed consistency between the two chromophores, a normalization coefficient (c = 1.56) was computed to adjust TR to match FU at a single relative time point in each time lapse pair, allowing for a comparison of flow rates before and after each intervention.
We then determined the fluorescent intensities for FU and TR in each eye at the same relative time points of half-maximum fluorescence of FU as measured in ImageJ. This kept the time factor constant and allowed to compare outflow rates per quadrant in microliters per minute. For this computation, the aqueous humor flow rate of three microliters per minute of porcine eyes was divided by the relative fluorescence of each quadrant measured in control eyes and used as the baseline to compare postsurgical flow.
Differential Canalograms
The fluorescent tracer reperfusion technique was used in 41 eyes to quantify outflow changes from TMB implantation or AIT. Whole pig eyes were prepared, mounted, and pre-perfused with DMEM as described above. Fluorescein was then gravity-infused at a concentration of 0.0332 mg/ml in clear DMEM for 15 minutes. The chromophore flow pattern was recorded as a time lapse using a stereo dissecting microscope (Olympus SZX16 and DP80 Monochrome/Color Camera; Olympus Corp., Center Valley, PA) equipped for fluorescent imaging (Chroma 49002 GFP cube and Chroma 49004 DSRED cube, Chroma Technology Corp, Bellow Falls, VT). En face images were obtained every 20 seconds at 580 × 610 pixel resolution with 2 × 2 binning and 14 bit depth (CellSens, Olympus Life Science, Tokyo, Japan). FU infusion was then stopped and the needle removed. A surgeon experienced in microincisional glaucoma surgery (NAL) performed all AITs and TMB implantations as described below (Fig. 3). The temporal, clear corneal incision was sealed in a watertight fashion using cyanoacrylate. Clear DMEM containing TR at a concentration of 0.28 mg/ml was infused for 15 minutes with a time lapse recorded. At conclusion, the eyes were processed for histology. TMB eyes were marked at the site of implantation and the stent was removed. All eyes were rinsed in PBS, hemisected, and fixed with 4% paraformaldehyde at room temperature followed by PBS for 48 hours before being placed in 70% ethanol. A corneoscleral wedge from the surgical site was paraffin-embedded for histological processing, cut at 10 μm thickness, and stained with hematoxylin and eosin (H&E).
Trabecular Meshwork Bypass Implantation Technique
Sixteen pig eyes underwent TMB implantation (iStent, Glaukos Corporation, Laguna Hills, CA, USA). The surgical technique was analogous to that used in human eyes45. With the temporal side of the eye facing the surgeon, a clear corneal incision was created 2 mm anterior to the temporal limbus with a 1.8 mm keratome. The loaded TMB applicator was inserted into the anterior chamber and advanced toward the TM. The stent was driven through the TM using a gentle sweeping motion. Only the proximal end of the stent remained visible in the anterior chamber (Fig. 3A). The stent was ejected from the applicator and the applicator tip was removed. A drop of cyanoacrylate was used to seal the temporal, clear corneal incision.
Ab Interno Trabeculectomy Technique
After infusion with FU, 17 eyes underwent AIT, performed analogous to AIT in human eyes1. Eyes were positioned under a surgical microscope with the temporal side directed toward the surgeon. A 1.8 mm keratome was used to create a clear corneal incision 2 mm anterior to the temporal limbus. The inner third was slightly flared to improve mobility and eliminate striae from torque. The eyes were then tilted by 30 degrees toward the nasal side and a goniolens (Goniolens ONT-L, #600010, NeoMedix Inc., Tustin, CA) was placed on the cornea to visualize the nasal chamber angle. The tip of the trabectome handpiece was inserted into the anterior chamber with constant irrigation, and gentle goniosynechiolysis with the smooth base plate was performed to disinsert pectinate ligaments (Fig. 3B). The TM was engaged and Schlemm’s canal entered with a left and upward movement. TM ablation at 1.1 mW ensued toward the left for 45 degrees with appropriate rotation of the goniolens to maintain visualization. The tip was then disengaged from the TM, rotated 180 degrees within the eye, and positioned at the original starting location. A 45 degree ablation was performed towards the right. The handpiece was removed, and the temporal, clear corneal incision closed watertight with a drop of cyanoacrylate.
Time Lapse Analysis
Individual time lapses with FU and TR were analyzed using ImageJ software46,47. For each fluorescein time lapse, the half-maximum perilimbal fluorescence was calculated, and the appropriate frame containing perilimbal fluorescence that best matched that value was selected for analysis. The same frame number was used in the corresponding Texas Red time lapse for each eye. A masked observer measured raw fluorescence intensities from these frames by outlining the quadrants containing fluorescent outflow channels. Each pig eye was divided into four quadrants: inferonasal (IN), superonasal (SN), superotemporal (ST), and inferotemporal (IT). Quadrant outlines began at the limbus of each quadrant to exclude quantification of fluorescence of the dye in the anterior chamber.
Statistics
Student’s paired two sample t-test was used to compare outflow changes in the same eyes before and after each intervention. The unpaired t-test was utilized to detect any significant differences between right and left eyes. A sample size of at least seven eyes was calculated as the minimum number of control eyes needed to detect a significant difference between the two-dye reperfusion technique with 80% statistical power and an alpha error of 0.05 on a two-tailed matched pair comparison. Similarly, pilot data in the experimental groups revealed a sample size of at least 16 eyes in each group to reliably detect significant changes before and after each intervention using the same test parameters for power and alpha error.
Additional Information
How to cite this article: Parikh, H. A. et al. Differential Canalograms Detect Outflow Changes from Trabecular Micro-Bypass Stents and Ab Interno Trabeculectomy. Sci. Rep. 6, 34705; doi: 10.1038/srep34705 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Katherine Davoli (Core Grant for Vision Research EY08098) for providing histology. This work was supported by grants from the National Institutes of Health (K08EY022737), Eye and Ear Foundation of Pittsburgh, American Glaucoma Society, Research to Prevent Blindness, and the Alpha Omega Alpha Carolyn L Kuckein Student Research Fellowship.
Footnotes
N.A.L. has received honoraria as a Trabectome trainer for NeoMedix Corporation. iStents were a gift from Glaukos Corporation. Neither NeoMedix nor Glaukos Corporation had any role in the design or conduct of this research.
Author Contributions N.A.L. conceived and supervised the study and provided the materials. H.A.P., R.T.L. and P.R. conducted the experiments. H.A.P. analyzed the results. H.A.P. and N.A.L. wrote the main manuscript text. K.L.L. assisted with experimental design and prepared Figure 2. J.S.S. advised on experimental design and critically reviewed the manuscript. R.T.L. greatly contributed to Figure 4. All authors reviewed the manuscript.
References
- Kaplowitz K., Schuman J. S. & Loewen N. A. Techniques and outcomes of minimally invasive trabecular ablation and bypass surgery. Br. J. Ophthalmol. 98, 579–585 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedde S. J. et al. In Am J Ophthalmol 153, 804–814.e1 (2012 Elsevier Inc, 2012).22244522 [Google Scholar]
- Kaplowitz K., Bussel I. I., Honkanen R., Schuman J. S. & Loewen N. A. Review and meta-analysis of ab-interno trabeculectomy outcomes. Br. J. Ophthalmol. 10.1136/bjophthalmol-2015-307131 (2016). [DOI] [PubMed] [Google Scholar]
- Bussel I. I., Kaplowitz K., Schuman J. S. & Loewen N. A. & Trabectome Study Group. Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br. J. Ophthalmol. 99, 914–919 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussel I. I. et al. Outcomes of ab interno trabeculectomy with the trabectome after failed trabeculectomy. Br. J. Ophthalmol. 99, 258–262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson T. W. et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology 118, 459–467 (2011). [DOI] [PubMed] [Google Scholar]
- Rosenquist R., Epstein D., Melamed S., Johnson M. & Grant W. M. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr. Eye Res. 8, 1233–1240 (1989). [DOI] [PubMed] [Google Scholar]
- Bill A. & Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm–an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol. 50, 295–320 (1972). [DOI] [PubMed] [Google Scholar]
- Mäepea O. & Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp. Eye Res. 54, 879–883 (1992). [DOI] [PubMed] [Google Scholar]
- Fellman R. L., Feuer W. J. & Grover D. S. Episcleral Venous Fluid Wave Correlates with Trabectome Outcomes: Intraoperative Evaluation of the Trabecular Outflow Pathway. Ophthalmology 122, 2385–2391.e1 (2015). [DOI] [PubMed] [Google Scholar]
- Nau C. B., Malihi M., McLaren J. W., Hodge D. O. & Sit A. J. Circadian variation of aqueous humor dynamics in older healthy adults. Invest. Ophthalmol. Vis. Sci. 54, 7623–7629 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F. et al. Mathematical Modeling of Outflow Facility Increase With Trabecular Meshwork Bypass and Schlemm Canal Dilation. J. Glaucoma. 10.1097/IJG.0000000000000248 (2015). [DOI] [PubMed] [Google Scholar]
- de Kater A. W., Melamed S. & Epstein D. L. Patterns of aqueous humor outflow in glaucomatous and nonglaucomatous human eyes. A tracer study using cationized ferritin. Arch. Ophthalmol. 107, 572–576 (1989). [DOI] [PubMed] [Google Scholar]
- Hann C. R. & Fautsch M. P. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest. Ophthalmol. Vis. Sci. 50, 1692–1697 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagemann L. et al. 3D visualization of aqueous humor outflow structures in-situ in humans. Exp. Eye Res. 93, 308–315 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. et al. Gold nanorods as a contrast agent for Doppler optical coherence tomography. PLoS One 9, e90690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm E. R. The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res. 88, 648–655 (2009). [DOI] [PubMed] [Google Scholar]
- Overby D. R., Stamer W. D. & Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp. Eye Res. 88, 656–670 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. M. Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthalmol. 69, 783–801 (1963). [DOI] [PubMed] [Google Scholar]
- Schuman J. S., Chang W., Wang N., de Kater A. W. & Allingham R. R. Excimer laser effects on outflow facility and outflow pathway morphology. Invest. Ophthalmol. Vis. Sci. 40, 1676–1680 (1999). [PubMed] [Google Scholar]
- Parikh H. A., Bussel I. I., Schuman J. S., Brown E. N. & Loewen N. A. Coarsened Exact Matching of Phaco-Trabectome to Trabectome in Phakic Patients: Lack of Additional Pressure Reduction from Phacoemulsification. PLoS One 11, e0149384 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover D. S. et al. Gonioscopy-Assisted Transluminal Trabeculotomy, Ab Interno Trabeculotomy. Ophthalmology 121, 855–861 (2014). [DOI] [PubMed] [Google Scholar]
- Sultan M. & Blondeau P. Episcleral venous pressure in younger and older subjects in the sitting and supine positions. J. Glaucoma 12, 370–373 (2003). [DOI] [PubMed] [Google Scholar]
- Jea S. Y., Francis B. A., Vakili G., Filippopoulos T. & Rhee D. J. Ab interno trabeculectomy versus trabeculectomy for open-angle glaucoma. Ophthalmology 119, 36–42 (2012). [DOI] [PubMed] [Google Scholar]
- Loewen R. T. et al. Quantification of Focal Outflow Enhancement Using Differential Canalograms. Invest. Ophthalmol. Vis. Sci. 57, 2831–2838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen R. T. et al. Regionally Discrete Aqueous Humor Outflow Quantification Using Fluorescein Canalograms. PLoS One 11, e0151754 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone M. A. et al. Effects of a Schlemm canal scaffold on collector channel ostia in human anterior segments. Exp. Eye Res. 119, 70–76 (2014). [DOI] [PubMed] [Google Scholar]
- Lewis R. A. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J. Cataract Refract. Surg. 40, 1301–1306 (2014). [DOI] [PubMed] [Google Scholar]
- Hoeh H. et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J. Cataract Refract. Surg. 39, 431–437 (2013). [DOI] [PubMed] [Google Scholar]
- McMenamin P. G. & Steptoe R. J. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J. Anat. 178, 65–77 (1991). [PMC free article] [PubMed] [Google Scholar]
- Tripathi R. C. Ultrastructure of the exit pathway of the aqueous in lower mammals: (A preliminary report on the ‘angular aqueous plexus’). Exp. Eye Res. 12, 311–314 (1971). [DOI] [PubMed] [Google Scholar]
- Suárez T. & Vecino E. Expression of endothelial leukocyte adhesion molecule 1 in the aqueous outflow pathway of porcine eyes with induced glaucoma. Mol. Vis. 12, 1467–1472 (2006). [PubMed] [Google Scholar]
- Tripathi R. C. Ultrastructure of the exit pathway of the aqueous in lower mammals: (A preliminary report on the ‘angular aqueous plexus’). Exp. Eye Res. 12, 311IN23313–312IN28314 (1971). [DOI] [PubMed] [Google Scholar]
- Loewen R. T. et al. A Porcine Anterior Segment Perfusion and Transduction Model With Direct Visualization of the Trabecular Meshwork. Invest. Ophthalmol. Vis. Sci. 57, 1338–1344 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y., Loewen R., Parikh H. A., Roy P. & Loewen N. A. Gene transfer to the outflow tract. Exp. Eye Res. 044396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin P. G. & Steptoe R. J. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J. Anat. 178, 65–77 (1991). [PMC free article] [PubMed] [Google Scholar]
- Alford R. et al. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol. Imaging 8, 341–354 (2009). [PubMed] [Google Scholar]
- Kagemann L. et al. Visualization of the conventional outflow pathway in the living human eye. Ophthalmology 119, 1563–1568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele Sandrian M. et al. Inflammatory response to intravitreal injection of gold nanorods. Br. J. Ophthalmol. 96, 1522–1529 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbault G. G. Practical fluorescence. 3, (CRC Press, 1990). [Google Scholar]
- Kadadevarmath J. S., Malimath G. H., Melavanki R. M. & Patil N. R. Static and dynamic model fluorescence quenching of laser dye by carbon tetrachloride in binary mixtures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 117, 630–634 (2014). [DOI] [PubMed] [Google Scholar]
- Bahler C. K., Smedley G. T., Zhou J. & Johnson D. H. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am. J. Ophthalmol. 138, 988–994 (2004). [DOI] [PubMed] [Google Scholar]
- Chowdhury U. R. et al. ATP-sensitive potassium (KATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest. Ophthalmol. Vis. Sci. 52, 6435–6442 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler C. K., Fautsch M. P., Hann C. R. & Johnson D. H. Factors influencing intraocular pressure in cultured human anterior segments. Invest. Ophthalmol. Vis. Sci. 45, 3137–3143 (2004). [DOI] [PubMed] [Google Scholar]
- Nichamin L. D. G iS Trabecular Micro-Bypass. Middle East Afr. J. Ophthalmol. 16, 138–140 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Rueden C. T., Hiner M. C. & Eliceiri K. W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 82, 518–529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. & Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.