Abstract
Aims
In this analysis, we utilized data from PARADIGM‐HF to test the hypothesis that participants who exhibited any dose reduction during the trial would have similar benefits from lower doses of sacubitril/valsartan relative to lower doses of enalapril.
Methods and results
In a post‐hoc analysis from PARADIGM‐HF, we characterized patients by whether they received the maximal dose (200 mg sacubitril/valsartan or 10 mg enalapril twice daily) throughout the trial or had any dose reduction to lower doses (100/50/0 mg sacubitril/valsartan or 5/2.5/0 mg enalapril twice daily). The treatment effect for the primary outcome was estimated, stratified by dose level using time‐updated Cox regression models. In the two treatment arms, participants with a dose reduction (43% of those randomized to enalapril and 42% of those randomized to sacubitril/valsartan) had similar baseline characteristics and similar baseline predictors of the need for dose reduction. In a time‐updated analysis, any dose reduction was associated with a higher subsequent risk of the primary event [hazard ratio (HR) 2.5, 95% confidence interval (CI) 2.2–2.7]. However, the treatment benefit of sacubitril/valsartan over enalapril following a dose reduction was similar (HR 0.80, 95% CI 0.70–0.93, P < 0.001) to that observed in patients who had not experienced any dose reduction (HR 0.79, 95% CI 0.71–0.88, P < 0.001).
Conclusions
In PARADIGM‐HF, study medication dose reduction identified patients at higher risk of a major cardiovascular event. The magnitude of benefit for patients on lower doses of sacubitril/valsartan relative to those on lower doses of enalapril was similar to that of patients who remained on target doses of both drugs.
Keywords: Chronic heart failure, Neprilysin inhibitor, Clinical trial, Sacubitril, Valsartan
Introduction
In the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial, sacubitril/valsartan (LCZ696) reduced the risk of cardiovascular death and of heart failure hospitalization compared with enalapril in patients with chronic heart failure.1 Sacubitril/valsartan, a complex containing the neprilysin inhibitor sacubitril and the ARB valsartan, augments endogenous compensatory vasoactive peptides by inhibiting their breakdown, and, in addition, blocks the renin–angiotensin system.2 The active run‐in phase ensured all patients were titrated to a target dose of enalapril 10 mg twice daily and then sacubitril/valsartan 200 mg twice daily before randomization. The majority of patients remained on target doses after randomization, with final mean achieved doses of 375 mg daily and 18.9 mg daily respectively.1 Nevertheless, not all patients were maintained on target doses of study medication during long‐term follow‐up. Whether sacubitril/valsartan confers benefit at lower than target doses similar to those taking lower than target doses of enalapril is unknown.
We utilized data from PARADIGM‐HF to test the hypothesis that participants who exhibited any dose reduction during the trial would have similar benefits from lower doses of sacubitril/valsartan relative to lower doses of enalapril.
Methods
Study design and patient selection
PARADIGM‐HF was a double‐blind, randomized, active controlled trial designed to assess the impact of the angiotensin receptor neprilysin inhibitor sacubitril/valsartan compared with enalapril on cardiovascular mortality and heart failure hospitalizations in patients with LVEF ≤40% and NYHA functional class II–IV heart failure. The protocol was approved at each participating site by an ethics committee or institutional review board. All participants provided written informed consent in accordance with established guidelines for the protection of human subjects.
Eligible subjects had at least mildly elevated natriuretic peptide levels and were treated with stable doses of ACE inhibitors or ARBs and beta‐adrenergic receptor blockers for at least 4 weeks prior to trial enrolment. Patients with symptomatic hypotension, estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, potassium concentration >5.2 mmol/L at screening, or history of angioedema were excluded. The study design and detailed inclusion and exclusion criteria have been previously reported.3
Participants underwent sequential single‐blind run‐in phases with enalapril at a dose of at least 10 mg twice daily for 2 weeks, followed by sacubitril/valsartan, first dosed at 100 mg twice daily, then 200 mg twice daily for 4–6 weeks. They were then randomized to receive enalapril 10 mg twice daily or sacubitril/valsartan 200 mg twice daily, and were followed for a median duration of 27 months. During the double‐blind phase of the study, investigators were allowed to down‐titrate study drug to one of three lower study medication dose levels that corresponded to 100 mg sacubitril/valsartan twice daily or 5 mg enalapril twice daily (dose level 2), or 50 mg sacubitril/valsartan twice daily or 2.5 mg enalapril twice daily (dose level 1). Patients could also discontinue study medication, temporarily, or permanently (dose level zero). At the time of dose reduction, study personnel recorded information regarding the reason for study medication dosage change on case report forms.
Statistical analyses
Participants were categorized according to whether or not they received the target dose of study drug for the entire duration of the double‐blind portion of follow‐up. Baseline characteristics were compared between participants who experienced a study medication dose reduction vs. those who were maintained on target study medication doses throughout the duration of the study regardless of treatment assignment. Characteristics were also compared among participants with a dose reduction between treatment arms. Between‐group assessments were performed using t‐tests for continuous variables, and χ2 or Fisher's exact tests, as appropriate, for categorical variables.
We constructed two time‐updated covariates: the first time‐updated covariate indicated any prior dose reduction, updated when a dose change occurred from the maximum dose to any other lower dose level, and was used to distinguish those patients with sustained maximum dosage from patients with any reduction in dose. The second time‐updated covariate represented ‘cumulative average’ dose received, updated daily after randomization. Risk factors for the likelihood of receiving subtarget dosage at any point during follow‐up were determined with a forward stepwise selection process using the following baseline characteristics: age, sex, geographic region, body mass index, NT‐proBNP, EF, eGFR, NYHA functional class, history of diabetes mellitus, myocardial infarction, and use of ACE inhibitors or ARBs, beta‐adrenergic blockers, mineralocorticoid receptor antagonists, and diuretics at screening. Potential interactions between predictors of dose reduction and treatment assignment were investigated by using a stepwise selection process separately for each treatment group. Treatment covariate interaction terms were created for all covariates found to be significant in either treatment arm, and interaction terms were considered to be significant if they could be added to the base model with a P‐value <0.05. To investigate the relationship between dose reduction status and the effectiveness of sacubtril/valsartan compared with enalapril, the hazard ratio (HR) for sacubitril/valsartan relative to enalapril for the primary outcome was estimated, censored at dose reduction, and separately starting after dose reduction. A sensitivity analysis was performed to examine effectiveness of sacubitril/valsartan compared with enalapril that focused on events occurring at least 30 days following dose reduction. To investigate the relationship between study medication dose levels and the effectiveness of sacubitril/valsartan compared with enalapril, time‐updated Cox proportional hazards regression models were used stratified by dose levels. We further assessed the occurrence of dose reduction by geographic region, and whether any differences were found in the treatment effect subsequent to dose reductions by region. A P‐value of <0.05 was considered statistically significant. All analyses were completed using Stata, version 13 (StataCorp LP, College Station, TX, USA).
Results
Out of 8399 participants in the PARADIGM‐HF trial, >99.9% in both arms achieved the target dose after randomization. In an intent to treat analysis, 43% of patients in the enalapril arm and 42% of patients in the sacubitril/valsartan arm reduced their dose at any time after randomization (P = 0.53). Median time to dose reduction was 255 days [interquartile range (IQR) 70, 516] for enalapril vs. 249 days (IQR 64, 506) for sacubitril/valsartan (P = 0.54). Of those with a dose reduction, 1332 (37.5%) subsequently returned to target study medication doses, and this occurred more frequently in patients randomized to sacubitril/valsartan than with enalapril (39.8% vs. 35.3%, P = 0.005). Individuals in both groups who experienced a dose reduction (Table 1) were older, had worse NYHA functional class, and higher serum creatinine levels and NT‐proBNP concentrations at baseline. These participants were also more likely to be taking diuretics and have implantable cardioverter defibrillators or CRT devices. The frequency of dose reduction varied by region (Supplementary material online, Table S1), such that dose reductions were most frequently seen in North America and least observed in Asia. Among those with a dose reduction, there were no significant differences in baseline characteristics by treatment assignment (Table 2).
Table 1.
Baseline characteristics by dose reduction status
| Characteristic | Any dose reduction (n = 3549) | No dose reduction (n = 4850) | P‐value |
|---|---|---|---|
| Baseline age, years | 65 ± 12 | 63 ± 11 | <0.001 |
| Female sex (%) | 764 (21.5%) | 1068 (22.0%) | 0.59 |
| Caucasian (%) | 2406 (67.8%) | 3138 (64.7%) | 0.003 |
| Baseline BMI, kg/m2 | 28.2 ± 5.6 | 28.2 ± 5.5 | 0.86 |
| NYHA class | <0.001 | ||
| I | 139 (3.9%) | 250 (5.2%) | |
| II | 2454 (69.3%) | 3465 (71.5%) | |
| III | 929 (26.2%) | 1089 (22.5%) | |
| IV | 20 (0.6%) | 40 (0.8%) | |
| Left ventricular ejection fraction (%) | 29.4 ± 6.5 | 29.5 ± 6.0 | 0.49 |
| Ischaemic aetiology (%) | 2208 (62.2%) | 2828 (58.3%) | <0.001 |
| History of hypertension (%) | 2528 (71.2%) | 3412 (70.4%) | 0.38 |
| History of DM (%) | 1332 (37.5%) | 1575 (32.5%) | <0.001 |
| Prior use of ACE inhibitor (%) | 2729 (76.9%) | 3803 (78.4%) | 0.10 |
| Systolic BP, mmHg | 120.7 ± 15.8 | 121.9 ± 14.9 | <0.001 |
| Heart rate (b.p.m.) | 72.7 ± 12.4 | 72.1 ± 11.7 | 0.035 |
| Serum creatinine, mg/dL | 1.18 ± 0.32 | 1.08 ± 0.27 | <0.001 |
| NT‐proBNP, pg/mL (IQR) | 1833 (976, 3834) | 1473 (834, 2877) | <0.001 |
| Current medications | |||
| Diuretics | 2912 (82.1%) | 3826 (78.9%) | <0.001 |
| Beta‐blockers | 3262 (91.9%) | 4549 (93.8%) | <0.001 |
| MRA | 1918 (54.0%) | 2753 (56.8%) | 0.013 |
| Digoxin | 1077 (30.3%) | 1462 (30.1%) | 0.84 |
| ICD | 657 (18.5%) | 586 (12.1%) | <0.001 |
| CRT | 312 (8.8%) | 262 (5.4%) | <0.001 |
BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist.
Table 2.
Baseline characteristics among those with dose reduction, by treatment group
| Characteristic | Sacubitril/valsartan (n = 1755) | Enalapril (n = 1794) | P‐value |
|---|---|---|---|
| Baseline age, years | 65.3 ± 11.9 | 65.2 ± 11.2 | 0.82 |
| Female sex (%) | 359 (20.5%) | 405 (22.6%) | 0.12 |
| Caucasian (%) | 1200 (68.4%) | 1206 (67.2%) | 0.46 |
| Baseline BMI, kg/m2 | 28.1 ± 5.8 | 28.2 ± 5.4 | 0.65 |
| NYHA class | 0.72 | ||
| I | 65 (3.7%) | 74 (4.1%) | |
| II | 1222 (69.9%) | 1232 (68.7%) | |
| III | 454 (26.0%) | 475 (26.5%) | |
| IV | 8 (0.5%) | 12 (0.7%) | |
| Left ventricular ejection fraction (%) | 29.5 ± 6.5 | 29.4 ± 6.6 | 0.57 |
| Ischaemic aetiology (%) | 1104 (62.9%) | 1104 (61.5%) | 0.40 |
| History of hypertension (%) | 1260 (71.8%) | 1268 (70.7%) | 0.46 |
| History of DM (%) | 661 (37.7%) | 671 (37.4%) | 0.87 |
| Prior use of ACE inhibitor (%) | 1363 (77.7%) | 1366 (76.1%) | 0.28 |
| Systolic BP, mmHg | 120.7 ± 15.8 | 120.7 ± 15.8 | 0.95 |
| Heart rate (b.p.m.) | 72.6 ± 12.5 | 72.7 ± 12.3 | 0.89 |
| Serum creatinine, mg/dL | 1.18 ± 0.32 | 1.18 ± 0.32 | 0.74 |
| NT‐proBNP, pg/mL (IQR) | 1829 (992, 3637) | 1839 (966, 3995) | 0.93 |
| Current medications | |||
| Diuretics | 1439 (82.0%) | 1473 (82.1%) | 0.93 |
| Beta‐blockers | 1621 (92.4%) | 1641 (91.5%) | 0.33 |
| MRA | 930 (53.0%) | 988 (55.1%) | 0.21 |
| Digoxin | 509 (29.0%) | 568 (31.7%) | 0.09 |
| ICD | 335 (19.1%) | 322 (17.9%) | 0.38 |
| CRT | 165 (9.4%) | 147 (8.2%) | 0.20 |
BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist.
In a multivariable regression model, many significant predictors of dose reduction were identified, including: higher serum creatinine, geographic region (North America, Latin America, and Western Europe), higher NT‐proBNP, higher heart rate, older age, and lower systolic blood pressure (Table 3). There was no significant misspecification (P = 0.12), and the associated area under the curve (AUC) was 0.64. Of 11 statistically significant predictors, one nominal interaction with randomized treatment was identified, in which history of myocardial infarction (MI) was a stronger predictor of dose reduction in the patients randomized to LCZ696 [odds ratio (OR) = 1.31] than in those randomized to enalapril (OR = 1.06, P for interaction = 0.021). In an analysis of 7156 study participants with available data of ACE inhibitor or ARB doses at the time of screening, there was no interaction between dose at screening and randomized treatment arm with respect to subsequent dose reduction (P for interaction = 0.26). Reasons reported by site investigators for dose reductions differed between the sacubitril/valsartan and enalapril arms, with hypotension responsible for more dose reductions among those taking sacubitril/valsartan, and cough more common in those randomized to enalapril (Table 4). A higher proportion of participants in the sacubitril/valsartan group compared with enalapril were re‐up‐titrated to target doses of study medication after down‐titration for hypotension or hyperkalaemia. For cough, a similar proportion of participants were re‐up‐titrated in each treatment arm (Table 4).
Table 3.
Multivariable predictors of any study medication dose reduction
| Characteristic | OR | 95% CI | χ2 |
|---|---|---|---|
| Serum creatinine (per mg/dL) | 2.38 | 2.01–2.82 | 101.8 |
| Age (per 10 years above 60) | 1.27 | 1.19–1.36 | 53.6 |
| Region (reference = Central Europe) | 49.9 | ||
| North America | 1.77 | 1.46–2.14 | |
| Latin America | 1.20 | 1.04–1.38 | |
| Western Europe and other | 1.25 | 1.11–1.42 | |
| Asia‐Pacific | 0.90 | 0.78–1.04 | |
| NT‐proBNP (per log) | 1.16 | 1.11–1.22 | 38.6 |
| SBP (per 10 mmHg decrease below 120) | 1.21 | 1.13–1.29 | 34.1 |
| Heart rate (per 10 b.p.m.) | 1.09 | 1.05–1.14 | 20.6 |
| NYHA class (reference = Class II) | 12.6 | ||
| I | 0.82 | 0.66–1.03 | |
| III | 1.15 | 1.03–1.29 | |
| IV | 0.68 | 0.39–1.20 | |
| History of MI | 1.17 | 1.07–1.29 | 11.2 |
| History of DM | 1.17 | 1.07–1.29 | 11.0 |
| Beta‐blocker | 0.76 | 0.64–0.91 | 9.4 |
| Sex: female | 1.17 | 1.04–1.32 | 7.3 |
CI, confidence interval; DM, diabetes mellitus; OR, odds ratio; MI, myocardial infarction; SBP, systolic blood pressure.
Table 4.
Reasons for dose reductions and proportion re‐uptitrated, by treatment group
| Reason for dose reduction | Sacubitril/valsartan (n = 1523)a | Enalapril (n = 1524) | P‐value |
|---|---|---|---|
|
Hyperkalaemia (proportion re‐up‐titrated) |
102 (6.7%) 61/102 (60%) |
124 (8.1%) 70/124 (56%) |
0.13 0.61 |
|
Hypotension (proportion re‐up‐titrated) |
330 (21.7%) 118/330 (36%) |
248 (16.3%) 67/248 (27%) |
<0.001 0.026 |
|
Patient request (proportion re‐up titrated) |
225 (14.8%) 27/225 (12%) |
230 (15.1%) 21/230 (9%) |
0.81 0.32 |
|
Renal dysfunction (proportion re‐up‐titrated) |
133 (8.7%) 55/133 (41%) |
150 (9.8%) 42/150 (28%) |
0.29 0.018 |
|
Angioedema (or angioedema‐like event) (proportion re‐up‐titrated) |
6 (0.4%) 5/6 (83%) |
4 (0.3%) 3/4 (75%) |
0.53 0.75 |
|
Cough (proportion re‐up‐titrated) |
28 (1.8%) 16/28 (57%) |
66 (4.3%) 34/66 (52%) |
<0.001 0.62 |
|
Other (proportion re‐uptitrated) |
556 (36.5%) 328/556 (59%) |
576 (37.8%) 330/576 (57%) |
0.46 0.56 |
Numbers excluded participants whose dose reduction led to permanent discontinuation of study medication.
Any dose reduction, regardless of treatment assignment, was associated with a higher subsequent risk of the primary event [HR 2.5, 95% confidence interval (CI) 2.2–2.7; Figure 1]. When the primary outcome events were censored at the time of dose reduction, participants taking sacubitril/valsartan had fewer events relative to enalapril prior to dose reduction (HR 0.79, 95% CI 0.71–0.88; Figure 2 A). In a landmark analysis beginning at the time of dose reduction, we observed a similar magnitude of benefit (HR 0.80, 95% CI 0.70–0.93; Figure 2 B). Similar results were observed after adjustment for characteristics reported in Table 1 (HR 0.80, 95% CI 0.69–0.92). A sensitivity analysis capturing events beginning 30 days after dose reduction revealed a similar reduction of events in participants taking sacubitril/valsartan (HR 0.79, 95% CI 0.67–0.92). Likewise, similar results were noted when the analysis was repeated while censoring participants who permanently discontinued study medication (unadjusted HR 0.81, 95% CI 0.69–0.95, P = 0.008; adjusted HR 0.82, 95% CI 0.70–0.95, P = 0.010). The treatment effect subsequent to dose reductions was not different between regions (P for interaction = 0.20). In an analysis that considered only patients who experienced an initial dose reduction but who did not subsequently permanently discontinue study drug or return to target dose, the association between sacubitril/valsartan and the primary outcome remained unchanged (unadjusted HR 0.78, 95% CI 0.65–0.95; adjusted HR 0.81, 95% CI 0.67–0.98). Patients who permanently discontinued were at lower risk of a primary event in the first 30 days following discontinuation if they were in the sacubitril/valsartan arm (HR 0.52, 95% CI 0.26–1.05, P = 0.07), that declined subsequent to 30 days (HR 0.84, 95% CI 0.55–1.28, P = 0.42), although there was no difference between treatment groups for mortality following discontinuation (HR 0.97, 95% CI 0.74–1.27).
Figure 1.
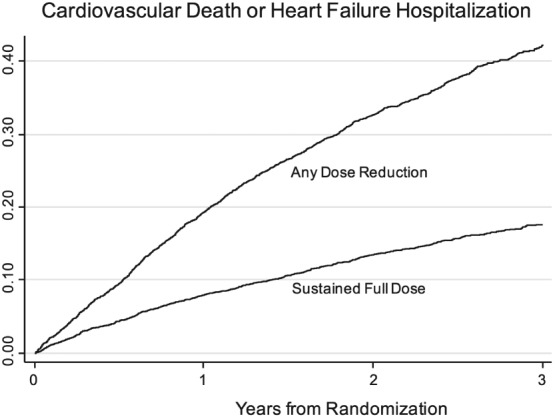
Kaplan–Meier curves showing primary outcome events by dose reduction status. Participants with a dose reduction had a higher risk of the primary event compared with those who remained on full study medication doses.
Figure 2.
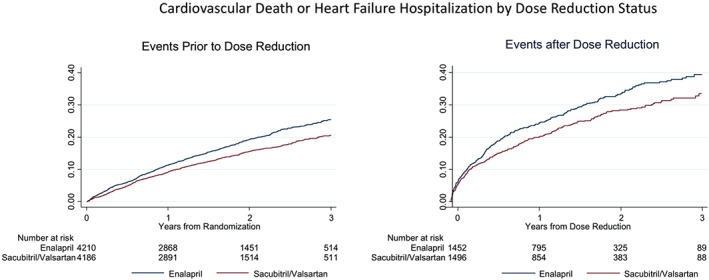
(A) Kaplan–Meier curves showing primary outcome events censored at dose reduction by treatment assignment. Individuals taking sacubitril/valsartan had fewer events compared with the enalapril group [hazard ratio (HR) 0.79, 95% confidence interval (CI) 0.71–0.88]. (B) Kaplan–Meier curves showing primary outcome events following dose reduction by treatment assignment. Individuals randomized to sacubitril/valsartan had fewer events relative to enalapril after dose reduction (HR 0.80, 95% CI 0.70–0.93).
When the risk for the primary outcome event was examined by cumulative mean dose level of study medications, those taking lower mean doses of sacubitril/valsartan had fewer events compared with participants taking lower mean doses of enalapril (Figure 3). As such, the relative reduction in events with sacubitril/valsartan compared with enalapril did not differ according to the cumulative mean dose of study drug received (P for interaction = 0.99). Nearly identical results were found when the less than target doses of sacubitril/valsartan were compared with less than target doses of enalapril with respect to cardiovascular death alone.
Figure 3.
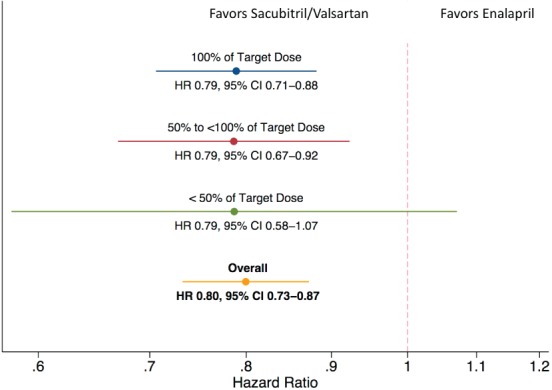
Hazard ratios (HR; sacubitril/valsartan relative to enalapril) of the primary outcome measure by time‐updated mean dose post‐randomization. Participants taking lower than target sacubitril/valsartan doses had a lower risk of the primary event compared with those taking lower than target doses of enalapril. CI, confidence interval.
Discussion
In patients with heart failure and reduced EF enrolled in the PARADIGM‐HF study, we found that dose reductions were frequent in both study medication groups, and that patients who required a dose reduction were at higher risk for major cardiovascular events than patients who did not have a dose reduction. Despite reducing the dose, the magnitude of benefit for patients on lower doses of sacubitril/valsartan relative to those on lower doses of enalapril was similar to that of patients who remained on target dose. These data suggest that patients with heart failure with reduced EF who are unable to tolerate target doses of sacubitril/valsartan or enalapril still benefit from lower doses of sacubitril/valsartan compared with lower doses of enalapril.
The design of PARADIGM‐HF, which included an active run‐in phase, during which patients were titrated to target doses of each study medication, ensured that a higher proportion of patients remained on enalapril 10 mg twice daily than in any previous trial, and that patients in PARADIGM‐HF attained the highest average dose of enalapril of any large trial.4, 5 Nevertheless, dose reductions in PARADIGM‐HF were frequent and were associated with a variety of patient characteristics. More advanced age, lower systolic blood pressure, more severe symptoms of heart failure, or greater renal impairment were more common among those who experienced a dose reduction, suggesting more significant disease burden or increased frailty, and these factors were similar between treatment arms, as was time to dose reduction. These findings are consistent with other studies that have reported that age and systolic blood pressure were predictors of failing to achieve target doses of neurohormonal blockers.6, 7, 8, 9 In an analysis of the IMPROVE‐HF study, characteristics associated with lower than target beta‐blocker doses included older age, Caucasian heritage, lower systolic blood pressure, and an ischaemic heart failure aetiology.6 Older adults with co‐morbidities were less likely to achieve target doses of ACE inhibitors and beta‐blockers in a retrospective cohort study of primary care practices in the UK.10
We found that the benefit of sacubitril/valsartan relative to enalapril was maintained even at lower than target doses. In the Metoprolol CR/XL Randomized Intervention Trial in Heart failure (MERIT‐HF), patients unable to achieve target doses of metoprolol had a higher event rate but a similar benefit from beta‐blockade compared with patients who were successfully titrated to target doses.11 In the Carvedilol Or Metoprolol European Trial (COMET) study, target doses of carvedilol and metoprolol tartrate were reached in 75% and 78% of participants, respectively. Failure to achieve target doses was associated with worse outcomes, but the benefit of carvedilol relative to metoprolol in lowering all‐cause mortality was maintained at lower doses of beta‐blocker.12 Data from several heart failure registries show that despite guideline recommendations, less than half of patients are treated with target doses for both ACE inhibitors and beta‐blockers.9, 13 Thus, despite the very large number of patients achieving target doses in PARADIGM‐HF, the number of patients who will achieve these targets in a real‐world setting will probably be lower. Nevertheless, our data suggest that even if dose reduction is indicated, sacubitril/valsartan remains effective compared with enalapril at reducing the composite of cardiovascular death or heart failure hospitalizations.
This analysis was post‐hoc and thus needs to be interpreted with caution. In particular, our comparison of patients who had dose reductions was a post‐randomization comparison; yet it is noteworthy that the baseline characteristics of the patients who underwent a dose reduction in the two treatment arms were similar. Furthermore, in earlier studies exploring achieved dose, MERIT‐HF and COMET, patients received subtarget doses of the study medication because they failed to be successfully up‐titrated during the first few weeks of the trial, and, thus, such a failure may have been a reflection of patient frailty. In the PARADIGM‐HF trial, only patients demonstrated to be able to tolerate target doses of the study medications could be randomized. Despite this, a substantial proportion of patients did require dose reductions following months of sustained treatment. Yet, the reasons for post‐randomization dose reduction in patients taking sacubitril/valsartan or enalapril were similar to the reasons for intolerance of target doses of these drugs during the run‐in period. If dose reduction resulted in any diminution of the advantage of sacubitril/valsartan over enalapril, we found no evidence for this in the patients studied in the PARADIGM‐HF trial.
In conclusion, in patients with heart failure with reduced EF enrolled in the PARADIGM‐HF trial, dose reductions of study medications were frequent, but the efficacy of sacubitril/valsartan relative to enalapril was maintained even among participants taking lower doses. These data suggest that patients taking less than target doses of these drugs would still derive greater benefit from sacubitril/valsartan when compared with enalapril.
Conflict of interest: O.V., J.R.T., S.D.S., M.P., J.J.V.M., M.R.Z., O.V., J.R.T., S.D.S., J.J.V.M., M.R.Z. J.R., A.S.D, K.S., J.R., and A.S.D have all consulted for Novartis. O.V., J.R.T., S.D.S., J.J.V.M., M.R.Z. J.R., and A.S.D. received research support from Novartis. V.S. and M.L. are employees of Novartis. B.C. has no relevant disclosures to declare.
Supporting information
Table S1. Dose reduction of study medication by region.
References
- 1. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin–angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail 2014;2:663–670. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2013;15:1062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 5.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 6. Gheorghiade M1, Albert NM, Curtis AB, Thomas Heywood J, McBride ML, Inge PJ, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW, Fonarow GC. Medication dosing in outpatients with heart failure after implementation of a practice‐based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail 2012;18:9–17. [DOI] [PubMed] [Google Scholar]
- 7. Heywood JT, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Gheorghiade M, Inge PJ, McBride ML, Mehra MR, O'Connor CM, Reynolds D, Walsh MN. Comparison of medical therapy dosing in outpatients cared for in cardiology practices with heart failure and reduced ejection fraction with and without device therapy: report from IMPROVE HF. Circ Heart Fail 2010;3:596–605. [DOI] [PubMed] [Google Scholar]
- 8. Verbrugge FH, Duchenne J, Bertrand PB, Dupont M, Tang WH, Mullens W. Uptitration of renin–angiotensin system blocker and beta‐blocker therapy in patients hospitalized for heart failure with reduced versus preserved left ventricular ejection fractions. Am J Cardiol 2013;112 : 1913–1920. [DOI] [PubMed] [Google Scholar]
- 9. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Dosing of beta‐blocker therapy before, during, and after hospitalization for heart failure (from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure). Am J Cardiol 2008;102:1524–1529. [DOI] [PubMed] [Google Scholar]
- 10. Calvert MJ, Shankar A, McManus RJ, Ryan R, Freemantle N. Evaluation of the management of heart failure in primary care. Fam Pract 2009;26:145–153. [DOI] [PubMed] [Google Scholar]
- 11. Wikstrand J, Hjalmarson A, Waagstein F, Fagerberg B, Goldstein S, Kjekshus J, Wedel H. Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT‐HF). J Am Coll Cardiol 2002;40:491–498. [DOI] [PubMed] [Google Scholar]
- 12. Metra M, Torp‐Pedersen C, Swedberg K, Cleland JG, Di Lenarda A, Komajda M, Remme WJ, Lutiger B, Scherhag A, Lukas MA, Charlesworth A, Poole‐Wilson PA. Influence of heart rate, blood pressure, and beta‐blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J 2005;26:2259–2268. [DOI] [PubMed] [Google Scholar]
- 13. Fiuzat M, Wojdyla D, Kitzman D, Fleg J, Keteyian SJ, Kraus WE, Piña IL, Whellan D, O'Connor CM. Relationship of beta‐blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF‐ACTION trial. J Am Coll Cardiol 2012;60:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dose reduction of study medication by region.


