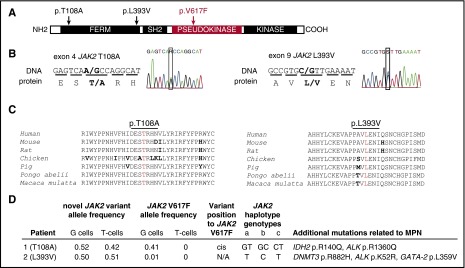
Figure 1.
Clinical and molecular features of 2 PV patients with germ line JAK2 mutations, T108A and L393V. (A) The schematic structure of the JAK2 domains with depicted locations of T108A and L393V mutations and V617F. (B) Sequencing analysis of germ line JAK2 mutations, causing an amino acid substitutions at codon 108 (threonine to alanine, p.T108A, c.A322G, novel mutation) and codon 393 (leucine to valine, p.L393V, c.C1177G, rs2230723; G allele presented in 1% of the population). (C) Alignment of amino acid sequences of JAK2 residues 93 to 125 and 379 to 407 (human JAK2 nomenclature) from different species shows highly conserved pattern. Conserved residues are colored in black, differences are in bold, and the residues at which the mutation occurs are in red. (D) The mutational screening was determined by whole exome sequencing (details published elsewhere4,8). G cells, granulocytes; T cells, CD3+ T lymphocytes. The chromosomal position of new mutation toward V617F was determined by cDNA cloning, followed by Sanger sequencing. JAK2 GGCC haplotype represents a set of single nucleotide polymorphisms (SNPs) that are associated with a predisposition to MPN.24 JAK2 GGCC haplotype was determined using polymerase chain reaction and TaqMan SNP assay on demand: (a) rs3780367, C_27515396_10, G/T, intron 10; (b) rs10974944, C_31941696_10, G/C, intron 12; (c) rs12343867, C_319416 89_10, C/T, intron 14. Several other acquired mutations that may have contributed to their MPN pathogenesis and clinical course were found in both patients. The allele frequencies of these mutations in patients’ granulocytes were as follows: IDH2 p.R140Q, 0.43; ALK p.R1360Q, 0.14; DNMT3 p.R882H, 0.31; ALK p.K52R, 0.13; GATA-2 p.L359V, 0.19 (sequencing, annotation, and validation details published elsewhere4,8).