Abstract
Patient‐reported benefits of research participation have been described by study participants; however, many studies have small sample sizes or are limited to patient groups with poor prognoses. The purpose of this study was to explore the effects of research participation on patient experience using survey responses from a large, national sample of cancer patients (N = 66 462) and interviews with breast cancer patients attending a London trust. Multivariate logistic regression was used to investigate associations between taking part in research and positive patient experience. Based on our analysis, patients who participated in research were more likely to rate their overall care and treatment as ‘very good/excellent’ (OR adj:1.64, 95%CI: 1.53–1.76, P < 0.001) and to describe positive patient experiences, such as better access to non‐standard care, better interactions with staff and being treated as an individual. However, findings from our interviews indicated that there was no common understanding of what constitutes cancer research and no clear delineation between research participation and standard care, from the patient perspective. Further work to explore how participation positively influences patient experience would be useful to develop strategies to improve care and treatment for all patients regardless of whether or not they choose, or have the opportunity, to take part in research.
Keywords: cancer, patient experience, research participation
Introduction
The UK has one of the highest levels of participation in cancer research in the world with more than 20% of newly diagnosed patients participating in a study in 2011 (Department of Health 2012). Taking part in research benefits the collective patient population by enabling the continued improvement of cancer care and treatment; however, direct benefits as a result of being involved in research have also been described by study participants. While improved clinical outcomes and quality of life have been associated with taking part in research (Peppercorn et al. 2004), most benefits described by participants relate to aspects of patient experience, for example having access to the latest treatments, drugs or specialist equipment that might not otherwise be available (Cox 1999; Kemeny et al. 2003). Better access to speciality oncologists or allied health professionals during study‐related visits can make participants feel they are in expert hands, and consequently taking part in research may appear synonymous with receiving the best treatment available. Being part of a research study can also facilitate better coordination of care due to increased continuity of staff and the involvement of trial managers or study coordinators (Maslin‐Prothero 2000; Moore 2001), creating a sense of safety for patients by providing a structured and routinised environment (Agrawal et al. 2006). Closer follow‐up and monitoring, in the form of more frequent diagnostic tests, scans and screens associated with research studies (Hallowell et al. 2010), may also act as a source of comfort and security to participants (Tolmie et al. 2004). Better patient–staff relationships are often described by patients involved in studies, perhaps because consultations are less time‐pressured and patients therefore feel they receive more attention than usual (Hutchison 1998; Maslin‐Prothero 2000). This more frequent positive contact with staff and greater continuity and coordination of care can help research participants to feel as if they are recognised and treated as individuals (Maslin‐Prothero 2000) and many patients who take part in research describe feeling special or privileged because of their involvement and position in a trial (Cox 1999; Tolmie et al. 2004). Another benefit described by some research participants is being better informed about their condition (Nurgat et al. 2005), which can allow them to feel more in control of their illness and/or care and treatment.
While the potential benefits of taking part in research have been described in the literature, many of the reported studies involve small numbers of patients (Moore 2001; Nurgat et al. 2005; Catt et al. 2011). Furthermore, these studies are often limited to patient groups with poor prognoses (i.e. those participating in phase I/II clinical trials (Hutchison 1998; Cox 1999; Agrawal et al. 2006) or palliative care studies (Barnett 2001; Shipman et al. 2008) and so their findings may not be generalisable to the wider cancer patient population. The National Cancer Patient Experience Survey (NCPES) is a series of surveys carried out annually on behalf of the Department for Health (DH) to assess patient experience in England. In NCPES 2012‐13, a new question about research participation was introduced thereby providing a unique opportunity to use national survey data to investigate the effects of research participation on patient experience among a large sample of cancer patients with a variety of prognoses. The overall aim of this study was to explore the effects of research participation on patient experience using a mixed‐methods approach by combining secondary analysis of a national quantitative data set with local interviews of breast cancer patients. On the basis of previous findings from the literature, we hypothesised that patients would have differing views on cancer research and that those who took part in research would report more positive experiences.
Methods
Quantitative data source
Cross‐sectional NCPES 2012‐13 data collected on behalf of the DH was used for secondary analysis in this study. In January 2013, the survey was sent to the discharge address of all patients with a primary diagnosis of cancer who attended an NHS hospital as an inpatient or day case between 1 September 2012 and 30 November 2012 (Department of Health 2013). Non‐responders were sent two follow‐up reminders and, accounting for individuals known to have died or moved, a 64% response rate was achieved. The NCPES 2012‐13 contained 82 questions in total; 70 multiple choice and three free text questions about aspects of patient experience and nine multiple choice questions about patients' clinical and demographic characteristics. Two questions related to research, namely Q30 ‘Since your diagnosis, has anyone discussed with you whether you would like to take part in cancer research?’ and Q31 ‘If yes, did you then go on to take part in cancer research?’ As patients who provided no response to these research‐related questions (n = 2775) could not be included in our analysis, the data set analysed in this study contained responses from 66 462 patients attending 155 hospital trusts across England. Methods for categorising patient, clinical and trust‐level factors have been described elsewhere (Bone et al. 2014). Briefly gender, age, ethnicity, employment status, long‐standing conditions, time since first treatment and response to treatment were derived from patients' responses to survey questions while tumour group and day case or inpatient status were extracted from hospital administration records. Hospital trusts were categorised by foundation status, location (inside or outside London) and type (large acute, medium acute, small acute, specialist and teaching). The largest groups were chosen as reference categories for regression analysis, with the exception of gender and long‐standing conditions, where men and not having the specific long‐standing condition were used respectively. The characteristics of patients and the trusts they attended can be found in Table S1.
Quantitative data analysis
First, relevant published literature was reviewed to develop a conceptual model of how research participation may affect cancer patient experience (Fig. 1). With reference to this model, the 70 multiple choice questions related to experiences of care and treatment contained in NCPES 2012‐13 were screened to identify those likely to be associated with research participation. In total, 29 questions were selected a priori for further analysis (Table 1). As the survey was designed to measure cancer patient experience generally, not all the proposed effects of research participation had related questions in the data set; for example, there were no questions on access to latest treatments, remuneration or clinical outcomes.
Figure 1.
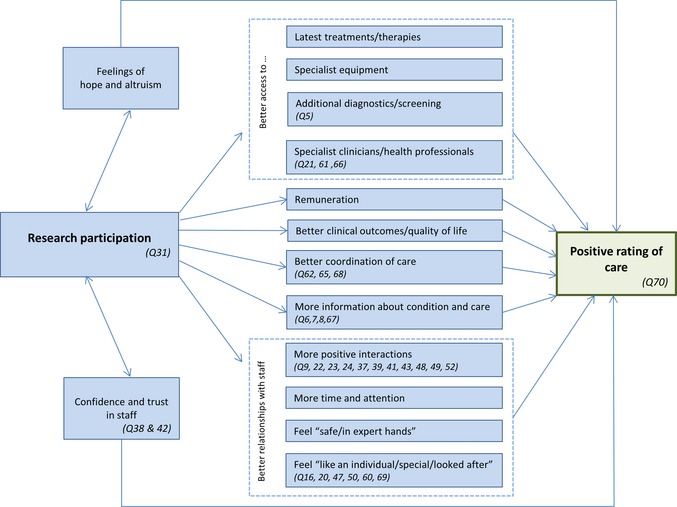
Models how research participation may affect cancer patient experience and overall rating of care based on the available published literature. Similar aspects of care are enclosed in dashed boxes. Questions from the NCPES 2012‐13 related to these a priori aspects of patient experience are indicated in italics and detailed in Table S2. NCPES, National Cancer Patient Experience Survey.
Table 1.
The effect of research participation on overall rating of care and a priori aspects of patient experience as measured by questions in the National Cancer Patient Experience Survey 2012‐13
| OR | 95% CI | P‐value | ORadj a | 95% CI | P‐value | ||
|---|---|---|---|---|---|---|---|
| Better overall rating of care | |||||||
| 70 | Overall how would you rate your care? | 1.60 | 1.50–1.72 | <0.001 | 1.64 | 1.53–1.76 | <0.001 |
| Better interactions with staff | |||||||
| 9 | Test results were explained in an understandable way | 1.16 | 1.11–1.23 | <0.001 | 1.24 | 1.18–1.31 | <0.001 |
| 22 | It was easy to contact my Clinical Nurse Specialist | 1.08 | 1.03–1.14 | 0.002 | 1.16 | 1.10–1.22 | <0.001 |
| 23 | My Clinical Nurse Specialist listened carefully to me | 1.21 | 1.12–1.31 | <0.001 | 1.29 | 1.19–1.39 | <0.001 |
| 24 | My Clinical Nurse Specialist gave understandable answers to my questions | 1.21 | 1.12–1.31 | <0.001 | 1.25 | 1.15–1.36 | <0.001 |
| 37 | Doctor gave understandable answers to my questions | 1.41 | 1.31–1.51 | <0.001 | 1.49 | 1.39–1.60 | <0.001 |
| 39 | Doctors didn't talk in front of me as if I wasn't there | 1.14 | 1.04–1.25 | 0.01 | 1.21 | 1.13–1.30 | <0.001 |
| 41 | Nurse gives understandable answers to my questions | 1.22 | 1.15–1.30 | <0.001 | 1.26 | 1.18–1.34 | <0.001 |
| 43 | Nurses didn't talk in front of me as if I wasn't there | 1.07 | 0.99–1.14 | 0.06 | 1.12 | 1.05–1.20 | 0.001 |
| 48 | I was given enough privacy when discussing my condition or treatment | 1.05 | 0.98–1.12 | 0.16 | 1.16 | 1.09–1.24 | <0.001 |
| 49 | I was given enough privacy when being examined or treated | 1.09 | 0.98–1.21 | 0.11 | 1.23 | 1.10–1.37 | <0.001 |
| 52 | I was treated with respect and dignity by the doctors, nurses and other hospital staff | 1.06 | 1.00–1.13 | 0.06 | 1.13 | 1.06–1.21 | <0.001 |
| Better informed about their condition and care | |||||||
| 6 | Staff explained the purpose of the test beforehand | 1.34 | 1.26–1.43 | <0.001 | 1.32 | 1.24–1.40 | <0.001 |
| 7 | Staff explained what would be done during the test procedure beforehand | 1.33 | 1.25–1.43 | <0.001 | 1.31 | 1.22–1.40 | 0.04 |
| 8 | I was given written information about the test beforehand | 1.50 | 1.38–1.62 | <0.001 | 1.46 | 1.33–1.57 | <0.001 |
| 67 | I was given the right amount of information about my condition and treatment | 1.21 | 1.14–1.29 | 0.71 | 1.28 | 1.20–1.37 | <0.001 |
| Better access to specialist staff and diagnostic tests | |||||||
| 5 | I had a diagnostic test for cancer in the last 12 months | 1.06 | 0.99–1.13 | 0.08 | 1.19 | 1.11–1.28 | <0.001 |
| 21 | I was given the name of a Clinical Nurse Specialist who would be in charge of my care | 1.94 | 1.80–2.08 | <0.001 | 1.84 | 1.70–1.98 | <0.001 |
| 61 | I had an appointment with a cancer doctor in the last 12 months | 1.92 | 1.74–2.13 | <0.001 | 1.61 | 1.44–1.79 | <0.001 |
| 66 | I had treatment from other allied health professionals, e.g. dietician for my cancer | 1.32 | 1.27–1.38 | <0.001 | 1.17 | 1.12–1.22 | <0.001 |
| Feel ‘like an individual/special/looked after’ | |||||||
| 16 | My views were taken into account when deciding on treatment | 1.19 | 1.13–1.24 | <0.001 | 1.24 | 1.19–1.30 | <0.001 |
| 20 | I was involved as much as I wanted to be in decisions about care and treatment | 1.28 | 1.22–1.34 | <0.001 | 1.35 | 1.29–1.41 | <0.001 |
| 47 | Doctors and nurses asked me what name I prefer to be called by | 1.03 | 0.98–1.08 | 0.23 | 1.13 | 1.08–1.19 | <0.001 |
| 50 | I was able to discuss my worries and fears with staff during hospital visit | 1.15 | 1.09–1.21 | <0.001 | 1.21 | 1.15–1.28 | <0.001 |
| 60 | I was given enough emotional support from hospital staff as an outpatient | 1.09 | 1.03–1.14 | <0.001 | 1.18 | 1.12–1.24 | <0.001 |
| 69 | I was treated as a whole person rather than ‘a set of cancer symptoms’ | 1.04 | 0.99–1.09 | 0.16 | 1.16 | 1.10–1.22 | <0.001 |
| Better coordination of care | |||||||
| 62 | Doctor had the right documents such as medical notes at last appointment | 1.21 | 1.09–1.35 | 0.001 | 1.29 | 1.15–1.44 | <0.001 |
| 65 | The different people treating and caring for me worked well together | 0.98 | 0.94–1.02 | 0.27 | 1.08 | 1.03–1.13 | 0.001 |
| 68 | I was offered a written assessment and care plan | 1.26 | 1.20–1.32 | <0.001 | 1.30 | 1.23–1.37 | <0.001 |
Adjusting for patient, clinical and trust‐level factors found to be associated with research participation, i.e. age, ethnicity, having a long‐standing illness, tumour group, time since first treatment and trust type.
Significant results highlighted in bold.
Given the benefits reported to be associated with research participation in other studies (see Introduction), it was hypothesised that research participants would be more likely than non‐participants to rate their overall care positively and to provide positive responses to the other a priori questions. To investigate this hypothesis, the recently added question ‘If [research participation was discussed], did you then go on to take part in cancer research?’ was used to identify research participants. Patients who had not been offered the opportunity to participate in research or who had not taken part were classed as ‘non‐participants’. Responses to the 29 selected survey questions were binarised in accordance with the official survey guidance (Quality Health 2013); for example, for the question ‘Overall, how would you rate your care?’, excellent and very good responses were categorised as ‘positive’ and good, fair and poor as ‘not positive’ (Table S2). Univariate logistic regression was then used to describe associations between being a research participant and a positive response to the a priori questions. To control for confounding, multivariate logistic regression was subsequently used; patient, clinical and trust‐level characteristics associated with research participation at a univariate level (P < 0.10) were included in the multivariate model. All statistical analyses were carried out using stata V.12 (StataCorp LP, College Station, Texas, USA).
Qualitative data source and analysis
In‐depth interviews were used to explore the experiences of cancer patients at a large London trust with the aim of exploring how patient experience may vary across the care pathway and to develop strategies to improve care and treatment. Participation was limited to breast cancer patients (the largest tumour group nationally and at the trust) as patient experience is known to vary by tumour group (Quality Health, 2013). In order to be maximally inclusive, a convenience approach to sampling was taken. To be eligible for interview, patients had to be ≥18 years of age and receiving treatment or follow‐up care for breast cancer at the trust. Patients did not have to have participated in cancer research and there were no other eligibility restrictions, e.g. ethnicity, prognosis and stage of treatment. All consecutive patients attending outpatient oncology clinics were informed of the study by a member of the research team and the study was also advertised in chemotherapy and radiotherapy suites using posters and leaflets. All eligible women who volunteered to participate in the study were offered an interview. In total, 26 in‐depth interviews were conducted between September 2012 and February 2013.
During the interviews, the topic of cancer research was introduced using the NCPES question, ‘Since your diagnosis, has anyone discussed with you whether you would like to take part in cancer research?’ To allow direct comparison of patients' interview responses with data from NCPES 2012‐13, the interviewer did not define ‘cancer research’, instead patients were free to interpret the question based on their beliefs as to what constituted research. Patients' attitudes to cancer research, reasons for participating, what taking part had involved and the effects of research participation on experiences of care and treatment were then explored. Interviews lasted 50–70 min and were recorded and audio‐transcribed. Transcripts were then examined iteratively and relevant data related to research participation coded using Nvivo software. The study had ethical approval (City & East REC: 12/LO/0685) and all local NHS research permissions.
Results
Does research participation affect patients' rating of care and other aspects of their experience?
Overall, 19.1% (n = 12 682) of NCPES 2012‐13 respondents reported that they had participated in cancer research. Analysis of their survey responses indicates that patients who participated in research were more likely than those that did not to rate their overall care as excellent or very good (OR: 1.60, 95% CI: 1.50–1.72, P < 0.001). This association remained even after patient, clinical and trust‐level factors associated with research participation (i.e. age, ethnicity, having a long‐standing illness, tumour group, time since first treatment and trust type) were controlled for (ORadj: 1.64, 95% CI: 1.53–1.76, P < 0.001). Adjusting for confounding factors, all of the other a priori questions were also found to be positively associated with research participation (Table 1). Research participants were more likely to report feeling informed about their care and condition and that they were treated like an individual. Research participants also reported better interactions with staff, coordination of care and access to specialist staff and diagnostic tests. For example, a greater proportion had treatment from an allied health professional for their cancer (33.3% of research participants vs. 27.4% of non‐participants, P < 0.001) and patients who took part in research were almost twice as likely to have been assigned a CNS (ORadj: 1.84, 95% CI: 1.70–1.98, P < 0.001).
Characteristics of interview participants
Of the 26 women we interviewed, 18 had been asked if they would like to participate in cancer research and 10 reported that they had subsequently taken part in a research study. One woman was unsure if she had participated in research or not. Participants ranged in age from 38 to 79 years (median: 58.7 years) and most were White (n = 19). The majority of women were being treated for first occurrences of breast cancer (n = 20) and began treatment less than 5 years ago (n = 18).
What does research participation mean to cancer patients?
Patients' responses to the question ‘Since your diagnosis, has anyone discussed with you whether you would like to take part in cancer research?’ indicated that there was significant variation in individuals' interpretation of what constituted cancer research. For some, it was synonymous with participating in a clinical trial, while for others it had a broader meaning encompassing local service improvement surveys, donation of biological samples and the interview conducted as part of our study.
What, for being in trials for treatment and things like that? [Participant 1]
They just keep a sample of my genetics. Then whatever research they do in terms of what comes out, that's a sample that they can use. [Participant 2]
Someone talked to me about [the service], like what you're doing. [Participant 3]
Participation in cancer research differed among these women. One woman described making great efforts to participate in a study that required 6‐monthly bone marrow donations over a 7‐year period, while others attended multiple additional appointments for heart screening while undergoing chemotherapy treatment. Other women presented their contribution to research in a way that suggested it required little additional effort on their part. One woman described how she was participating in a trial to compare having 6 with 12 months of Herceptin treatment. She had been assigned to the standard treatment group of 12 months and took this to mean that she was not doing anything she would not be doing otherwise. Another woman who had been randomised to the control arm of an intervention study giving her standard care did not consider that she participated in research at all. Several women described how, during a routine biopsy, additional tissue was removed and would later be used for research purposes or how additional blood was collected during standard pre‐chemotherapy tests as part of a study to identify biological predictors of side effects. In this way, the boundaries between research participation and standard treatment were often not clearly differentiated by patients.
When (I) had the biopsy, they said they were going to get a little (specimen) from that tissue and that's it. [Participant 4]
It was basically that they took extra blood from me, at the time when they were taking blood before the chemo. Also just talking about what side effects you would have and how you feel. [Participant 5]
What are the patient‐reported effects of research participation?
Participation in research was seen as inherently positive and beneficial by some patients who chose to attend a teaching hospital so that they could potentially be involved in research.
One of the things that appealed to me, of course my main reasons why I wanted to be at a teaching hospital is to be involved in possibly, this or that trial [Participant 6]
Women described a range of benefits that varied with the type of research in which they participated. For the women assigned to the intervention arm of a trial comparing 1 week of radiotherapy to the standard 3, the main benefit they described was having a shortened duration of treatment.
I was fully informed by the woman who was organising the trial or administrating it, of potential side effects… then I weighed that up against having three weeks' worth of radiotherapy, which I had sort of pretty much decided “No way, thank you!” [Participant 6]
Another benefit identified by patients was access to additional monitoring. One woman who took part in a study of the effects of chemotherapy on the heart felt the regular echocardiograms were beneficial, not just for providing checks on her cardiovascular health, but also because they made her more conscious of maintaining a healthy lifestyle in general. However, the additional monitoring was not without disadvantages as she also found herself feeling anxious and inferring the worst from clinicians' actions during the monitoring procedures.
So, I have my echoes, but every time I go, that's another thing. I go and they do you on that machine. They make one little noise, I say, “What, have you seen something?” They say, “No, we're just checking” … They said my heart is fine, but that makes you eat healthier. I've been eating much more healthily. [Participant 7]
The woman who had donated bone marrow every 6 months over a 7‐year period described how participating in research had affected her relationship with her oncologist, who was also running the study. Years after the study had been completed, her arthritis was aggravated intolerably by the hormonal cancer treatment she was undergoing. While other doctors dismissed her complaints and felt she should continue with the treatment regardless, she felt that the personal relationship she had built with her oncologist meant that he listened to her complaints seriously and took steps to adjust her treatment so as to improve her quality of life.
I was under his trial for 7 years to‐ing and fro‐ing and I put myself out in lots of different ways and I used to see a lot of him. I think he knew that I wasn't making a fuss. [Participant 8]
A good relationship with staff meant one woman enrolled in a trial did not have to wait to have her routine pre‐chemotherapy blood tests done; instead these were done by the research nurse at the same time as the trial samples were being collected.
They cut a few corners for me every now and again. The blood tests, I always have them done anyway. So what [the research nurse] did was, she did them herself, so I didn't have to go down to the phlebotomy and have it done there. So I sort of gained a little bit on that really, because she did the whole lot in one go. [Participant 9]
Among the women who took part in studies that did not involve ongoing monitoring or a change in their treatment plan, the reported effects of their participation in research were more abstract; for example, the women who had taken part in interviews or surveys felt glad to have the opportunity to express their opinions or tell their story. There was also a sense of pride among the women who had participated in research and a feeling that they were helping others. This was especially clear in one case where the drug a woman had trialled subsequently became standard treatment. A young woman who donated tissue samples for genetic research also gained comfort from her belief that, if the research team made any breakthroughs or discoveries, she may potentially benefit herself in the future from improved treatments and therapies specific to her tumour type.
Because if you don't have people doing trials then they can't do the research properly, can they, and then you can't get better treatment. [Participant 5]
If it's going to help other people, why not? I'm not selfish… It's for helping others; I don't mind. [Participant 4]
I did my five years on Arimidex. Thanks to me … and thousands of others it got its licence. So by the time I was … finished all my other treatments, Arimidex was top of the list. [Participant 10]
Discussion
To the authors' knowledge, this is the first large study to explore the effects of research participation on cancer patient experience using quantitative and qualitative data sources. As hypothesised, patients who took part in cancer research reported a more positive patient experience. Patients who participated in research were more likely than those who did not to describe their overall care and treatment as very good or excellent and to rate all of the a priori aspects of patient experience in our conceptual model positively. For example, research participants had more positive interactions with staff, better coordination of care and were more likely to feel that they were treated as individuals. However, there was no common understanding of what constitutes cancer research, and no clear delineation between research participation and standard care, from the patient perspective.
A strength of this study is that it analyses data from a large, national sample of cancer patients and is not limited to patients with poor prognoses. Furthermore, the interview data provide insight into patients' understanding of what constitutes cancer research and how research participation affects their experience (though as interviews were limited to breast cancer patients, these findings may not be generalisable to the entire patient population). The other main limitations of this study relate to potential biases in the data which may also affect the generalisability of our study findings. For example, ethnic minorities and young patients are known to be less likely to respond to NCPES and are therefore under‐represented in the quantitative data set analysed in this study. Ethnic minorities were also under‐represented in the interview sample due to the approach used for recruitment. Furthermore, negative aspects of research participation may have been under‐reported by participants as interviewers did not specifically prompt on this topic.
The positive effect of research participation on relationships with staff was evident in both NCPES responses and our interviews. The more positive interactions with staff associated with participation may be due to the time and attention staff had for patients as a result of their involvement in research, benefits that have been reported elsewhere (Maslin‐Prothero 2000; Hussain‐Gambles et al. 2004). The quality of relationships that research staff and participants were able to develop may explain why research participants were more likely to report feeling that they were treated as individuals by staff. In NCPES data, participants were more likely to have been asked what name they preferred to be called and to have their views taken into account when deciding on treatment and patients also described better relationships with staff in the interviews. In particular, the woman who had been involved in a 7‐year trial described how her participation enabled her oncologist to get to know her as an individual. The effects of this relationship were evident years after her involvement in research, influenced subsequent treatment decisions and improved her quality of life. Access to non‐standard care and treatment was one of the benefits of research participation evident from analysis of NCPES data, with participants more likely to have access to specialist staff such as dieticians. In our patient interviews, the effects of research participation on access to non‐standard care and treatment were also evident though they often remained implicit in patients' descriptions of their care and treatment overall. For example, some patients described how taking part in a trial allowed them to have a shorter than standard duration of radiotherapy, something they would have preferred ordinarily if the choice had been available. Taking part in a study (even one that involved randomisation) provided patients with the opportunity to influence their treatment plan.
From the NCPES data, patients who took part in research were more likely to have been better informed by staff about some elements of their care and treatment (specifically tests) and to feel that they received the right amount of information about their condition and treatment overall, perhaps because staff were more likely to view participants as individuals or partners and therefore to make greater efforts to listen and explain carefully when dealing with them. It is also possible that patients participating in research felt more empowered to ask questions and seek information about their care and treatment. Research participants also benefited from better coordination of care, which may be attributable to closer follow‐up or better organisation of care within a research study (Catt et al. 2011). There was no mention of having better coordination of care or receiving more information about their condition in our interviews. However, the open approach taken in our in‐depth interviews may have contributed to the narrow range of effects described by patients as women were not prompted to comment on any specific effects of research participation. This methodology contrasts with many other studies that are survey or questionnaire‐based, where patients are asked to select from, rank or rate pre‐specified items, a procedure that introduces its own bias. The limited descriptions of the effects of research can also be attributed to the fact that there was no common understanding of what constitutes cancer research among the women we interviewed; for example, one woman assigned to the control arm of a trial did not think she had participated in research and while most women who participated in our interview study considered this to be ‘cancer research’, one woman did not. There was also no clear delineation between research participation and standard care and treatment from the patients' perspective as the research reported often took place in the same location as standard treatment and involved the same staff. One woman was unsure if she had participated in research or not, which is not uncommon among cancer patients (Joffe et al. 2001). It is unsurprising therefore that it was difficult for patients to describe the effects of research participation when they could not readily identify it or disentangle it from their normal care.
National Cancer Patient Experience Survey does not ask about motivations for participating in cancer research, something which was explored in our interviews. Being able to help others was the most frequently mentioned effect of research participation and was also a strong motivator to take part in a study. This desire to affect other patients in a positive way through involvement in research is often labelled altruism (Catt et al. 2011). It is more common among patients with good prognoses, such as the majority of women that we interviewed, and some have argued that this is because they are well enough to think beyond their own personal situations (Hallowell et al. 2010). However, when thinking about helping others, the nature of cancer as a potentially recurrent or lifelong illness with strong familial and genetic elements must also be considered. In a context where the possibility of future illness threatens women who have been treated for cancer, as well as their close family members, this desire to help others could also be viewed as a form of caring. By participating in research trials, cancer patients are hoping to improve care and treatment for future patients, who may include themselves or their relatives.
Analysis of both quantitative and qualitative data sources suggests that taking part in research is beneficial to patient experience with participants rating their care more positively. While the effects of taking part in research varied with the nature of the study, they did not appear to be limited to patients involved in clinical trials, those who took part in interviews or made one‐off donations of samples also appeared to benefit. The more positive patient experience evident in our study may be due to inherent differences among those who participate in research, with those who choose to take part being optimists (Agrawal et al. 2006) and therefore more likely to rate their experiences positively. However, it may also be due to differences in the provision of care and treatment experienced by patients as a result of being involved in cancer research. Further work to explore how participation positively affects patient experience would be useful to develop strategies aimed at improving care and treatment for all patients, regardless of whether or not they choose or have the opportunity to take part in research.
Funding
The research was supported by the Imperial College Healthcare Charity (7006/P31U) and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, NIHR or Department of Health. Halth.
Supporting information
Table S1. Characteristics of respondents and the hospital trusts they attended.
Table S2. NCPES 2012‐13 questions related to a priori aspects of patient experience.
MC Grath‐Lone L., Ward H., Schoenborn C. & Day S. (2016) European Journal of Cancer Care 25,1056–1064 The effects of cancer research participation on patient experience: a mixed‐methods analysis
References
- Agrawal M., Grady C., Fairclough D., Meropol N., Maynard K. & Emanuel E. (2006) Patients' decision‐making process regarding participation in phase I oncology research. Journal of Clinical Oncology 24, 4479–4484. [DOI] [PubMed] [Google Scholar]
- Barnett M. (2001) Interviewing terminally ill people: is it fair to take their time? Palliative Medicine 15, 157–158. [DOI] [PubMed] [Google Scholar]
- Bone A., McGrath‐Lone L., Day S. & Ward H. (2014) Inequalities in the care experiences of patients with cancer: analysis of data from the National Cancer Patient Experience Survey 2011‐2012. BMJ Open 4, e004567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt S., Langridge C., Fallowfield L., Talbot D.C. & Jenkins V. (2011) Reasons given by patients for participating, or not, in Phase 1 cancer trials. European Journal of Cancer 47, 1490–1497. [DOI] [PubMed] [Google Scholar]
- Cox K. (1999) Researching research: patients' experiences of participation in phase I and II anti‐cancer drug trials. European Journal of Oncology Nursing 3, 143–152. [Google Scholar]
- Department of Health (2013) Cancer Patient Experience Survey 2013 National Report. Available at: http://www.quality-health.co.uk/resources/surveys/national-cancer-experience-survey/2013-national-cancer-patient-exerience-survey/2013-national-cancer-patient-experience-survey-reports (accessed May 30, 2014).
- Department of Health (2012) Improving Outcomes?: A Strategy for Cancer Second Annual Report 2012. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/136551/Improving_outcomes_second_annual_report.pdf (accessed May 30, 2014).
- Hallowell N., Cooke S., Crawford G., Lucassen L., Parker M. & Snowden C. (2010) An investigation of patients' motivations for their participation in genetics‐related research. Journal of Medical Ethics 36, 37–45. [DOI] [PubMed] [Google Scholar]
- Hussain‐Gambles M., Leese B., Atkin K., Brown J., Mason S. & Tovey P. (2004) Involving South Asian patients in clinical trials. Health Technology Assessment 8, 1–128. [DOI] [PubMed] [Google Scholar]
- Hutchison C. (1998) Phase I trials in cancer patients: participants' perceptions. European Journal of Cancer Care 7, 15–22. [DOI] [PubMed] [Google Scholar]
- Joffe S., Cook E.F., Cleary P., Clark J. & Weeks J. (2001) Quality of informed consent in cancer clinical trials: a cross‐sectional survey. Lancet 358, 1772–1777. [DOI] [PubMed] [Google Scholar]
- Kemeny M.M., Peterson B., Kambith A., Muss H., Wheeler J., Levine E., Bartlett N., Fleming G. & Cohen H. (2003) Barriers to clinical trial participation by older women with breast cancer. Journal of Clinical Oncology 21, 2268–2275. [DOI] [PubMed] [Google Scholar]
- Maslin‐Prothero S.E. (2000) Factors affecting recruitment to breast cancer clinical trials?: an examination of the British Association of Surgical Oncology II trial and the International Intervention Study Breast Cancer. PhD Thesis, University of Nottingham. Available at: http://www.worldcat.org/title/factors-affecting-recruitment-to-breast-cancer-clinical-trials-an-examination-of-the-british-association-of-surgical-oncology-ii-trial-and-the-international-breast-cancer-intervention-study/oclc/53571286 (accessed May 30, 2014).
- Moore S. (2001) A need to try everything: patient participation in phase I trials. Journal of Advanced Nursing 33, 738–747. [DOI] [PubMed] [Google Scholar]
- Nurgat Z.A., Craig W., Campbell N.C., Bisset J.D., Cassidy J. & Nicolson N.C. (2005) Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. British Journal of Cancer 92, 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppercorn J.M., Weeks J., Cook E.F. & Joffe S. (2004) Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet 363, 263–270. [DOI] [PubMed] [Google Scholar]
- Quality Health (2013) National Cancer Patient Experience Survey Programme Guidance Manual. Available at: http://discover.ukdataservice.ac.uk/catalogue?sn=7400 (accessed May 30, 2014).
- Shipman C., Hotopf M., Richardson A., Murray S., Koffamn J., Speck P. & Higginson I.J. (2008) The views of patients with advanced cancer regarding participation in serial questionnaire studies. Palliative Medicine 22, 913–920. [DOI] [PubMed] [Google Scholar]
- Tolmie E.P., Mongal M., Louden G., Lindsay G. & Gaw A. (2004) Understanding why older people participate in clinical trials: the experience of the Scottish PROSPER participants. Age and Ageing 33, 374–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of respondents and the hospital trusts they attended.
Table S2. NCPES 2012‐13 questions related to a priori aspects of patient experience.


