To the editor:
Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs) can be viewed as clonal premalignant disorders that precede development of acute leukemia wherein the order of mutation acquisition, together with environmental insults such as inflammation, matters. MPN disease-defining somatic mutations are in Janus kinase 2 (JAK2), calreticulin (CALR), and myeloproliferative leukemia virus oncogene (MPL; a gene encoding thrombopoietin receptor) genes. Several studies have revealed other somatic mutations affecting intracellular signaling of hematopoietic stem cells in MPNs, but these are considered as phenotype-modifying, not disease-initiating, mutations (reviewed by Tefferi and Pardanani1), such as Tet methylcytosine dioxygenase 2 (TET2) mutations, which may precede or follow acquisition of JAK2V617F.2-4 However, the order of TET2 mutation occurrence alters the transcriptional program in hematopoietic stem cells and patients’ clinical outcome.5
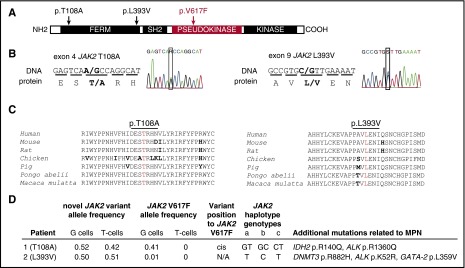
In polycythemia vera (PV), the JAK2V617F mutation is present in the vast majority of patients, yet is not always the disease-initiating mutation and constitutes only part of the clone.6,7 We reported in 2014 the mutational landscape of 31 JAK2V617F-positive PV patients4,8 and identified 2 patients with an acquired polycythemia phenotype carrying JAK2 variants at conserved residues of JAK2: first (T108A) in the 4-point, ezrin, radixin, moesin (FERM) domain, and the other (L393V) in 7 amino acids upstream of the Src homology 2 (SH2) domain (Figure 1A-C). Both mutations were verified by Sanger sequencing in DNA isolated from patients’ granulocytes, and then proven to be heterozygous germ line mutations by their presence in T cells and nail DNA. Protein prediction algorithms (SIFT,9 AGVGD,10 and PolyPhen11) considered both variants as tolerated, benign, and light, respectively, but both variants were predicted to be damaging by MutationTaster12 and possibly damaging by LoFtool. Nevertheless, the functional significance of these germ line JAK2 variants and their putative predisposition to PV are not known.
Figure 1.
Clinical and molecular features of 2 PV patients with germ line JAK2 mutations, T108A and L393V. (A) The schematic structure of the JAK2 domains with depicted locations of T108A and L393V mutations and V617F. (B) Sequencing analysis of germ line JAK2 mutations, causing an amino acid substitutions at codon 108 (threonine to alanine, p.T108A, c.A322G, novel mutation) and codon 393 (leucine to valine, p.L393V, c.C1177G, rs2230723; G allele presented in 1% of the population). (C) Alignment of amino acid sequences of JAK2 residues 93 to 125 and 379 to 407 (human JAK2 nomenclature) from different species shows highly conserved pattern. Conserved residues are colored in black, differences are in bold, and the residues at which the mutation occurs are in red. (D) The mutational screening was determined by whole exome sequencing (details published elsewhere4,8). G cells, granulocytes; T cells, CD3+ T lymphocytes. The chromosomal position of new mutation toward V617F was determined by cDNA cloning, followed by Sanger sequencing. JAK2 GGCC haplotype represents a set of single nucleotide polymorphisms (SNPs) that are associated with a predisposition to MPN.24 JAK2 GGCC haplotype was determined using polymerase chain reaction and TaqMan SNP assay on demand: (a) rs3780367, C_27515396_10, G/T, intron 10; (b) rs10974944, C_31941696_10, G/C, intron 12; (c) rs12343867, C_319416 89_10, C/T, intron 14. Several other acquired mutations that may have contributed to their MPN pathogenesis and clinical course were found in both patients. The allele frequencies of these mutations in patients’ granulocytes were as follows: IDH2 p.R140Q, 0.43; ALK p.R1360Q, 0.14; DNMT3 p.R882H, 0.31; ALK p.K52R, 0.13; GATA-2 p.L359V, 0.19 (sequencing, annotation, and validation details published elsewhere4,8).
The first patient (JAK2T108A) had a JAK2V617F allele burden of 41% in granulocytes. He initially presented 13 months prior to our encounter with hemoglobin 19.4 g%, thrombocytosis of 535 × 109/L, mild leukocytosis (11.3 × 109/L), and recent history of pulmonary embolism. Detailed family history was not available, but there was an undocumented history of increased hematocrit in maternal uncle. Subsequent therapy with hydroxyurea normalized his blood counts. He moved to a different state, and several months later, he developed bone pain, prostration, thrombocytopenia, and transfusion-requiring anemia and died with fulminant blast transformation. The second patient (JAK2L393V) was ethnic Japanese who had a preceding 12-year history of initially isolated thrombocytosis followed within an ensuing year also by erythrocytosis. He had a history of coronary thromboses. He achieved normalization of blood counts by hydroxyurea therapy. At the time of the study, he had very low JAK2V617F allele burden at 1% (confirmed by sensitive quantitative JAK2V617F assay6). A year later, he became anemic, and hydroxyurea therapy was stopped. His marrow showed ∼10% of blasts. In the ensuing 2 years, he developed progressive pancytopenia with increasing blasts; he then refused supporting therapy, at which time he developed leukocytosis and peripheral blood blast count exceeding 75%, refused further supportive therapy, and died. Their other mutations detected at the time of the study that may have contributed to their PV phenotype are depicted in Figure 1D.4
We first analyzed individual burst-forming unit erythroid colonies (BFU-Es) for erythropoietin (EPO)–independent growth (EECs) from the patient with JAK2L393V. All individually genotyped colonies had wild-type (wt) and JAK2L393V alleles in similar proportions, and only 1 EEC (1/16) had an equal proportion of JAK2V617F and wt alleles (heterozygous). Material from the JAK2T108A patient was not available for BFU-E analyses; however, we cloned and sequenced his complementary DNA (cDNA) from his granulocytes and found that JAK2T108A and JAK2V617F were cis. In the patient with the JAK2L393V mutation, a paucity of material and low allelic burden of JAK2V617F precluded determination of a JAK2L393V/V617F configuration.
Unfortunately, in silico modeling of potential interactions of JAK2 germ line variants cannot be obtained because the structure of the JAK2 FERM domain is not yet available, whereas in the structure of the TYK2 FERM domain, these residues are exposed and do not appear to interact with other residues that lack the kinase and pseudokinase domains or with partners of other receptor subunits.13 Interestingly, our JAK2 variants contain precise residues of TYK2 at the 108 (Ala) and 393 (Val) positions, suggesting that, in themselves, they are compatible with functional JAK kinases. TYK2 functions in heteromeric receptor complexes, whereas JAK2 functions in homodimeric receptor complexes, and it is possible that these exposed residues play a role in limiting JAK2 self-activation.
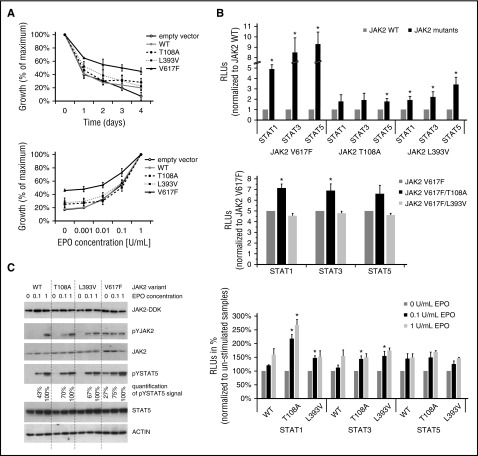
In order to determine the functional consequences of JAK2 germ line mutations on the PV phenotype, we generated stably transfected Ba/F3 cell lines expressing erythropoietin receptor (EPOR) and JAK2 variants (T108A, L393V, V617F, and wt). Cells expressing JAK2V617F were EPO independent, and their viability was significantly increased in comparison with JAK2WT-expressing cells, which did not grow in the absence of EPO and proliferated less at various EPO concentrations. The 2 interrogated JAK2 mutants were dependent on EPO but were hypersensitive (Figure 2A). Response to a JAK2 inhibitor (LY2784544)14 was similar for both germ line and wt JAK2 variants at different concentrations of LY2784544 after 72 hours of exposure (data not shown).
Figure 2.
Functional modeling and characterization of 2 JAK2 germ line mutations, T108A and L393V. The methods are described in the supplemental Methods (available on the Blood Web site). The Ba/F3-EPOR cells (5 × 105 cells per sample) were nucleofected with 2 μg pCMV6-AC-IRES-GFP-Puro “empty” vector or the construct encoding wt or mutated forms of JAK2 kinase (as indicated) using AMAXA II device (program X_001). Transfected cells were selected with 2 μg/mL puromycin for 2 weeks. (A) Proliferation of cells in the absence of EPO was quantified by CellTitre-Blue reagent (Promega, Madison, WI) and Perkin-Elmer Envision analyzer (top). Data are expressed as a percentage of maximum value at starting point (day 0). Results are shown as the mean (± standard deviation [SD]) of 3 independent experiments performed in triplicate. Proliferation of cells in increased concentrations of EPO (0, 0.001, 0.01, 0.1, and 1 U/mL) were quantified by CellTitre-Blue reagent (Promega) and Perkin-Elmer Envision analyzer (bottom). Data are expressed as a percentage of maximum value (EPO 1 U/mL). Results are shown as the mean (± SD) of 3 independent experiments performed in triplicate. (B) Luciferase reporter assay. STAT-dependent transcriptional activity induced by JAK2 variants was measured using firefly luciferase STAT reporter (pGl4.26/GRR4.CZ) in HCT116 cells. Reporter plasmid was constructed with STAT responsive elements,25 when single-stranded oligos were annealed, ligated, and cloned to pGl4.26 reporter backbone. The ratios of constructs in the transfection mixtures (ie, plasmids producing JAK2, EPOR, STATs, the luciferase reporter, and the Renilla control vector) were determined by titration experiments. Luminescence was measured 48 hours after transfection, and Renilla counts were used as internal control (top). Shown are averages ± standard error of the mean (SEM) of 5 independent experiments performed in duplicate. Two-tailed Student t test: *P > .05. JAK2 double mutants were created by site directed mutagenesis of the pCMV6-JAK2V617F-IRES-GFP-Puro expression plasmid (middle). Luminescence was measured 48 hours after transfection, and Renilla counts were used as internal control. Shown are averages ± SEM of 3 independent experiments performed in duplicate. Two-tailed Student t test: *P > .05. Luminescence was measured 24 hours after EPO stimulation (48 hours after transfection), and Renilla counts were used as internal control (bottom). Shown are averages ± SEM of 3 independent experiments performed in duplicate. Two-tailed Student t test: *P > .05. (C) Protein assay. The level of human JAK2 protein in stably transfected Ba/F3-EPOR cell-lines is equal, as indicated by western blot using anti-DDK antibody (Cell Signaling Technology). Ba/F3-EPOR cells expressing different JAK2 wt and mutant variants were cytokine starved for 6 hours and then shortly stimulated. Western blots were performed with rabbit JAK2, pYJAK2 (Tyr 1007/1008), pYSTAT5 (Tyr 694), and STAT5 (Cell Signaling Technology) antibodies, and actin (Sigma-Aldrich, St. Louis, MO) was used as a loading control. JAK2 V617F samples for JAK2/pJAK2 were run on separate gel than the rest of the samples. Signal for pYSTAT5 was quantified using densitometric analysis by ImageJ software.
We evaluated signal transduction of JAK2 mutant and wt kinases, as measured by STAT activation, using dual luciferase assays with STAT responsive elements reporter plasmid. STAT activation was markedly increased without EPO for JAK2V617F and less so, but still more, compared with wt in both germ line variants (P < .05; Figure 2B, top). Using double mutants in the luciferase assay, only cis configuration of T108A/V617F increased the STAT activation above JAK2V617F signal (P < .05; Figure 2B, middle). The L393V mutation does not seem to synergize in augmenting V617F signaling, which is consistent with only 1% V617F allele burden in the patients’ granulocytes. It is possible that JAK2L393V variant synergizes with other acquired mutations with higher allele frequency in this patient (Figure 1D), but without further study, the existence of such cooperation of these mutations should be interpreted with caution. We then stimulated cells with different EPO concentrations (0,1, and 1 U/mL, EPO added 24 hours after transfection) and determined STATs activation. Increased STAT activation by EPO was significant only at low concentrations of EPO (P < .05; Figure 2B, bottom) for both germ line mutants, whereas no further increase of STAT activation was observed in already markedly hyperactive JAK2V617F-transfected cells after addition of EPO (data not shown).
We also investigated JAK/STAT signaling in stably transfected Ba/F3-EPOR cell lines. Cells were cytokine starved for 6 hours and then stimulated with an increased concentration of EPO for 15 minutes. Similar levels of human JAK2 protein in all cell lines were verified by western blot using anti-DDK antibody (Cell Signaling Technology, Danvers, MA). Constitutive activation of the STAT5 pathway was obvious in JAK2V617F-expressing cells in the absence of EPO. We repeatedly observed stronger phosphorylation of JAK2 and STAT5 at low concentrations of EPO in both mutant variants (T108A, L393V) in comparison with JAK2WT cell line (Figure 2C).
Because JAK2 germ line mutations in MPNs were identified at the same time as oncogenic JAK2V617F,15 their impact on JAK2 kinase activity and effect on disease initiation or progression have not been fully evaluated. Several germ line mutations in addition to the somatic JAK2V617F mutation in JAK2 pseudo-kinase domains can induce MPN phenotype (eg, R564Q16 and V625F17) or isolated thrombocytosis (V617I18) and erythrocytosis (E846D and R1063H),19 but our data also suggest that germ line mutations outside the core regulatory domain can alter the EPO-sensing properties of cells and therefore may provide proliferation advantage. Because the JAK2 FERM domain is required for EPOR association and consequent relief of inhibitory conformation of the kinase domain that leads to JAK2 activation,20,21 the molecular basis of this activation remains to be elucidated.
To our knowledge, this is the first description of an association of gain-of-function germ line JAK2 mutations coexisting with JAK2V617F in subjects with PV phenotypes. Interestingly, these propositi had documented normal blood counts for decades prior to their PV diagnosis. However, after developing the PV phenotype, both eventually died of acute myeloid leukemia (AML) or myelodysplastic syndrome transforming within months to AML. Whether these germ line mutations increase the probability of acquiring PV is suggestive; both mutations were found in analysis of 31 unrelated PV subjects (statistically unlikely to be a random occurrence), and, in 1 subject, JAK2T108A and JAK2V617F were in cis. In addition, the L393V mutation was found with increased frequency among diffuse large B-cell tumors,22 and T108A has been reported in an adenocarcinoma cell line,23 further supporting their potential to predispose to malignancy.
In conclusion, we hypothesize that JAK2 germ line mutations may represent a mechanism that may precede acquisition of JAK2V617F lesion during the evolution of PV phenotype and may contribute to further genomic alterations in PV clone and perhaps even leukemic transformation.
Footnotes
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Stefan Constantinescu and Emilie Leroy for their insights into the JAK2 protein interactions and valuable input to this manuscript. This work was supported by research funding from the Czech Science Foundation, project GACR 15-18046Y; the Ministry of Education, Youth and Sports, Czech Republic, Program NPU I, Project LO1419 (L.L., O.B., and V.K.); and Myeloproliferative Disorders Research Consortium (National Cancer Institute, National Institutes of Health [grant P01CA108671] [J.T.P.]).
Contribution: L.L., V.D., and J.T.P. designed and conceived the project and the experiments; L.L., O.B., and S.S. performed the experiments; D.A.W., L.W., and J.T.P. performed and analyzed the whole exome sequencing; L.L., V.D., and J.T.P. wrote the manuscript; and S.S., D.A.W., L.W., and V.K. critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Josef T. Prchal, Division of Hematology, 30 N 1900 E, 5C402 SOM, University of Utah, Salt Lake City, UT 84132; e-mail: josef.prchal@hsc.utah.edu.
References
- 1.Tefferi A, Pardanani A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncol. 2015;1(1):97–105. doi: 10.1001/jamaoncol.2015.89. [DOI] [PubMed] [Google Scholar]
- 2.Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 3.Schaub FX, Looser R, Li S, et al. Clonal analysis of TET2 and JAK2 mutations suggests that TET2 can be a late event in the progression of myeloproliferative neoplasms. Blood. 2010;115(10):2003–2007. doi: 10.1182/blood-2009-09-245381. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Swierczek SI, Drummond J, et al. Whole-exome sequencing of polycythemia vera revealed novel driver genes and somatic mutation shared by T cells and granulocytes. Leukemia. 2014;28(4):935–938. doi: 10.1038/leu.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortmann CA, Kent DG, Nangalia J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015;372(7):601–612. doi: 10.1056/NEJMoa1412098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nussenzveig RH, Swierczek SI, Jelinek J, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35(1):32.e1–32.e9. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108(4):1377–1380. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Swierczek SI, Lanikova L, et al. The relationship of JAK2(V617F) and acquired UPD at chromosome 9p in polycythemia vera. Leukemia. 2014;28(4):938–941. doi: 10.1038/leu.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 10.Tavtigian SV, Deffenbaugh AM, Yin L, et al. Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. J Med Genet. 2006;43(4):295–305. doi: 10.1136/jmg.2005.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30(17):3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 13.Wallweber HJ, Tam C, Franke Y, Starovasnik MA, Lupardus PJ. Structural basis of recognition of interferon-α receptor by tyrosine kinase 2. Nat Struct Mol Biol. 2014;21(5):443–448. doi: 10.1038/nsmb.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma L, Clayton JR, Walgren RA, et al. Discovery and characterization of LY2784544, a small-molecule tyrosine kinase inhibitor of JAK2V617F. Blood Cancer J. 2013;3:e109. doi: 10.1038/bcj.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Etheridge SL, Cosgrove ME, Sangkhae V, et al. A novel activating, germline JAK2 mutation, JAK2R564Q, causes familial essential thrombocytosis. Blood. 2014;123(7):1059–1068. doi: 10.1182/blood-2012-12-473777. [DOI] [PubMed] [Google Scholar]
- 17.Marty C, Saint-Martin C, Pecquet C, et al. Germ-line JAK2 mutations in the kinase domain are responsible for hereditary thrombocytosis and are resistant to JAK2 and HSP90 inhibitors. Blood. 2014;123(9):1372–1383. doi: 10.1182/blood-2013-05-504555. [DOI] [PubMed] [Google Scholar]
- 18.Mead AJ, Rugless MJ, Jacobsen SE, Schuh A. Germline JAK2 mutation in a family with hereditary thrombocytosis. N Engl J Med. 2012;366(10):967–969. doi: 10.1056/NEJMc1200349. [DOI] [PubMed] [Google Scholar]
- 19.Kapralova K, Horvathova M, Pecquet C, et al. Cooperation of germline JAK2 mutations E846D and R1063H in hereditary erythrocytosis with megakaryocytic atypia. Blood. 2016;128(10):1418–1423. doi: 10.1182/blood-2016-02-698951. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Ma Y, Seemann J, Huang LJ. A regulating role of the JAK2 FERM domain in hyperactivation of JAK2(V617F). Biochem J. 2010;426(1):91–98. doi: 10.1042/BJ20090615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funakoshi-Tago M, Pelletier S, Moritake H, Parganas E, Ihle JN. Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity. Mol Cell Biol. 2008;28(5):1792–1801. doi: 10.1128/MCB.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witzig TE, Price-Troska TL, Stenson MJ, Gupta M. Lack of JAK2 activating non-synonymous mutations in diffuse large B-cell tumors: JAK2 deregulation still unexplained. Leuk Lymphoma. 2013;54(2):397–399. doi: 10.3109/10428194.2012.708931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson AM, Yates T, Li Y, et al. Discrepancies in cancer genomic sequencing highlight opportunities for driver mutation discovery. Cancer Res. 2014;74(22):6390–6396. doi: 10.1158/0008-5472.CAN-14-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olcaydu D, Harutyunyan A, Jäger R, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41(4):450–454. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- 25.Pearse RN, Feinman R, Ravetch JV. Characterization of the promoter of the human gene encoding the high-affinity IgG receptor: transcriptional induction by gamma-interferon is mediated through common DNA response elements. Proc Natl Acad Sci USA. 1991;88(24):11305–11309. doi: 10.1073/pnas.88.24.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]