Summary
Background
Willis‐Ekbom disease/restless legs syndrome (WED/RLS) seems to be a frequent cause of intractable chronic insomnia (ICI) but is under‐recognized in children/adolescents with neurodevelopmental conditions (NDCs), as many patients do not have the ability to express the underlying “urge‐to‐move”. In light of this, we aim to develop a protocol for behavioral observations supporting the diagnosis of WED/RLS.
Methods
We investigated 26 pediatric patients (age 1–16 years, median 8) with NDCs, ICI and evidence of familial WED/RLS employing (1) “emplotted narratives” for description of the various “urge‐to‐move” presentations and (2) self‐description and “behavioral observations” during a “suggested clinical immobilization test” (SCIT).
Results
Parental narratives reflected typical WED/RLS‐related “urge‐to‐move” symptoms during day‐, bed‐, and nighttime in all patients. Fifteen out of 26 patients could describe the “urge‐to‐move” during the SCIT. Ten out of 26 patients, unable to describe their symptoms due to cognitive disabilities, showed patterns of “relieving‐movements” upon observation. Sensory processing abnormalities were reported in all patients, with tactile sensitivities (26/26) (including shifted pain threshold) as the most common sensory domain.
Conclusion
“Emplotted narratives” and structured “behavioral observations” support recognition of familial WED/RLS associated movement patterns and provide a useful tool for the diagnosis of WED/RLS in children with NDCs in a clinical office setting.
Keywords: Attention deficit hyperactivity disorder, Iron deficiency, Periodic limb movements in sleep, Polysomnography, Sleep problem
Introduction
Despite the high prevalence of intractable and chronic insomnia (ICI) in children with neurodevelopmental conditions (NDCs), a majority of patients remain undiagnosed, causing a significant burden on the child and his/her caregiver family's well‐being 1, 2, 3. This shortcoming is compounded by the fact that, in the face of multiple comorbidities, sleep problems often remain undiagnosed and opportunities to treat are missed 4, 5, 6.
Willis‐Ekbom disease/restless legs syndrome (WED/RLS) is one of the common causes of insomnia in adults 7, 8, affecting 2–4% of the population. In children and adolescents, the prevalence is equally high, and 0.5% to 1% experience moderate to severe WED/RLS 9, 10, 11. Recently, the existence of WED/RLS in early childhood and its impact on early onset insomnia has been demonstrated using clinical observations and polysomnography 12. Still, the impact of WED/RLS on ICI has not been fully recognized in the pediatric population 13, 14. Even less is known when it comes to children with NDCs 15.
WED/RLS is a neurologic disorder characterized by discomfort (up to pain) of feet, legs, hands, arms, and/or other body parts. This discomfort often worsens during periods of rest and toward the night, and is relieved by movements. WED/RLS occurs as a primary (idiopathic) or secondary disorder on the basis of other clinical conditions. Patients with idiopathic WED/RLS often show a positive family history. Furthermore, patients with a positive family history tend to present their first symptoms at a younger age compared to those with a negative family history 16. WED/RLS is frequently associated with iron deficiency 17, and serum ferritin levels are inversely associated with the severity of WED/RLS symptoms 18.
The diagnosis is mainly based on verbalized self‐reporting. The essential diagnostic criteria include (1) an urge to move the legs, usually accompanied by uncomfortable or unpleasant sensations in the legs; (2) symptoms begin or worsen during rest; (3) symptoms are relieved by movement; (4) symptoms occur mainly in the evening/night; and (5) symptoms cannot be solely accounted for by another medical condition. Based on the explanatory model of self‐reporting 8, these criteria equally apply to children and adolescents 14.
The diagnosis of WED/RLS relies on the child's perception of their own symptoms 19. However, symptom description becomes a major challenge in young children 12, and in patients with NDCs who are unable to express themselves or to use the “right words” 20. This phenomenon has also been acknowledged as a limiting diagnostic factor in adults with language and cultural barriers when describing their symptoms 21. Additionally, children or adults with chronic WED/RLS may be missing a reference point due to early onset, or impact of other comorbidities. For such complex cases, the pediatric section of the International‐RLS‐Study‐Group has suggested the use of supportive criteria such as positive family history of WED/RLS, periodic limb movements in sleep (PLMS), and “behavioral observations” 14. While interviewing parents about their sleep history is possible in the clinical setting, and the assessment of PLMS is possible via polysomnography, no protocols exist to guide clinicians on how to conduct “behavioral observations”.
Recently, an association between sensory processing abnormalities (SPAs) and insomnia in children 22 with fetal alcohol spectrum disorders 23 and autism 24 has been recognized. However, knowledge about the causal interconnections between the impact of SPAs on insomnia and WED/RLS is limited in both children and adults 3, 5, 25.
In response to the need of a diagnostic protocol in children with NDCs, we have developed an observational approach for assessment of WED/RLS in a clinical setting, employing parents’ emplotted narratives, 1 4, 26 and a clinical immobilization test, which encourages patients to describe their “urge‐to‐move” and allows for structured observations of “urge‐to‐move” patterns 27, 28. In this study, we describe this approach and the results obtained in children with NDCs and early onset ICI and a history of familial WED/RLS after four years of experience.
Patients and Methods
The methods were developed over an interdisciplinary PhD research endeavor 15, utilizing qualitative methodologies in order to optimize clinical best practice (REB #: H10‐03466).
Sleep/Wake‐Behavior Assessments
Methods were integrated in sleep/wake‐behavior assessments as part of the clinical evaluations of children with NDCs and ICI. Data are presented from assessments performed between 2010–2014 at the Sleep/Wake‐Behavior Clinic (Division of Developmental Pediatrics, Department of Pediatrics, Faculty of Medicine, British Columbia Children's Hospital, University of British Columbia). Patients were referred by community‐based pediatricians or psychiatrists.
Assessments included:
Clinical and narrative Sleep/Wake‐behavior history;
An extended family sleep history;
A Suggested Clinical Immobilization Test (SCIT);
Exploration of sensory processing;
A sleep/Wake‐Behavior report.
Clinical observations and direct quotations by parents and patients were documented by a second observer throughout the duration of the assessment.
The clinical and narrative sleep/wake‐behavior history is conducted as a semi‐structured interview using the concept of therapeutic emplotment and narrative schema 26, 29. Parents are encouraged to describe the sleep‐ and wake‐related behaviors of their child in their own words and in the context of everyday routines 4, 15. Special emphasis is given to transitioning behaviors at day‐, bed‐, and nighttime, as well as daytime resting activities. BEARS domains (bedtime, excessive daytime sleepiness, awakenings, regularity/routines, and snoring) 30 are explored with standard questions such as how often? and since when?, with special emphasis on urge‐to‐move‐patterns in the first four domains. To further support the clinical assessment and grasp a more comprehensive clinical picture, some adaptations were made to the BEARS 31 (Table 1). In positive cases, symptoms are further investigated with the Pediatric Sleep Questionnaire (PSQ) 35. Additional areas elucidated in the reports included medical and functional diagnoses of comorbidities, on‐going therapies, medications and medication effects (psychotropic medications in particular), and scales for subjective assessment of the impacted well‐being of the child and caregivers.
Table 1.
Clinical sleep/wake‐history taking. As a qualitative exploratory interviewing approach of best/worst sleep/wake‐situations we use the modified expanded BEARS mnemonic (Vancouver Polar BEARS) 31, which also includes questions about (1) family ecology 32, 33, 34, e.g., “can you give some descriptions related to the child's strengths and problem behavior and how these affect the child, you, and your family?” 4; (2) child development, e.g., describing his/her development and behavior?, e.g., describe sleep patterns and any breathing or sensory problems?; (3) any sleep/wake‐behavior treatments, e.g., what efforts have been made to improve sleep?; and (4) as well as the impact of sleep problem on family, e.g., to how did your child's sleep problem impact your life / the life of your family and the life of the child?
| Clinical assessment categories | Descriptions |
|---|---|
| B | Bedtime situations which positively facilitate the patient's ability to fall asleep
|
| E | Excessive daytime sleepiness was altered to excessive daytime behaviors, as hyperactive‐like behaviors are explored ex aequo., also perceptions about stressful daytime situations in accounts that relate to the well‐being of the child (and themselves) |
| A | Awakenings, parasomnias and rhythmic movement disorders are explored. Parents are encouraged to elaborate about their perceptions of stressful nighttime situations and restorative/non‐restorative sleep perception in accounts that relate to the well‐being of the child (and themselves) |
| R | Routines and regularity (e.g., hours of sleep) are asked with special focus on transitioning situations (i.e., from movement to rest and vice versa, e.g., at school or during dinner), in addition to sleep health measures |
| S | Snoring was changed to sleep disordered breathing and signs of sleep disordered breathing, such as open mouth posture, signs of non‐restorative sleep (restless/sweating), and problems in getting up in the morning, were screened |
| Non‐restorative sleep | Waking up not refreshed despite having enough hours of sleep |
| Well‐being (Quality of life) | Ranking of current well‐being and well‐being if sleep problems improved, on a scale from 0(lowest)‐10(highest) for patient and parent/caregiver(s) |
The extended sleep/wake‐behavior family history includes questions addressing sleep disturbances and WED/RLS‐related symptoms (e.g., behaviors during TV watching as an example for restful activity, quality of sleep, e.g., deep or light sleeper, restful or restless sleeper, and getting up situations), as well as history of iron deficiency. This extended history captures the familial dimension of insomnia and the family's sleep habits, thus supporting the development of a shared language (Table 1).
The Suggested Clinical Immobilization Test (SCIT) is an adaptation of the laboratory‐based SIT (Suggested Immobilization Test), which is used during the montage of polysomnography leads 21, 36, and allows for standardized neurophysiological observations of behaviors and movements with an electromyography (EMG). The SCIT is administered to both the child and the parent(s) and comprises four steps (Figure 1). In cases where the SCIT cannot be administered (e.g., due to lack of comprehension, behavioral compliance or motor ability), observations of the child (with shoes and socks removed) while moving around, coming to rest, and again starting movements in the examination room were used instead (informal SCIT). Explaining the observations from the SCIT to the parents usually triggers additional narratives of related information about similar situations at home.
Figure 1.
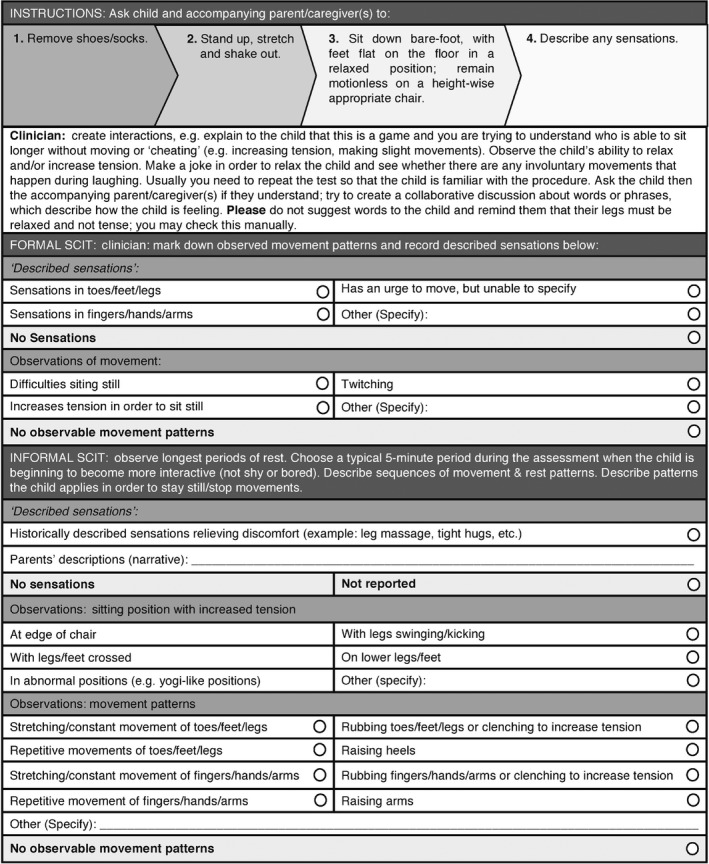
Suggested clinical immobilization test procedure.
Exploration of Sensory Processing Abnormalities (SPA). During the assessment (usually following the SCIT), parents were asked to identify: (1) If their child had experienced any SPAs (“Does your child have any sensory processing challenges?” and “Have sensory processing challenges been mentioned by any health care professional?”). (i) If yes, the types of experienced SPAs were further explored with narratives provided by the parent(s) (e.g., “He/she must wear clothes with the labels removed.”). The pain threshold of the child and affected family members is further explored in each case. (ii) If no, sensory challenges were further explored by specific questions which addressed inability to integrate and respond to sensory stimuli appropriately (“Does your child show any responses to touch or auditory stimuli which you consider as different from your other children or his/her peers?”). (2) Whether a formal sensory assessment had been conducted by an occupational therapist trained in assessing sensory problems.
Sleep/Wake‐Behavior Reports. The end product of the assessment was a sleep/wake‐behavior report, including: (1) a detailed description and summary of sleep/wake‐behaviors (including excerpts of original quotations by patients/parents); (2) our interpretations, incorporating the parents’ emplotted narrative in a de‐medicalized language; (3) recommendations for parents, and involved community‐based support teams. We used inclusive language comprehensible for any interested lay person at a grade five reading level 4, 15. Parents were asked to review and edit the reports in collaboration with the health care professionals involved in the assessment 37, 38. Complex cases were followed up on and discussed with involved community‐based pediatricians and therapy teams.
Patients
For the purpose of this study, we retrospectively analyzed the sleep/wake‐behavior assessment reports of patients seen in our clinic between 2010 and 2014 who were diagnosed with WED/RLS and fulfilled the following criteria:
Completed a clinical sleep/wake‐behavior assessment;
Evidence of familial WED/RLS through maternal history of a formal diagnosis of WED/RLS or reported experience of WED/RLS‐related discomfort either continuously or at some point in their lives, e.g., during pregnancy, as well as a self‐reported history of chronic or pregnancy‐related iron deficiency and/or anemia 27, 28;
Evidence of WED‐related insomnia, through patient‐based expression of the “urge‐to‐move” in his/her own words or the patient showed “urge‐to‐move” behaviors during the assessment, which were supported by parental narratives about their child's sleep/wake‐behaviors.
Data Analysis
Clinic reports were analyzed to capture (1) typical core clinical features of sleep/wake‐ and sensory processing behaviors; (2) original quotations out of parents’ narratives about these behaviors; and (3) behavioral observations, made during the assessment and during the SCIT test.
For the SCIT analysis, quotations from the assessment were organized into three categories; clinician's observations of (1) sitting position, (2) toe/feet/leg movements, and (3) patient's descriptions and parental narratives of sensations during formal/informal SCIT. Particular focus was given on descriptions of the patient's experienced sensations and observed associated behaviors in association with the three essential WED/RLS criteria: (1) the “urge‐to‐move” (lower/upper limbs, and the body in general); (2) their change and development (evolvement) during rest; and (3) relieving movement patterns. The experienced sensations, behaviors and clinical observations of toe/feet/leg movements, and clinicians’ observations of sitting position(s) were summarized in the report.
Information about sensory processing was categorized in the main domains (1) auditory, (2) tactile, (3) visual, and (4) oral. The selection of descriptive subcategories was guided by the Sensory Profile™, a standardized questionnaire completed by caregivers and teachers to assess a child's sensory processing patterns 39.
Results
Out of 463 patients seen in the sleep/wake‐behavior clinic between 2010 and 2014, 31 (7%) patients met the inclusion criteria. Five cases were excluded due to incomplete information (three lacking sensory processing assessments; two lacking a formal/informal SCIT assessment, or language barriers due to missing interpreter during the assessment), leaving 26 patients for analysis (21 males, 5 females; mean & median age 8 years, range 1–16).
Amongst the 26 patients, there were 43 neurodevelopmental and 45 mental health presentations with typically more than one presentation per patient (Table 2). The most common neurodevelopmental presentations were developmental delay/intellectual disability (confirmed: 18/26; suspected: 3/26) and autism spectrum disorder (confirmed: 10/26; suspected: 0/26). Ten out of 26 had a confirmed (14/26 a suspected) externalizing disorder or disorders of disruptive challenging behaviors, with attention deficit hyperactivity disorder (ADHD) being the most common presentation. Nine out of 26 had a confirmed (8/26 a suspected) internalizing disorder, with anxiety disorders being the most common. All patients met the International Classification of Sleep Disorders (Third Edition) criteria 40 for chronic insomnia, 26/26 (100%) had falling asleep problems and 23/26 (88%) sleep maintenance problems. All patients fulfilled the criteria for circadian rhythm sleep disorder (CRSD), delayed sleep onset subtype, which led to irregular sleep/wake‐rhythms in five cases (19%). Sixteen out of 26 (62%) had reported parasomnias, and 24/26 (92%) had signs of sleep disordered breathing (Table 2).
Table 2.
Neurodevelopmental Conditions & Comorbidities. Neurodevelopmental and mental health diagnoses as well as type of sleep problems in 26 children with evidence of familial WED/RLS. 1n = 21: patients assessed for ferritin levels in their medical history
| Demographics of patient cohort (n = 26, mean 8 year/median 8 year; min 1 year; max 16 year) | No. of patients with confirmed diagnosis, n (%) | No. of patients with suspected/under investigation diagnosis, n (%) |
|---|---|---|
| Neurodevelopmental Conditions | 26 (100) | 9 (35) |
| Autism Spectrum Disorder (ASD) | 10 (38) | 0 |
| Fetal Alcohol; Spectrum Disorder/Alcohol‐ related Neurodevelopmental Disorder | 2 (8) | 0 |
| Developmental Delay/Intellectual Disability (including Down syndrome) | 18 (69) | 3 (12) |
| Motor Disorder (Developmental Coordination Disorder, repetitive movements, Tourette syndrome, etc.) | 4 (15) | 6 (23) |
| Visual Impairment | 1 (4) | 0 |
| Hearing impairment | 0 | 0 |
| Mental Health Comorbidities | 11 (42) | 16 (62) |
| Externalizing Disorders or Disorders of Disruptive Challenging Behavior | 10 (38) | 14 (54) |
| ADHD | 8 (31) | 13 (50) |
| Oppositional Defiant Disorder | 3 (12) | 0 |
| Conduct Disorder | 0 | 0 |
| Attachment Disorder | 0 | 0 |
| Neurobehavioral Disorder | 1 (4) | 1 (4) |
| Internalizing Disorders | 9 (35) | 8 (31) |
| Anxiety Disorder | 8 (31) | 7 (27) |
| Depression | 2 (8) | 1 (4) |
| Hyperphagia | 1 (4) | |
| Sleep Disorders | 26 (100) | 0 |
| Insomnia | 26 (100) | 0 |
| Falling asleep problems | 26 (100) | 0 |
| Sleep maintenance | 23 (88) | 0 |
| Restless sleep (interpreted as periodic limb movements in sleep) | 23 (88) | 1 (4) |
| Circadian Rhythm Sleep Disorder (CRSD) | 26 (100) | 0 |
|
Delayed sleep onset leading to irregular/biphasic sleep patterns |
26 (100) 5 (19) |
0 |
| Clinical sleep‐disordered breathing | 24 (92) | |
| Parasomnias | 16 (62) | 0 |
| WED/RLS diagnosis & SCIT Results (+result) | ||
| Willis‐Ekbom Disease/Restless Legs Syndrome (WED/RLS) | 26 (100) | 0 |
| Positive formal SCIT result | 15 (58) | |
| Positive informal SCIT result (no formal SCIT conducted) | 10 (38) | |
| Negative SCIT result (informal) | 1 (4) | |
| Ferritin Levels (n = 21)1 | ||
| Ferritin level lower than 10 μg/L | 0 | |
| Ferritin level between 10–20 μg/L | 7 (33) | |
| Ferritin level between 20–30 μg/L | 5 (24) | |
| Ferritin level between 30–40 μg/L | 6 (29) | |
| Ferritin level between 40–50 μg/L | 3 (14) | |
| Ferritin level over 50 μg/L | 0 | |
Sleep/Wake‐Behavior Narratives
Quotations by patients/parents were assigned to the following categories of WED/RLS symptomatology: motor and sensory, as well as descriptive behaviors during day‐ and nighttime. Daytime motor and behavior characteristics included descriptions such as “always on the go”, “motor driven”, “fidgety” and were reported in 100% of patients. Nighttime motor and behavior characteristics included “restless sleep” and “kicking movements”, and were noted in 88% and 77% of the patients respectively (Table 3).
Table 3.
WED/RLS Indicators in 26 Pediatric Patients with NDCs and chronic insomnia and evidence of familial WED/RLS. The table structures the core symptom “urge‐to‐move” (A) by parental report at day‐ and nighttime; (B) patient descriptions of sensations and self‐reflected behaviors during the formal and informal SCIT; (C) clinical observations during formal and informal SCIT; (D) experienced insomnia symptoms; and (E) the repetitive behaviors worsening towards the night. 1n = 25: patients with a positive formal/informal SCIT result
| WED/RLS indicators | Quoted by patients/parents/clinician n = 26 (%) patients | Keywords and examples of representative quotations from the patient, parents and/or assessing teams observations; original quotations from the reports. |
|---|---|---|
| (A) “Urge‐to‐move” | ||
| Motor daytime | 14 (54) | Motor driven (by parental report and observations) |
| Motor night time | 23 (88) | Restless sleep, kicking movements (by parental report) |
| (B) “Urge‐to‐move” during (formal/informal) SCIT: Patient descriptions of sensations & associated behaviors in reports | ||
| Sensations in feet/toes | 5 (19) |
|
| Sensations in legs | 5 (19) |
|
| Difficulties sitting still | 25 (96) |
|
| (C) ‘Urge‐to‐move’ associated behavioral observations | ||
| Observations of positions in Formal SCIT/Observations of positions in Informal SCIT (n = 25)1 | ||
| Sitting position | ||
| At edge of chair | 5 (19) |
|
| w/legs/feet crossed | 11 (42) | “She always had her legs crossed (e.g., crossed at the ankles, crossed with one ankle on her knee, or crossed at the knees).” (Patient #7, observations) |
| w/legs swinging/kicking | 12 (46) | “When sitting, P#24 preferred to continuously swing her legs back and forth.” (Patient #24, observations) |
| On lower legs/feet | 8 (31) | “P#10 ‘always sits with one leg under’ and has ‘sat like that for years’, which his school reported as well.” (Patient #10, parental description) |
| In abnormal positions | 7 (27) | “At one point during the assessment, he sat awkwardly with his right leg hanging over the right arm rest. We observed him swinging his right leg.” (Patient #19, observations) |
| Leg/feet/toe movements | ||
| Stretching/constant movements | 17 (65) |
|
| Repetitive movements | 7 (27) | “He moved his legs/feet (e.g., tapping feet up and down, flexing and un‐flexing toes, rocking forward on toes and burrowing his toes inside of a shoe). (Patient #3, observations). |
| Rubbing together/clenching to increase tension | 14 (54) |
|
| Raising heels | 17 (65) | “When his feet were on the ground, he would put pressure on his toes or heels to increase the tension in his legs, or lean back by pressing on his toes.” (Patient #3, observations) |
| (D) “Urge‐to‐move” at night | ||
| Falling asleep problems | 25 (96) | |
| Sleep maintenance problems | 23 (88) | |
| (E) Repetitive “urge‐to‐move” patterns: increasing toward bedtime and/or worsening at rest | ||
| Challenging daytime behaviors: Excessive daytime sleepiness and enhanced hyperactive like behaviors towards nights | 26 (100) | “When A becomes increasingly hyper during the day, he will use a small trampoline to get rid of some of his hyperactivity, which will allow him to concentrate better on his school work. (Patient #18, parental descriptions) |
| Challenging nighttime behaviors: Secondary behavioral insomnia/limit setting insomnia | 22 (85) | “Refuses to go to bed every night, and it is reported that he does not seem anxious at bedtime. He tends to be highly active and restless before falling asleep, and is very reluctant to remain in his bed; he play(s) on (his) iPad or watches television, which reduces his restlessness so he can sit relatively still; (A parent) must stay with him during the falling‐asleep period, as he often becomes distressed (e.g., “freaks out”) if (left alone), often screaming or crying. (Patient #23, parental descriptions) |
Suggested Clinical Immobilization Test
Sixteen (62%) patients participated actively in the formal SCIT; 15/16 (94%) patients reported various descriptions of “urge‐to‐move” and showed positive signs of involuntary movements of toes/feet/legs (Table 3, section C). Patient #6, a boy younger than six years old with insomnia (treated with clonidine 0.1 mg/daily at nighttime), parasomnia and anxiety disorder (treated with fluoxetine 4mgs/daily), was the only individual in our cohort who did not show/report motor signs and sensory discomfort during the SCIT. The remaining 10/26 (38%) patients could not participate actively in the SCIT due to insufficient comprehension (age or intellectual disability). In these patients, observation‐based involuntary motor movements at random rest situations were utilized as an informal SCIT. In Table 2, patient reported descriptions of symptoms or parent reported (triggered) descriptions from similar “resting” situations during the SCIT are summarized.
Sensory Processing Abnormalities
Sensory processing abnormalities were stratified based on the type of parental reports and observations: 100% (n = 26) of the patients had a tactile sensitivity; 77% (n = 20) presented with a shifted pain threshold. Auditory, visual, and oral sensitivities were reported in 23% (n = 6) of the patients (Table 4). The most common descriptive categories of tactile sensitivity were a shifted pain threshold (n = 19); sensitivity to clothing tags, closed shoes, socks, fabrics (n = 14); and “other” tactile‐seeking behaviors (n = 13). Eleven patients had tactile sensitivities that fell into two or more of the aforementioned categories, within a single sensory domain. Within the “other tactile‐seeking behaviors” category, parents described their children biting their own hands and arms, stubbing their toes on purpose, banging and thrashing their head against the wall, and “picking at sores”. Within the “shifted pain threshold” category, some parents told stories of their child getting an injury, such as a “broken arm” or “sliced hand” (both original quotations), and not noticing or reacting appropriately to the incident. Six patients fell within the auditory sensitivity domain, with a heightened sensitivity to loud or unexpected sounds (n = 5) most commonly reported. Five patients also had difficulty focusing in noisy environments, particularly with multiple different sounds occurring at the same time. Within the visual sensitivity domain, parents of four children reported a heightened sensitivity to bright lights, particularly fluorescent lights or sunlight. Parents also described children having difficulty finding objects in competing or complex backgrounds (n = 2). Within the oral sensitivity domain, all children had varying degrees of difficulty with the taste, texture, and smells of their foods (n = 5).
Table 4.
Parental Descriptions of Sensory Processing Abnormalities. Quotations taken from sleep/wake‐behavior assessment reports. (n = 26, mean 8 year/median 8 year; min 1 year; max 16 year). Parents have received and reviewed original copies of the assessment reports from which quotations of their own wording have been taken
| Sensory domain (% of children with SPAs) | Examples of parent‐reported descriptions of child's sensory processing abnormalities (child's age, gender, patient ID). |
|---|---|
| Tactile (n = 26, 100%) | |
| Shifted pain threshold (n = 19) |
|
| Sensitivity to clothing tags, shoes, and fabrics (n = 14) |
|
| “Other” tactile‐seeking behaviors (n = 13) |
|
| Auditory (n = 6, 23%) | |
| Heightened sensitivity to unexpected or loud noises (n = 5) |
|
| Is distracted/has trouble functioning if there is a lot of noise around (n = 5) |
|
| Visual (n = 6, 23%) | |
| Bothered by bright lights after others have adapted (n = 4) |
|
| Difficulty visualizing objects in complex/crowded background (n = 2) | “Has a hard time finding objects in competing backgrounds, such as shoes in a messy room. He misses written or demonstrated directions more than other students in the classroom.” (9 year, male, 12) |
| Oral (n = 5, 19%) | |
| Avoids certain food tastes/smells/textures that are typically part of children's diets (n = 5) |
|
Conclusion
The main reason why the medical community needs a new perspective on WED/RLS in children (and most probably also in geriatric patients) is because the current essential diagnostic criteria are based on self‐description and inherently do not include patients who are not able to verbalize their complaints. Polysomnographic investigations have proven that PLMS can additionally support the diagnosis of WED/RLS 14; however, the myriad of WED/RLS presentations in children with NDCs has not been unveiled yet. Furthermore, access to polysomnography is limited in many geographical regions around the world, and children with NDCs typically have difficulties complying. Our concept of structured observations in context with emplotted narratives can be applied during an office visit and provides information on all four clinical diagnostic WED/RLS criteria.
Exploratory interviewing captured typical characteristic descriptions of significant day‐ and nighttime restlessness, compatible with WED/RLS: (1) restlessness or inability to relax over the day in any situation associated with rest, and (2) restlessness at the falling asleep situation contributing to significant bedtime problems. The following quotations by a mother of a five year old patient (Patient #24) with an FASD‐diagnosis, describes that characteristically:
(Patient #24, quotations are from the medical report:) ‘… She is “constantly on‐the‐go,” and even “needs to stand up and eat at the dinner table.” Her “feet and/or hands are always moving.” … Even when she is sleeping you “can't slow her down”.’
As an objective measure to demonstrate the motor restlessness characteristic of WED/RLS, we adapted the laboratory‐based SIT 21, 36. The SCIT provokes a rest situation allowing behavioral observations as a part of the clinical exam. Forty‐six percent of the patients in this cohort had the ability to describe the perceived symptoms in their own wording while performing the SCIT. As an unexpected and fun activity during the assessment, it motivated children to report subjective symptoms experienced during the test in a more easy‐going way than in the typical clinical examination situation, where they might feel stressed or become shy, as the “black sheep” in the family. Administering the test to all family members present during the assessment gave information on affected family members, triggered reporting of familial anecdotes and speaking about previously or simultaneously made observations.
Home video recordings have shown that children with WED/RLS may have strong kicking movements of their legs during falling asleep situations 41, 42. We found similar movements in 10 (38%) of our patients during the observational phase of our clinical assessment (informal SCIT), while they were playing in a sitting or reclined position. During the formal SCIT, when children reported their distress to keep their feet tightly attached to the ground, these kicking movements were obviously suppressed.
Most interestingly, we found an overlap of SPA with ICI caused by probable WED/RLS in all our patients. Parent reported and SCIT‐based observations mainly referred to tactile sensitivities. The following excerpt from a medical report demonstrates the patients’ wording about the sensations during the SCIT, and the parental descriptions of sensory problems.
(Patient #19, quotations are from the medical report:): ‘When asked to sit in a relaxed manner, patient described that he felt “energy” in his legs and body (he felt that “something” is moving through his legs and “controlling” his body). (Parental description:) “…picks at sores until it bleeds, and won't let anyone else touch it… He is also upset by loud sounds and has trouble focusing in a noisy environment..”. (Clinical observations at SCIT:) ‘We observed him flexing his toes and shifting around, he was unable to keep still …’
Central disinhibition of nocioreceptive pathways has been postulated as the cause of painful sensation and discomfort in adult patients with primary WED/RLS and mechanic type of hyperalgesia 43, 44. Likewise, a central nervous dysfunction is the most plausible cause of the multisensory nature of integration abnormalities in our patients. A systematic investigation of SPAs in patients with proven WED/RLS will elucidate whether and which category of SPAs are part of the WED/RLS spectrum.
A majority of patients had mental health comorbidities with attention deficit hyperactivity disorder (ADHD) and anxiety disorder being the most common presentations. These findings are in line with other studies demonstrating the co‐occurrence of comorbid psychiatric conditions, including externalizing (e.g., ADHD; aggression) 14, 45 and internalizing (e.g., anxiety; depression) 8, 46 behaviors. Fifteen out of 26 (58%) patients were trialed with at least one psychotropic medication prior to the sleep/wake‐behavior assessment, while the narratives unveiled that insomnia had always (often since early infancy) been “an issue”. In a recent analyses, we could demonstrate that ICI due to familial WED/RLS, can lead to overmedication, even polypharmacy, for the purpose to treat challenging daytime behaviors resulting from WED/RLS discomfort per se and the chronic primary sleep deprivation 47.
Arbuckle et al. 48 and Pichietti et al. 49 have established descriptors and scales for the diagnosis of WED/RLS in the pediatric population, based on analysis of interviews with children with WED/RLS, who had the ability to describe their complaints verbally and via drawings. Our explorative approach provides guidance for how to observe children at risk for WED/RLS, who are unable to express themselves verbally. Involving both the child and the accompanying family member, the use of plain language and the opportunity for parents to review and edit the reports helped to avoid misinterpretations of our finding, thus assuring the validity of our findings. The SCIT performed jointly by the partent(s) and the child, motivated both parties to describe their symptoms with their own words 15. For many patients and for the majority of parents, the shared language promoted the comprehension of the final diagnostic interpretation and for the first time unveiled the connection between their daytime restlessness and ICI.
Our skills in behavioral observations improved over the years. For example, we noticed that with growing ease of giving instructions, the SCIT became a “fun” activity for the kids, promoting their collaboration and compliance. Thus, in earlier reports, the full spectrum of self‐reported descriptions might not have been captured.
Iron deficiency and low brain iron levels with abnormal dopaminergic consequences, against the background of genetic predisposion, are currently considered the main hallmark in the pathogenesis of WED/RLS 50. In this cohort, ferritin levels, as a marker of systemic iron homeostasis, could be obtained for 21 out of the 26 children. All 21 had results below 50 μg/L, fulfilling the critical threshold for iron supplementation of symptomatic children with WED/RLS (Table 2) 14.
Naturally, the retrospective analysis nature of the study affects its quality. Therefore, we deferred from reporting any quantifications (e.g., how many seconds passed until the first “urge‐to‐move” was reported or observed during the SCIT). Furthermore, this study is limited to a small cohort of patients with evidence of familial WED/RLS. Larger cohorts of patients need to be investigated prospectively in order to validate the findings of this retrospective study.
The WED/RLS story in children with NDCs is a modern parable. While conventional medicine facilitates a spectrum of diagnoses that are applied based on training culture, symptoms not in alignment with the standard repertoire are not recognized and diagnoses are missed. Understanding this parable and finding applicable answers for how such systemic errors can be avoided will add value to the well‐being of the patients and their caregivers/families, but is still a work in progress. Given the results of this study, we suggest using a standardized protocol including family sleep history, narratives of best/worst‐case scenario situations and structured observations during a test‐resting of the child, as demonstrated with the SCIT, and to explore further symptoms suggestive of SPAs as an additional criteria to diagnose familial WED/RLS.
We are currently developing downloadable assessment forms for multicentric use of our tool. Insights gained through studies utilizing our tool will result in a better understanding of the pathophysiology and, thus, of treatment options for WED/RLS‐related ICI.
Conflict of Interest
Dr. Ipsiroglu collaborates with the Austrian Institute of Technology, Vienna, a start‐up company of the Technical University of Vienna, Austria and the Department of Electrical and Computer Engineering, University of British Columbia for developing automated software for the analysis of restless day‐/nighttime movement patterns.
Acknowledgments
The authors would like to thank all patients and their families who supported the implementation of qualitative methodology in pediatric sleep medicine with their narratives which were initially unbelievably challenging but coherent. Without their eye‐opening stories, needless suffering would continue to be perpetuated. The research at the Sleep/Wake‐Behavior Clinic has been funded since 2007 by BC Children's Foundation; 2007–2012 by Victoria Foundation FASD Fund; in 2012 by Peter Wall Institute for Applied Sciences (UBC) with an Exploratory Workshop grant; 2011–2015 by the Treatable Intellectual Disability Endeavour (Child Family Research Institute, BC Children's Hospital and Provincial Health Service Authority, Vancouver); and since 2014 by NeuroDevNet, a Pan‐Canadian Centers of Excellence Network. We would particularly like to thank Dr. Suresh Kotagal, Mayo Clinic, Rochester, for his thoughtful and constructive pre‐review and critical comments, which guided us in our manuscript editing endeavour.
Note
Emplotted stands for collaboratively working out presentations of challenging/disruptive sleep‐ and wake‐behaviors with parents/caregivers (via exploration and negotiation of symptoms) and then sharing the final summary for quality control 15.
References
- 1. Jan JE, Owens JA, Weiss M, et al. Sleep hygiene for children with neurodevelopmental disabilities. Paediatrics 2008;122:1343–1350. [DOI] [PubMed] [Google Scholar]
- 2. Krakowiak P, Goodlin‐Jones B, Hertz‐Picciotto I, Croen L, Hansen R. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population‐based study. J Sleep Res 2008;17:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malow B, Byars K, Johnson K, et al. A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics 2012;130(Suppl 2):S106–S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ipsiroglu OS, McKellin WH, Carey N, Loock C. “They silently live in terror…” why sleep problems and night‐time related quality‐of‐life are missed in children with a fetal alcohol spectrum disorder. Soc Sci Med 2013;79:76–83. [DOI] [PubMed] [Google Scholar]
- 5. Jan JE, Bax M, Owens JA, Ipsiroglu OS, Wasdell M. Neurophysiology of circadian rhythm sleep disorders of children with neurodevelopmental disabilities. Eur J Paediatr Neurol 2012;16:403–412. [DOI] [PubMed] [Google Scholar]
- 6. Mayer S. Analysis of Sleep Encounters in Complex Care [Undergraduate honours thesis]. Vancouver: University of British Columbia, 2012. [Google Scholar]
- 7. Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 2005;165:1286–1292. [DOI] [PubMed] [Google Scholar]
- 8. Allen RP, Picchietti DL, Garcia‐Borreguero D, et al. International Restless Legs Syndrome Study Group. Restless legs syndrome/Willis‐Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria – history, rationale, description, and significance. Sleep Med 2014;15:860–873. [DOI] [PubMed] [Google Scholar]
- 9. Kinkelbur J, Hellwig J, Hellwig M. Frequency of RLS symptoms in childhood. Somnologie 2003;7(Suppl 1):34. [Google Scholar]
- 10. Yilmaz K, Kilincaslan A, Aydin N, Kor D. Prevalence and correlates of restless legs syndrome in adolescents. Dev Med Child Neurol 2011;56:803–807. [DOI] [PubMed] [Google Scholar]
- 11. Picchietti D, Allen R, Walters A, Davidson J, Myers A, Ferrini‐Strambi L. Restless legs syndrome: Prevalence and impact in children and adolescents – the Peds REST study. Pediatrics 2007;120:253–266. [DOI] [PubMed] [Google Scholar]
- 12. Tilma J, Tilma K, Norregaard O, Ostergaard J. Early childhood‐onset restless legs syndrome: Symptoms and effect of oral iron treatment. Acta Paediatr 2013;102:221–226. [DOI] [PubMed] [Google Scholar]
- 13. Lee‐Chiong T. Foreword. Sleep Med Clin 2007;2:xi–xii. [Google Scholar]
- 14. Picchietti DL, Bruni O, de Weerd A, et al. Pediatric restless legs syndrome diagnostic criteria: An update by the International Restless Legs Syndrome Study Group. Sleep Med 2013;14:1253–1259. [DOI] [PubMed] [Google Scholar]
- 15. Ipsiroglu OS. Applying ethnographic methodologies & ecology to unveil dimensions of sleep problems in children & youth with neurodevelopmental conditions [Dissertation]. Vancouver: University of Bristish Columbia, 2016. [Google Scholar]
- 16. Winkelman J, Wetter TC, Collado‐Seidel V, et al. Clinical characteristics and frequency of the hereditary restless legs syndrome in a population of 300 patients. Sleep 2000;23:597–602. [PubMed] [Google Scholar]
- 17. Kotagal S, Silber MH. Childhood‐onset restless legs syndrome. Ann Neurol 2004;56:803–807. [DOI] [PubMed] [Google Scholar]
- 18. Sun ER, Chen CA, Ho G, Earley CJ, Allen RP. Iron and the restless legs syndrome. Sleep 1998;21:371–377. [PubMed] [Google Scholar]
- 19. Picchietti D, Arbuckle R, Abetz L, et al. Pediatric restless legs syndrome: Analysis of symptom descriptions and drawings. J Child Neurol 2011;26:1365–1376. [DOI] [PubMed] [Google Scholar]
- 20. Ipsiroglu OS, Hung Y‐HA, Soo S, et al. Different restless legs syndrome/Willis Ekbom disease (RLS/WED) phenotypes. A missed co‐morbidity in children and youth with neurodevelopmental disorders that can aggravate challenging behaviour? [Abstract]. Sleep 2012;35:A367. [Google Scholar]
- 21. Garcia‐Borreguero D, Kohnen R, Boothby L, Tzonova D, Larrosa O, Dunkl E. Validation of the multiple suggested immobilization test: A test for the assessment of severity of restless legs syndrome (Willis‐Ekbom Disease). Sleep 2013;36:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vasak M, Williamson J, Garden J, Zwicker JG. Sensory processing and sleep in typically developing infants and toddlers. Am J Occup Ther 2015;69:6904220040. [DOI] [PubMed] [Google Scholar]
- 23. Wengel T, Hanlon‐Dearman A, Fjelsted B. Sleep and sensory characteristics in young children with fetal alcohol spectrum disorder. J Dev Behav Pediatr 2011;32:384–392. [DOI] [PubMed] [Google Scholar]
- 24. Mazurek MO, Petroski GF. Sleep problems in children with autism spectrum disorder: Examining the contributions of sensory over‐responsivity and anxiety. Sleep Med 2015;16:270–279. [DOI] [PubMed] [Google Scholar]
- 25. Winkelman J, Gagnon A, Clair A. Sensory symptoms in restless legs syndrome: The enigma of pain. Sleep Med 2013;14:934–942. [DOI] [PubMed] [Google Scholar]
- 26. Mattingly C. The concept of therapeutic ‘emplotment’. Soc Sci Med 1994;38:811–822. [DOI] [PubMed] [Google Scholar]
- 27. Ipsiroglu OS. Autismus‐Spektrum‐Störungen und Willis‐Ekbom‐Erkrankung. Ein Plädoyer für explorative Anamnesen [Autism Spectrum Disorders and Willis Ekbom disease. A plea for explorative histories] In: Paditz E, Sauseng W, editors. Schlafmedizin Kompendium [Sleep medicine compendium]. Dresden: Kleanthes, 2015;49–65. [Google Scholar]
- 28. Wagner A. Familial Willis Ekbom Disease/Restless Legs Syndrome: Presentations in Children with Neurodevelopmental Conditions and their Mothers (Medical doctorate thesis). Salzburg: Paracelsus Medical University, 2015. [Google Scholar]
- 29. Lawlor MC, Mattingly CF. The complexities embedded in family‐centered care. Am J Occup Ther 1998;52:259–267. [DOI] [PubMed] [Google Scholar]
- 30. Owens JA, Dalzell V. Use of the BEARS sleep screening tool in a pediatric residents continuity clinic: A pilot study. Sleep Med 2005;6:63–69. [DOI] [PubMed] [Google Scholar]
- 31. Ipsiroglu OS, Carey N, Collet J, et al. De‐medicalizing sleep: sleep assessment tools in the community setting for clients (patients) with FASD & prenatal substance exposure. National Organisation for Fetal Alcohol Syndrome – UK (NOFAS‐UK): Fetal Alcohol Forum, London, 2012.
- 32. Lucyshyn J, Albin R. Comprehensive support to families of children with disabilities and problem behaviors: Keeping it “friendly” In: Singer G, Powers L, editors. Families, disabilities, and empowerment: Active coping skills and strategies for family interventions. Baltimore: Paul H Brookes, 1993;365–407. [Google Scholar]
- 33. Lucyshyn J, Albin R, Nixon C. Embedding comprehensive behavioral support in family ecology: An experimental single‐case analysis. J Consult Clin Psychol 1997;65:241–251. [DOI] [PubMed] [Google Scholar]
- 34. Stockler S, Moeslinger D, Herle M, Wimmer B, Ipsiroglu OS. Cultural aspects in the management of inborn errors of metabolism. J Inherit Metab Dis 2012;35:1147–1153. [DOI] [PubMed] [Google Scholar]
- 35. Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep‐disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med 2000;1:21–32. [DOI] [PubMed] [Google Scholar]
- 36. Michaud M. Is the suggested immobilization test the “golden standard” to assess restless legs syndrome? Sleep Med 2006;7:541–543. [DOI] [PubMed] [Google Scholar]
- 37. Cicourel A. Hearing is not believing: Language and the structure of belief in medical communication In: Fisher S, Todd AD, editors. The social organization of doctor‐patient communication. Washington: Center for Applied Linguistics, 1983;221–239. [Google Scholar]
- 38. Fontana A, Frey J. The Interview. From Structured Questions to Negotiated Text In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research, 2nd edn Thousand Oaks: Sage Publications, 2000;645–672. [Google Scholar]
- 39. Dunn W, Westman K. The sensory profile: The performance of a national sample of children without disabilities. Am J Occup Ther 1997;51:25–34. [DOI] [PubMed] [Google Scholar]
- 40. American Academy of Sleep Medicine . International classification of sleep disorders, 3rd edn Darien: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 41. Bruni O, Angriman M, Luchetti A, Ferri R. Leg kicking and rubbing as a highy suggestive sign of pediatric restless legs syndrome. Sleep Med 2016;16:1576–1577. [DOI] [PubMed] [Google Scholar]
- 42. Ipsiroglu OS, Hung YH, Chan F, et al. “Diagnosis by behavioral observation” home‐videosomnography ‐ a rigorous ethnographic approach to sleep of children with neurodevelopmental conditions. Front Psychiatry 2015;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stiasny‐Kolster K, Magerl W, Oertel WH, Moller JC, Treede RD. Static mechanicalhyperalgesis without dynamic allodynia in patients with restless legs syndrome. Brain 2004;127:773–782. [DOI] [PubMed] [Google Scholar]
- 44. Bachmann CG, Rolke R, Scheidt U, et al. Thermal hyperesthesia differentiates secondary restless legs syndromes associated with small fibre neuropathy from primary restless legs syndrome. Brain 2010;133:762–770. [DOI] [PubMed] [Google Scholar]
- 45. Walters A, Silvestri R, Zucconi M, et al. Review of the possible relationship and hypothetical links between attention deficit hyperactivity disorder (ADHD) and the simple sleep related movement disorders, parasomnias, hypersomnias, and circadian rhythm disorders. J Clin Sleep Med 2008;15:591–600. [PMC free article] [PubMed] [Google Scholar]
- 46. Pullen S, Wall C, Angstman E, Munitz G, Kotagal S. Psychiatric comorbidity in children and adolescents with restless legs syndrome: A retrospective study. J Clin Sleep Med 2011;7:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ipsiroglu OS, Berger M, Lin T, Elbe D, Stockler S, Carleton B. Pathways to Overmedication and Polypharmacy: Case Examples from Adolescents with Fetal Alcohol Spectrum Disorders In: Di Pietro N, Illes J, editors. The science and ethics of antipsychotic use in children. Waltham: Elsevier, 2015;125–148. [Google Scholar]
- 48. Arbuckle R, Abez L, Durmer JS, et al. Development of the Pediatric restless legs syndrome severity scale (P‐RLS‐SS)©: A patient‐reported outcome measure of pediatric RLS symptoms and impact. Sleep Med 2010;11:897–906. [DOI] [PubMed] [Google Scholar]
- 49. Pichietti MA, Pichietti DL. Advances in pediatric restless legs syndrome: Iron, genetics, diagnosis and treatment. Sleep Med 2010;11:643–651. [DOI] [PubMed] [Google Scholar]
- 50. Dauvilliers Y, Winkelmann J. Restless legs syndrome: Update on pathogenesis. Curr Opin Pulm Med 2013;19:594–600. [DOI] [PubMed] [Google Scholar]


