Summary
Grain weight is the most important component of rice yield and is mainly determined by grain size, which is generally controlled by quantitative trait loci (QTLs). Although numerous QTLs that regulate grain weight have been identified, the genetic network that controls grain size remains unclear. Herein, we report the cloning and functional analysis of a dominant QTL, grain length and width 2 (GLW2), which positively regulates grain weight by simultaneously increasing grain length and width. The GLW2 locus encodes OsGRF4 (growth‐regulating factor 4) and is regulated by the microRNA miR396c in vivo. The mutation in OsGRF4 perturbs the OsmiR396 target regulation of OsGRF4, generating a larger grain size and enhanced grain yield. We also demonstrate that OsGIF1 (GRF‐interacting factors 1) directly interacts with OsGRF4, and increasing its expression improves grain size. Our results suggest that the miR396c‐OsGRF4‐OsGIF1 regulatory module plays an important role in grain size determination and holds implications for rice yield improvement.
Keywords: rice, grain size, growth‐regulating factor, miR396, GRF‐interacting factors
Introduction
Grain weight, which is mainly determined by grain size (length, width and thickness), is a highly important component of rice yield. Grain size is predominantly controlled by quantitative trait loci (QTLs). Recently, several QTLs regulating grain size have been molecularly identified and functionally analysed, providing useful information for understanding their mechanisms and use in rice breeding (Zuo and Li, 2014). A number of studies have identified QTLs that negatively regulate the grain size in rice, such as GW2 (Song et al., 2007), GW5/qSW5 (Shomura et al., 2008; Weng et al., 2008), GS3 (Fan et al., 2006; Mao et al., 2010), TGW6 (Ishimaru et al., 2013) and GL3.1/qGL3 (Qi et al., 2012; Zhang et al., 2012). Recently, several positive regulators have been well characterized. Amongst these regulators, GS5 encodes a putative serine carboxypeptidase (Li et al., 2011), GW8 encodes OsSPL16 (Wang et al., 2012), GW6a encodes a new‐type GNAT‐like protein (Song et al., 2015) and GW7/GL7 encodes a TONNEAU1‐recruiting motif protein (Wang et al., 2015a,b). Most recently, GS2 has been reported to positively regulate both grain width and length by promoting cell division and cell expansion (Hu et al., 2015). Interestingly, overexpression of rice OsmiR397 enlarges grain size and promotes panicle branching by down‐regulating OsLAC, a laccase‐like protein, which shows a regulatory role of miRNA in controlling rice seed size (Zhang et al., 2013). Although numerous QTLs regulating grain weight have been currently identified, the understanding of the precise mechanisms remains elusive.
Growth‐regulating factors (GRFs) are plant‐specific transcription factors (Omidbakhshfard et al., 2015) that were firstly identified for their roles in stem and leaf development (Horiguchi et al., 2005; Kim and Kende, 2004; Kim et al., 2003; van der Knaap et al., 2000). However, emerging data have demonstrated such transcription factors to be also important for other various developmental processes, such as root development (Bazin et al., 2013; Hewezi et al., 2012; Rodriguez et al., 2015; Vercruyssen et al., 2011), floral organ development (Liang et al., 2014; Liu et al., 2014), seed formation (Hu et al., 2015; Liu et al., 2012), longevity (Debernardi et al., 2014) and stress response (Casadevall et al., 2013; Hewezi et al., 2012; Kim et al., 2012).
A total of nine GRF members exist in Arabidopsis thaliana (Omidbakhshfard et al., 2015), and twelve exist in rice (Choi et al., 2004a; Omidbakhshfard et al., 2015). The majority of plant GRFs consist of two conserved domains: the QLQ domain, which is considered important for protein–protein interactions, and the WRC domains, which is expected to be involved in DNA binding (Choi et al., 2004b; Kim et al., 2003; van der Knaap et al., 2000). These two classically conserved domains suggest at least two important aspects of GRF molecular functions in its signal network. These aspects include protein–protein interactions with its partner and direct DNA binding of the downstream target for expression regulation (Choi et al., 2004a; Omidbakhshfard et al., 2015). GRF‐interacting factors (GIFs) are the major partners of GRFs through their conserved QLQ domains (Horiguchi et al., 2005; Kim and Kende, 2004). GIF1 has been mainly reported to participate in cell proliferation control during leaf development by interacting with GRFs (Horiguchi et al., 2005; Kim and Kende, 2004). GIF1 also functions in adaxial/abaxial patterning (Horiguchi et al., 2011; Iwakawa et al., 2007; Xu et al., 2003), establishment of cotyledon identity (Kanei et al., 2012) and chromatin remodelling by the interactions to other proteins (Debernardi et al., 2014; Vercruyssen et al., 2014). In contrast to the protein–protein interactions of GRFs, research that focuses on the downstream target of GRFs is currently limited. Only few target genes, such as the KNOX gene (Kuijt et al., 2014; Osnato et al., 2010), or OsCR4 and OsJMJ706 (Liu et al., 2014), were identified.
Another important mechanism underlying GRF function is the extensive post‐transcriptional control of GRF by microRNA396, an ancient miRNA family (Omidbakhshfard et al., 2015). Most, but not all, GRFs are miR396 targets. MiR396 directly targets GRF transcripts, thereby negatively regulating the latter's expression levels. Several reports, mostly in A. thaliana, have established a miR396‐GRF regulatory module, which operates in various developmental processes, such as stem/leaf development (Das Gupta and Nath, 2015; Debernardi et al., 2014; Liu et al., 2009; Mecchia et al., 2013; Rodriguez et al., 2010; Wang et al., 2010), root development (Bazin et al., 2013; Hewezi et al., 2012; Rodriguez et al., 2015), reproductive organ development (Baucher et al., 2013; Liang et al., 2014; Liu et al., 2014) and environmental response (Casadevall et al., 2013; Hewezi et al., 2012). However, reports of this regulatory module in other plants, especially in more economically important crops, are relatively lacking. Recently, the miR396d–GRF regulatory module is reported to be involved in floral organogenesis in rice (Liu et al., 2014). Interestingly, GRFs may also affect miR396 transcript levels and the expression of other GRFs possibly by a reciprocal feedback regulation (Hewezi and Baum, 2012); however, the underlying molecular details are unknown.
Herein, we report the map‐based cloning and functional analysis of a dominant QTL, GLW2, which positively regulates grain weight by simultaneously increasing grain length and width. The GLW2 locus encodes OsGRF4 and is regulated by miRNA OsmiR396 in vivo. The mutation in OsGRF4 perturbs OsmiR396‐directed regulation of OsGRF4, generating a larger grain size and enhanced grain yield. We also demonstrate that OsGIF1 directly interacts with OsGRF4, and increasing its expression improves grain size. Our results suggest that the miR396‐OsGRF4‐OsGIF1 regulatory module plays an important role in grain size determination and may help improve rice grain yield in rice.
Results
GLW2 allele from 307R significantly increases rice grain weight by simultaneously regulating grain length and width
An extra‐large grain rice line designated as 307R, with a 1000‐grain weight (TGW) of 64 g (Figure 1a), was identified from our breeding materials. To verify the usefulness of this trait, we crossed 307R with three elite rice restorer lines, IR24 (with a TGW of 28 g), MH63 (with a TGW of 30 g) and 527R (with a TGW of 35 g), respectively, which have been widely used as the male parents of commercial hybrid rice (Figure 1a). Three near‐isogenic lines (NILs; NIL‐IR24, NIL‐MH63 and NIL‐527R) were developed from different backgrounds (Figure 1b–d). The grains of NILs were significantly larger than those of the recurrent parents (Figure 1h), showing apparently increased grain length (from 21.42% to 31.69%) (Figure S1b), grain width (from 22.43% to 27.38%) (Figure S1c) and grain weight (from 26.91% to 52.97%) (Figure S1a). As a result, these improvements led to a 14.93%–26.0% increase in grain yield per plant in NILs compared with the recurrent parents (Figure S1e). Moreover, NILs also exhibited a tendency to improve in grain number per panicle, although the effect was not significant (Figure S1d). These results suggest that the allele from 307R can increase rice grain weight by regulating grain length and width. Grain size from heterozygous plants is close to that from the larger homozygous plants; the allele from the 307R is likely an incomplete dominant allele for rice grain size and weight control.
Figure 1.

Comparisons between near‐isogenic lines (NILs) and the recurrent parents. (a) Grains of 307R, IR24, MH63 and 527R; scale bar, 10 mm; (b–d) plant comparisons of NILs and the recurrent parents, IR24/NIL‐IR24 (b); MH63/NIL‐MH63 (c); 527R/NIL‐527R (d); scale bar, 30 cm; (e–g) panicle comparisons of NILs and the recurrent parents, IR24/NIL‐IR24 (e); MH63/NIL‐MH63 (f); 527R/NIL‐527R (g); scale bar, 10 cm; (h) grain size phenotype of NILs and the recurrent parents; scale bar, 10 mm.
GLW2 primarily regulates grain size by promoting cell expansion
The spikelet hull of NIL‐IR24 was apparently larger than that of IR24 both in length and width (Figure S2a). Histological sectioning analysis of the hull indicated that the number of the outer parenchyma cells was significantly increased by 9.4% in NIL‐IR24 compared with IR24 (Figures S2b,c). In addition, scanning electron microscopy of the grain husk revealed that NIL‐IR24 exhibited a significantly enlarged cell volume than that of IR24 (Figure S2d), showing a sharp decrease in epidermal cell numbers of outer glume per unit area (43.12%) (Figure S2e). Consistent with this result, the length and width of epidermal cells of the outer glumes increased by 59.82% and 30.36%, respectively, in NIL‐IR24 compared with those in IR24 (Figures S2f, g). These results suggest that the large grain gene allele of NILs predominantly promotes cell expansion but also increases cell proliferation.
GLW2 encodes OsGRF4, a functional transcription factor
Using 180 F2 short‐grain individuals generated from a cross of 307R/IR24, we firstly mapped the QTL to chromosome 2 and designated as GLW2. The GLW2 locus was further narrowed down to a 160‐kb interval between the markers H2 and Z4 using 2500 short‐grain individuals generated from a BC3F2 population of the same cross. Finally, we limited the GLW2 locus to a 15.3‐kb interval, which contains only one candidate gene LOC_Os02g47280, between the markers M2 and M8 by fine genotyping of eight fixed recombinants. Sequence comparison revealed that the coding regions of the LOC_Os02g47280 gene contained four polymorphisms between Nipponbare and 307R. However, only one polymorphism (TC487‐488AA) was conserved between IR24/MH63/527R and their NILs, which suggest that the TC487‐488AA mutation is the causal mutation for the large grain size (Figure S3).
This LOC_Os02g47280 gene encodes OsGRF4, which is preferentially expressed in young panicles; however, compared with Nipponbare, 307R exhibits an obviously elevated level of OsGRF4 transcripts in most tissues (Figure 4a). This fact suggests that the 2‐bp mutation may lead to an elevated expression of OsGRF4 and the final large grain in 307R.
Figure 4.
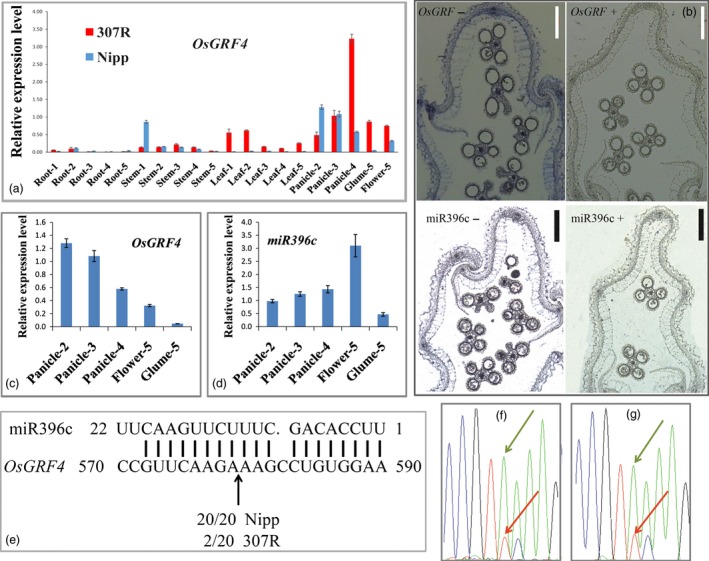
OsmiR396c regulates OsGRF4 in rice. (a) Differences in expression pattern of OsGRF4 between Nipponbare and 307R; 1, 2, 3, 4 and 5 indicate a plant with a panicle length of 0, 2, 5, 10 and 15 cm, respectively; (b) in situ hybridization analysis of OsGRF4 and OsmiR396c in rice spikelet hull; scale bar, 200 μm; (c) OsGRF4 expression pattern during panicle development in Nipponbare; (d) OsmiR396c expression pattern during panicle development in Nipponbare; (e) RLM‐RACE analysis of the OsmiR396c cleavage sites in the panicle of Nipponbare and of 307R; (f–g) sequencing chromatogram of the RT‐PCR products in the heterozygous plants of NIL‐527R/640A (f) and of NIL‐MH63/106A (g), with green arrows indicating mRNA in the NIL forms, whereas red arrows indicate the normal grain forms; values are all shown as means ± SEM.
To confirm whether OsGRF4 corresponds to GLW2, we firstly generated an overexpression construct in which the OsGRF4 from the IR24 background was driven by the 2 × 35S promoter and introduced into Nipponbare. Investigation indicated that the transgenic plants were increased apparently in grain size and weight (Figures 2a,c–e). The increased grain size and weight were further confirmed to be a consequence of OsGRF4 overexpression (Figure 2b). We next generated two gRNA constructs, which were introduced into NIL‐527R to knock out (KO) the OsGRF4 gene in a CRISPR/CAS9 strategy (Miao et al., 2013). Several independent bi‐allelic or homozygous KO plants were obtained (Figures 3a,b) and showed obvious decreases in grain size and weight (Figures 3b–e). These results demonstrate that OsGRF4 is responsible for the grain size and weight phenotype and its elevated expression benefit to a large and heavy grain.
Figure 2.
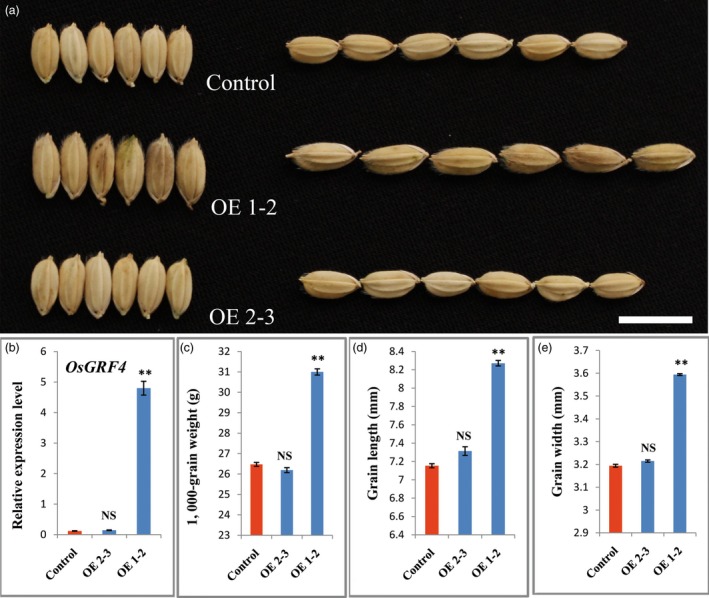
OsGRF4 overexpression increases rice grain size and weight. (a) Grain comparisons of the control and OsGRF4 overexpression plants; scale bar, 10 mm; (b) OsGRF4 expression levels of plants in (a); (c) TGW of plants in (a); (d) grain length of plants in (a); (e) grain width of plants in (a); values are all shown as means ± standard error of the mean (SEM, **P < 0.01.)
Figure 3.
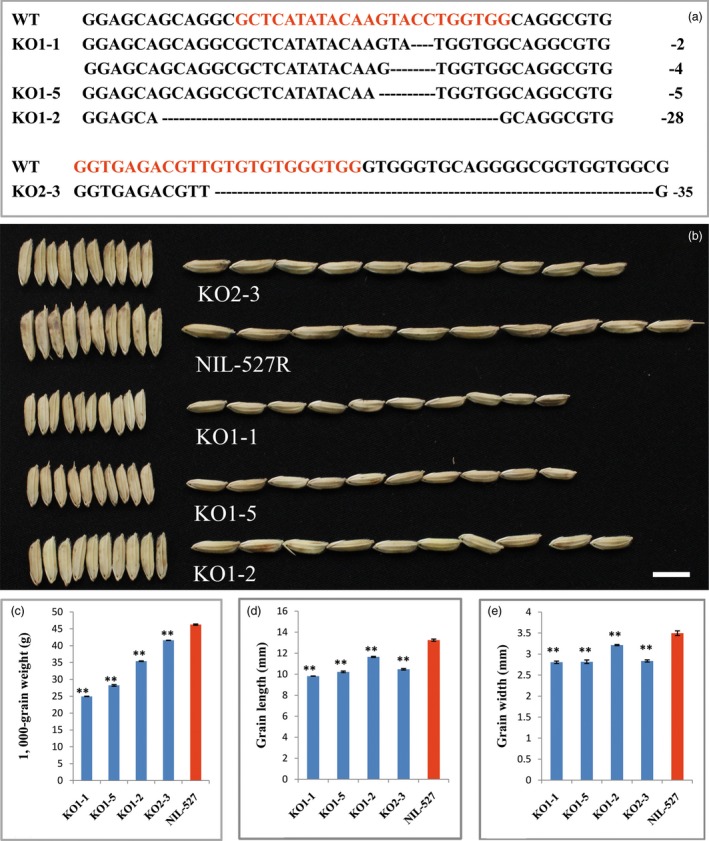
Knock out (KO) of OsGRF4 in NIL‐527R decreases grain size and weight. (a) Sequence comparisons of the targeting site of the KO plants; the sequence in red is the gRNA target site. Note that KO1‐1 plant is bi‐allelic and others are homozygous. (b) Grain comparisons of the control and OsGRF4 KO plants; scale bar, 10 mm; (c) KGW of plants in (b); (d) grain length of plants in (b); (e) grain width of plants in (b); values are all shown as means ± SEM (**P < 0.01).
The transient expression of a GLW2–YFP (yellow fluorescent protein) fusion protein in rice protoplasts showed that GLW2–YFP localized mainly to the nucleus (Figure S4a), which is consistent with the finding that OsGRF4 is a plant‐specific transcription factor (Omidbakhshfard et al., 2015). We then demonstrated that OsGRF4 holds a transcription activation activity, and the activation domain was located in its C‐terminal region as demonstrated by a series of truncation analyses in yeast cells (Figure S4b).
GLW2 is directly regulated by OsmiR396c
Growth‐regulating factor genes are known to be substantially regulated by miR396 (Debernardi et al., 2014; Hewezi et al., 2012; Liu et al., 2014; Omidbakhshfard et al., 2015; Rodriguez et al., 2015). Interestingly, the TC487‐488AA mutation of the large grain rice 307R occurred within the binding site of OsmiR396 (Figure 6a), which might suggest that the OsmiR396 also directly regulated OsGRF4. However, the cleaving efficiency of small grains might was different from that of large grains. In situ hybridization results showed that OsGRF4 and OsmiR396c both expressed in rice spikelet hulls (Figure 4b). Detailed quantitative PCR (qPCR) analysis indicated that the expression pattern of GLW2 was complementary to that of OsmiR396c during panicle and grain development in Nipponbare (Figures 4c,d). An RNA ligase‐mediated rapid amplification of cDNA ends (RLM‐RACE) analysis showed that miR396 could directly cleave OsGRF4 mRNA in vivo at one site within the miR396 pairing region in Nipponbare (20/20). However, the 2‐bp substitutions of GLW2 sharply down‐regulated the cleaving efficiency in 307R (2/20) (Figure 4e). We further demonstrated this notion by performing reverse transcription PCR using mRNAs from the heterozygous (NIL‐527R/640A, NIL‐MH63/106A) plants. Sequencing results clearly showed that the mRNAs existed predominantly as the NIL forms (Figures 4f,g), suggesting that cleavage of the NIL‐527R transcripts by OsmiR396 was disrupted. This result was consistent with OsGRF4 expressed higher in 307R than in Nipponbare (Figure 4a).
Figure 6.
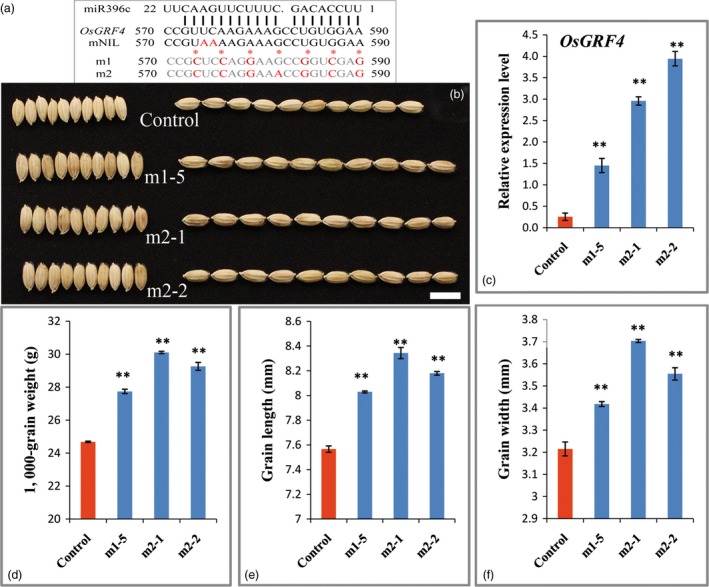
Blocking the target regulation of OsGRF4 by OsmiR396c leads to a large and heavy grain. (a) Mutant sequence of the two mOsGRF4 constructs; (b) grains of the control and mOsGRF4 overexpression plants; Scale bar, 10 mm; (c) OsGRF4 expression level of plants in (b); (d) TKW of plants in (b); (e) grain length of plants in (b); (f) grain width of plants in (b); values are all shown as means ± SEM (**P < 0.01).
To gain more genetic evidence for the regulation of OsmiR396 on OsGRF4, we generated an overexpression construct in which the OsmiR396c was driven by the 2 × 35S promoter and introduced it into Nipponbare. Investigations indicated an apparent decrease in the grain size and weight of the transgenic plants (Figures 5a,d,e). The decreased grain size and weight were further confirmed to be a consequence of the substantial decrease in OsGRF4 transcripts (Figure 5c), which was caused by the overexpression of OsmiR396c (Figure 5b). To confirm whether perturbation of miR396c regulation on OsGRF4 leads to large grain sizes, two miR396‐resistant variants of OsGRF4 (mOsGRF4) that disrupts the miR396 recognition without changing any amino acid and controlled by the 2 × 35S promoter were introduced into Nipponbare (Figure 6a). The transgenic plants exhibited an obviously larger grain size and weight (Figures 6b–f), suggesting that the blocked down‐regulation of OsGRF4 by miR396c caused the large grain phenotype.
Figure 5.
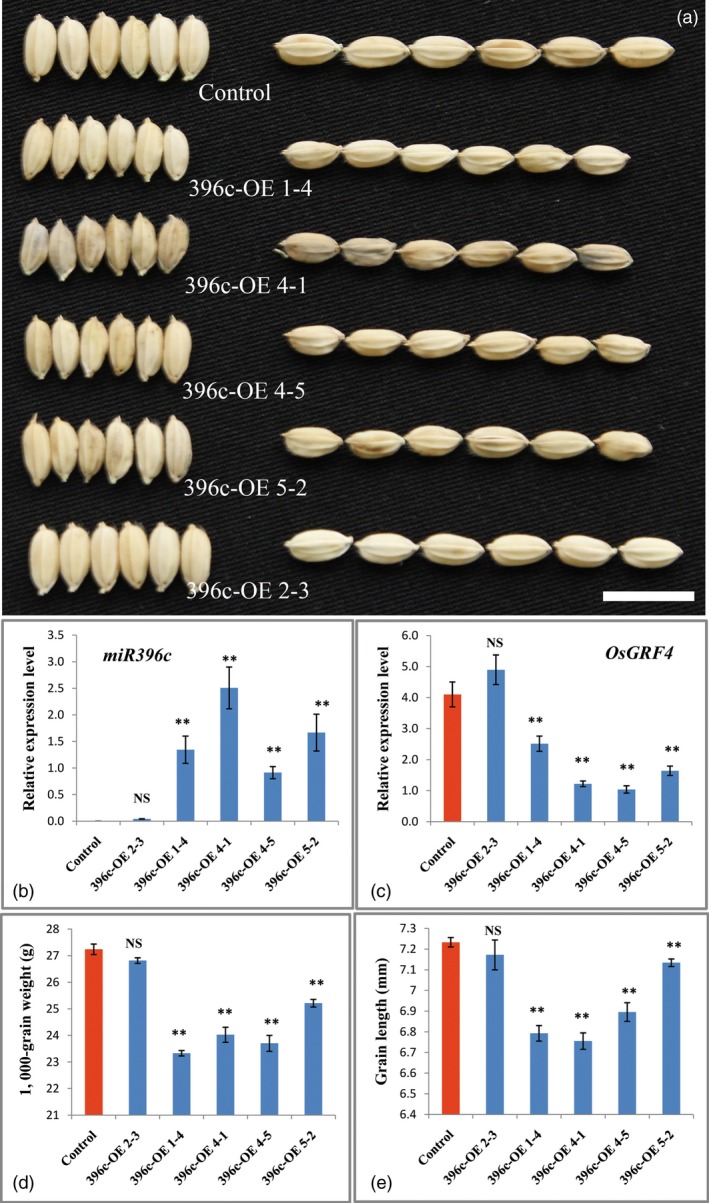
OsmiR396c overexpression decreases rice grain size and weight. (a) Grains of the control and OsmiR396c overexpression plants; scale bar, 10 mm; (b) OsmiR396c expression level of plants in (a); (c) OsGRF4 expression level of plants in (a); (d) TKW of plants in (a); (e) grain length of plants in (a); values are all shown as means ± SEM (**P < 0.01).
GLW2 directly interacts with OsGIF1 to regulate grain size
Growth‐regulating factors have been widely reported to interact directly with transcription coactivator GIFs (Horiguchi et al., 2005; Kim and Kende, 2004; Liu et al., 2014). We thus investigated whether GLW2 could interact with rice GIFs using a yeast two‐hybrid system. As shown in Figure 7a, we examined direct interactions between GLW2 and OsGIF1 in yeast cells. Further truncation analysis showed that GLW2 interacted with OsGIF1 at their N‐terminal domains, which is consistent with previous reports on GRFs interacting with partners by the QLQ domain (Horiguchi et al., 2005; Kim and Kende, 2004; Liu et al., 2014). Bimolecular fluorescence complementation (BiFC) assays further revealed that GLW2 interacted with OsGIF1 in plants (Figure 7b). This result suggests a conserved mechanism between Arabidopsis and rice.
Figure 7.
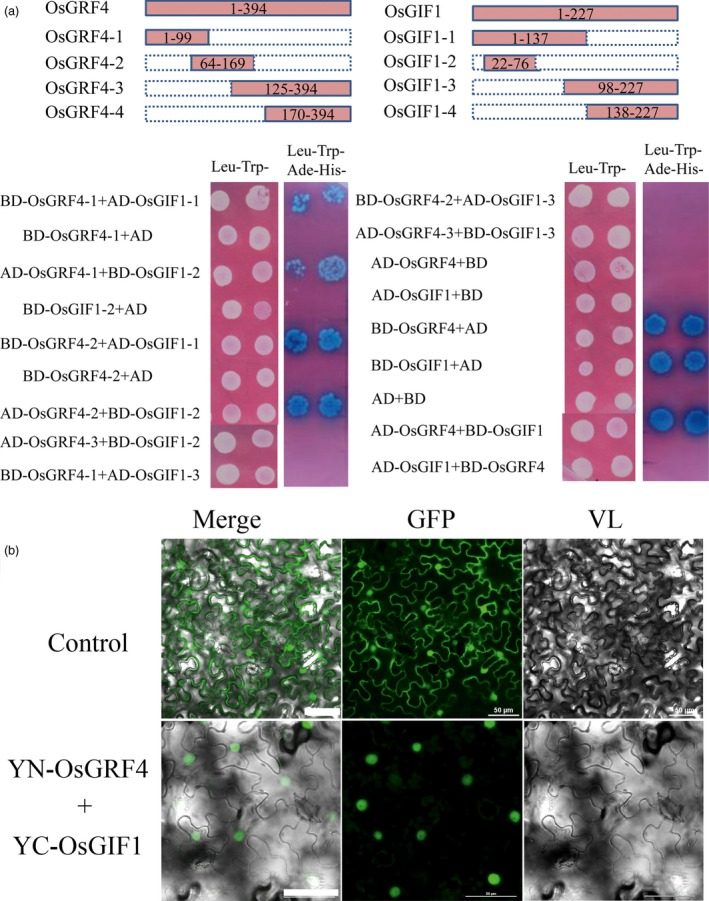
OsGRF4 directly interacts with OsGIF1 in rice. (a) GLW2 interacts with OsGRF4 in yeast. Both GLW2 and OsGIF1 exhibit transcription activation activity; thus, a series of truncations and deletions analyses for both genes were carried out depending on their conserved domains. (b) BiFC analysis of GLW2–OsGIF1 interaction in tobacco leaf. 35S‐YFP was employed as control; scale bar, 50 μm.
To determine whether OsGIF1 is involved in the seed development process in rice, we then overexpressed OsGIF1, which is controlled by the 2 × 35S promoter, in Nipponbare. Investigations showed that transgenic plants overexpressing OsGIF1 (Figure 8b) produced larger and heavier grains than those of Nipponbare (Figures 8a,c–e), indicating that OsGIF1 positively regulated grain growth in rice. Given the above‐mentioned findings, we summarized that GLW2 directly interacted with OsGIFs to manipulate grain size and weight in rice.
Figure 8.
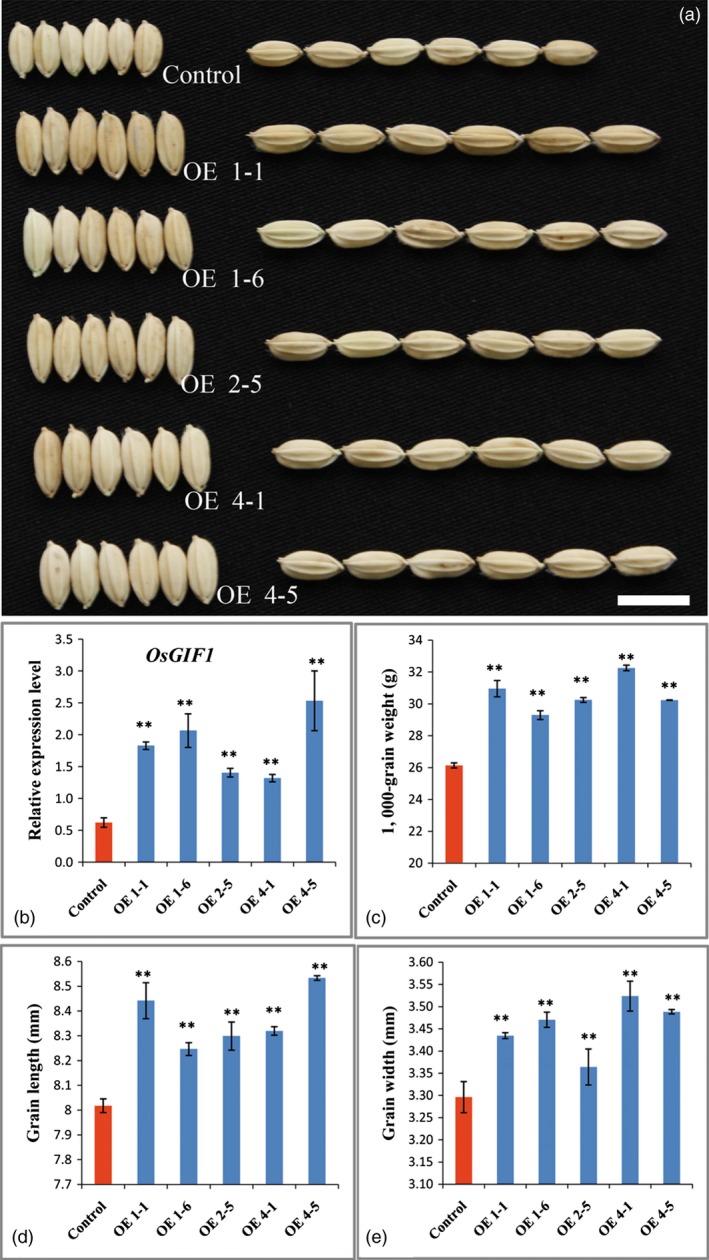
OsGIF1 overexpression increases rice grain size and weight. (a) Grain comparisons of the control and OsGIF1 overexpression plants; scale bar, 10 mm; (b) OsGIF1 expression levels of plants in (a); (c) TGW of plants in (a); (d) grain length of plants in (a); (e) grain width of plants in (a); values are all shown as means ± SEM (**P < 0.01).
GLW2 significantly increases hybrid rice yield
Because all the NILs were generated from restorer backgrounds, we can directly use these NILs to evaluate whether GLW2 can improve the yield of hybrid rice. We crossed those NILs to two currently used cytoplasmic male sterility lines, 106A and 640A, and thus generated several F1 hybrid rice lines. Field performance indicated that the GLW2 locus could significantly increase the plot yield of hybrid rice lines generated from NILs when compared to those generated from recurrent parents (showed a 13.7%–28.0% increase). This increase in plot yield was mainly achieved by increasing the grain length, width, weight and yield per hybrid rice plant (Table S1). Besides, the NIL‐generated hybrid rice was apparently longer in panicle length than that generated from recurrent parents. These data suggest that GLW2 holds a potential for direct application in high‐yield hybrid rice breeding.
Discussion
Rice is a highly important food crop because of its role as the main staple food of more than half of the world's population. Grain weight, mainly determined by grain size, is the most important component of rice yield and is generally controlled by QTLs. Although numerous QTLs regulating grain weight have been identified, our understanding of grain size regulation is fragmented. To reveal more genes related to rice grain size and weight, we identified an extra‐large grain rice line designated as 307R from our breeding materials. Genetic analysis indicates that the large grain trait is controlled by an incomplete dominant QTL, suggesting that this locus holds a potential application in rice breeding. As expected, introducing this allele into different backgrounds of rice significantly increases rice grain weight by simultaneously increasing grain length and width. Field performance indicates that GLW2 locus can also significantly increase the plot yield of hybrid rice lines generated from the NILs by 13.7%–28.0%. Thus, these results suggest that GLW2 can be exploited in high‐yield hybrid rice breeding.
Map‐based cloning of GLW2 demonstrated that GLW2 encodes OsGRF4, a plant‐specific transcription factor. We found that an elevated expression of OsGRF4 generated enlarged grains, and loss of function resulted in smaller grains. This finding suggests a positive role of GLW2 in grain size regulation. Very recently, two alleles of GLW2, GS2 (Duan et al., 2015) and GL2 (Che et al., 2015) have been reported to positively regulate grain size in rice. GRFs have been well documented for their roles in stem and leaf development (Horiguchi et al., 2005; Kim and Kende, 2004; Kim et al., 2003; van der Knaap et al., 2000), root development (Bazin et al., 2013; Hewezi et al., 2012; Rodriguez et al., 2015; Vercruyssen et al., 2011), floral organ development (Liang et al., 2014; Liu et al., 2014), longevity (Debernardi et al., 2014) and stress response (Casadevall et al., 2013; Hewezi et al., 2012; Kim et al., 2012). Our results, along with those of other reports (Che et al., 2015; Duan et al., 2015; Hu et al., 2015; Liu et al., 2012), demonstrate a new and important role for GRF genes in seed size regulation. The study findings suggest GRF functions in various plant developmental processes. However, similar to GS2/GL2, GLW2 predominantly promotes cell expansion but also slightly increases cell proliferation in organ size determination, which is different from the case of Arabidopsis, in which several GRFs have been reported to mainly function in promoting cell proliferation (Horiguchi et al., 2005; Omidbakhshfard et al., 2015). Further investigations are currently needed to understand the functional distinction of GRFs in organ size regulation.
Growth‐regulating factor genes are substantially regulated by miR396 (Debernardi et al., 2014; Hewezi et al., 2012; Liu et al., 2014; Omidbakhshfard et al., 2015; Rodriguez et al., 2015), which promotes us to survey whether GLW2 is regulated by OsmiR396. Coincidently, the 2‐bp mutations of the large grain rice 307R occurred within the binding site of OsmiR396. Besides, GLW2 expression level during grain development in 307R was obviously higher than in Nipponbare. Furthermore, GLW2 and OsmiR396c were co‐expressed in rice spikelet hull and exhibited a complementary pattern during panicle development in Nipponbare. These data strongly suggest that GLW2 may serve as the direct target of OsmiR396. We then confirmed this idea when we noted that the cleavage of GLW2 in vivo and the blocking of the target regulation of OsGRF4 by OsmiR396 generated large and heavy grains. However, eight isoforms (from a–h) exist in the OsmiR396 family; which isoform(s) actually regulate(s) OsGRF4 is currently unknown (Che et al., 2015; Duan et al., 2015). The expression of OsmiR396c was consistent with a regulatory role of OsGRF4; hence, we further overexpressed the OsmiR396c isoform and found that the overexpression of OsmiR396c significantly decreases the grain size and weight. This finding suggests the regulatory role of the OsmiR396c isoform on OsGRF4. However, whether other isoform(s) of this family also play roles in the regulation of GLW2 remains to be clarified in future study.
Growth‐regulating factors have been widely reported to interact directly with transcription coactivator GIFs (Horiguchi et al., 2005; Kim and Kende, 2004; Liu et al., 2014). Our results have also demonstrated that GLW2 directly interacts with OsGIF1 both in vitro and in vivo. Most importantly, overexpression of OsGIF1 in rice also significantly increases grain size and weight by 13.4%–21.8%, which suggests that OsGIF1 holds an important role in rice grain size regulation. Overall, our results thus establish that the OsmiR396c‐OsGRF4‐OsGIF1 regulatory module plays roles in grain size determination and enhances rice yield. Considering that OsGRF4 is a plant‐specific transcription factor with intrinsic transcription activation, another important aspect of its function lies in the direct regulation of its downstream target gene. The identification and characterization of the DNA‐binding domain of GLW2 and the GLW2 target gene may offer additional information on the mechanism by which the GRFs contribute to the regulation of grain size and yields in rice.
Experimental procedures
Plant materials and growth conditions
The indica varieties 307R, IR24, MH63 and 527R and the japonica variety of Nipponbare were used in this study. Three NILs for GLW2 were generated by backcrossing 307R with IR24, MH63 and 527R as the recurrent parents, respectively. All plants were planted in the experimental field of the Rice Research Institute, Sichuan Agricultural University, Wenjiang. Phenotypic data were collected at the maturing stage.
Map‐based cloning of GLW2
Normal‐sized F2 plants generated from the cross of 307R and IR24 were used for the primary mapping of GLW2. For fine mapping, approximately 2500 normal‐sized BC3F2 plants from the same class were used. Recombinants were self‐crossed and several fixed recombinants were then generated and genotyped with the newly developed markers. All of the new molecular markers used in this process are listed in Table S2.
Morphological and cellular analyses
The grain length, width and 1000‐grain weight were measured by an automatic seed‐size‐analysing system (SC‐G, Wanshen, Hangzhou, china). For histological analysis, spikelet hulls were placed in Formalin‐acetic acid‐alcohol (FAA) solution (50% alcohol: formalin: glacial acetic acid; 18:1:1) overnight at 4 °C, then dehydrated in a graded ethanol series, followed by substitution using 3‐methylbutyl acetate. The samples were dissected and then observed under a microscope (80I; Nikon, Kanagawa, Japan). An environmental scanning electronic microscope (QUANTA 450; Czech Republic) was employed to observe the outer glume cells.
Plasmid construction and plant transformation
To verify the GLW2 function, we firstly generated an overexpression construct by inserting full‐length GLW2 cDNA from IR24 into the plant binary vector pHB (Mao et al., 2005) and introduced the plasmid into Nipponbare. We then generated two gRNA constructs, in which the gRNA was driven by the rice U6 promoter and the plant‐optimized CAS9 was driven by the UBI promoter (Miao et al., 2013), and introduced the constructs into NIL‐527R. To verify the miR396c function, a full sequence of pri‐miRNA396c was inserted into pHB and introduced into Nipponbare. Two miR396‐resistant variants of OsGRF4 (mOsGRF4) generated by point mutation were inserted into pHB and introduced into Nipponbare. We also generated an OsGIF1 overexpression construct, which we introduced into Nipponbare for functional analysis. All constructs were introduced into the Agrobacterium tumefaciens strain EHA105. The primer sequences for these constructs are listed in Table S3. Rice transformation was performed in accordance with a previously published method (Hiei et al., 1997). To investigate the subcellular localization of GLW2, we constructed a pA7–GLW2–YFP (yellow fluorescent protein) fusion construct whose expression was driven by the CaMV 35S promoter for transiently transforming the rice protoplast. A laser scanning confocal microscope (Nikon A1, Kanagawa, Japan) was used to observe the rice protoplast.
Transcription activation assay and yeast two‐hybrid assay
The transcription activation assays were conducted with the Matchmaker GAL4 Two‐Hybrid System 2 (Clontech, Dalian, China). The full length and truncations of GLW2 were fused to the GAL4 DNA‐binding domain. The vectors were then transformed into yeast strain Y2H Gold for transcription activation assays. The full length and truncations of GLW2 and OsGIF1 were amplified and subcloned into the pGBKT7 or pGADT7 vectors. The prey and bait plasmids were cotransformed into the yeast strain Y2H Gold and cultured on SD‐Leu‐Trp media for 3 days at 30 °C. Clones were grown on SD‐Ade‐His‐Leu‐Trp medium for 2–3 days at 30 °C for interaction detections.
BiFC assay
The full‐length cDNA of GLW2 was cloned into the pXY106 (nYFP) vector, and OsGIF1 was cloned into the pXY104 (cYFP) vector. These plasmids were co‐expressed in tobacco leaf epidermis cells by Agrobacterium‐mediated infiltration. Yellow fluorescent protein was visualized with a confocal scanning microscope (Nikon A1, Kanagawa, Japan) 72 h after infiltration.
qPCR analysis
Total RNAs from various rice tissues were isolated using the TriPure isolation reagent (Roche, Indianapolis, USA). cDNAs were reverse‐transcribed using the Transcriptor First‐Strand cDNA Synthesis kit (Roche, Indianapolis, USA). For OsmiR396c detection, the microRNAs were isolated using the EASYspin Plant microRNA Kit (Aidlab, Beijing, China). Reverse transcription was conducted using a stem‐loop RT primer ST‐RT1 as described previously (Kramer, 2011). qPCR experiments were carried out in 10 μL reaction mixtures containing 0.3 μL of reverse‐transcribed product, 0.08 mm gene‐specific primers and 5.0 mL of Sso Advanced TM SYBR Green Supermix (Bio‐Rad, California, USA) using a Bio‐Rad CFX96 Real‐Time PCR System (California, USA) in accordance with the manufacturer's instructions.
RNA in situ hybridization
For RNA in situ hybridization, spikelet hulls were fixed in FAA solution for 24 h at 4 °C, then dehydrated using a graded ethanol series, followed by a xylene series, and embedded in Paraplast Plus (Sigma‐Aldrich, St Louis, USA) for sections. The sense and antisense RNA probes used in this test are listed in Table S3. Digoxigenin‐labelled RNA probes were prepared using a DIG RNA Labelling Kit (SP6/T7) (Roche, Mannheim, Germany) according to the manufacturer's instructions. A sense probe was used as a negative control.
RLM‐RACE
RLM‐RACE was conducted in accordance with a GeneRacer kit (Invitrogen, Carlsbad, CA, USA). In general, total RNAs were extracted and the first and second PCRs were performed, with the primers GSP1 and GSP2 (Table S3), respectively. The products from the second PCR were purified by agarose gel electrophoresis and then cloned for sequencing.
Supporting information
Figure S1 Yield‐related trait comparisons of NILs and the recurrent parents.
Figure S2 GLW2 increases grain size and weight mainly by promoting cell expansion.
Figure S3 Map‐based cloning of GLW2.
Figure S4 GLW2 is a transcription factor.
Table S1 Yield performance of the hybrid rice generated by NILs.
Table S2 Primers used in map‐based cloning.
Table S3 Primers (or probes) used in molecular cloning, construction and gene expression analysis.
Acknowledgements
We thank H. Q. Yang for providing the pHB and pXY106/104 vectors. This work was supported by the National Natural Science Foundation of China (31471474, 91435102 and 31570004), the Open Research Fund of State Key Laboratory of Hybrid Rice (Hunan Hybrid Rice Research Centre, 2013KF05) and The Sichuan Provincial Funding for Distinguished Young Scholars (2015JQ0048).
References
- Baucher, M. , Moussawi, J. , Vandeputte, O.M. , Monteyne, D. , Mol, A. , Perez‐Morga, D. and El Jaziri, M. (2013) A role for the miR396/GRF network in specification of organ type during flower development, as supported by ectopic expression of Populus trichocarpa miR396c in transgenic tobacco. Plant Biol. 15, 892–898. [DOI] [PubMed] [Google Scholar]
- Bazin, J. , Khan, G.A. , Combier, J.P. , Bustos‐Sanmamed, P. , Debernardi, J.M. , Rodriguez, R. , Sorin, C. et al (2013) miR396 affects mycorrhization and root meristem activity in the legume Medicago truncatula. Plant J. 74, 920–934. [DOI] [PubMed] [Google Scholar]
- Casadevall, R. , Rodriguez, R.E. , Debernardi, J.M. , Palatnik, J.F. and Casati, P. (2013) Repression of growth regulating factors by the microRNA396 inhibits cell proliferation by UV‐B radiation in Arabidopsis leaves. Plant Cell, 25, 3570–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, R. , Tong, H. , Shi, B. , Liu, Y. , Fang, S. , Liu, D. , Xiao, Y. et al (2015) Control of grain size and rice yield by GL2‐mediated brassinosteroid responses. Nature Plants, 2, 15195. [DOI] [PubMed] [Google Scholar]
- Choi, D. , Kim, J.H. and Kende, H. (2004a) Whole genome analysis of the OsGRF gene family encoding plant‐specific putative transcription activators in rice (Oryza sativa L.). Plant Cell Physiol. 45, 8. [DOI] [PubMed] [Google Scholar]
- Choi, D. , Kim, J.H. and Kende, H. (2004b) Whole genome analysis of the OsGRF gene family encoding plant‐specific putative transcription activators in rice (Oryza sativa L.). Plant Cell Physiol. 45, 897–904. [DOI] [PubMed] [Google Scholar]
- Das Gupta, M. and Nath, U. (2015) Divergence in patterns of leaf growth polarity is associated with the expression divergence of miR396. Plant Cell, 27, 2785–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debernardi, J.M. , Mecchia, M.A. , Vercruyssen, L. , Smaczniak, C. , Kaufmann, K. , Inze, D. , Rodriguez, R.E. et al (2014) Post‐transcriptional control of GRF transcription factors by microRNA miR396 and GIF co‐activator affects leaf size and longevity. Plant J. 79, 413–426. [DOI] [PubMed] [Google Scholar]
- Duan, P. , Ni, S. , Wang, J. , Zhang, B. , Xu, R. , Wang, Y. , Chen, H. et al (2015) Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nature Plant, 2, 15203. [DOI] [PubMed] [Google Scholar]
- Fan, C. , Xing, Y. , Mao, H. , Lu, T. , Han, B. , Xu, C. , Li, X. et al (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171. [DOI] [PubMed] [Google Scholar]
- Hewezi, T. and Baum, T.J. (2012) Complex feedback regulations govern the expression of miRNA396 and its GRF target genes. Plant Signal. Behav. 7, 749–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewezi, T. , Maier, T.R. , Nettleton, D. and Baum, T.J. (2012) The Arabidopsis microRNA396‐GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection. Plant Physiol. 159, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei, Y. , Komari, T. and Kubo, T. (1997) Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol. Biol. 35, 205–218. [PubMed] [Google Scholar]
- Horiguchi, G. , Kim, G.T. and Tsukaya, H. (2005) The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 43, 68–78. [DOI] [PubMed] [Google Scholar]
- Horiguchi, G. , Nakayama, H. , Ishikawa, N. , Kubo, M. , Demura, T. , Fukuda, H. and Tsukaya, H. (2011) ANGUSTIFOLIA3 plays roles in adaxial/abaxial patterning and growth in leaf morphogenesis. Plant Cell Physiol. 52, 112–124. [DOI] [PubMed] [Google Scholar]
- Hu, J. , Wang, Y. , Fang, Y. , Zeng, L. , Xu, J. , Yu, H. , Shi, Z. et al (2015) A rare allele of GS2 enhances grain size and grain yield in rice. Mol. Plant, 8, 1455–1465. [DOI] [PubMed] [Google Scholar]
- Ishimaru, K. , Hirotsu, N. , Madoka, Y. , Murakami, N. , Hara, N. , Onodera, H. , Kashiwagi, T. et al (2013) Loss of function of the IAA‐glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 45, 707–711. [DOI] [PubMed] [Google Scholar]
- Iwakawa, H. , Iwasaki, M. , Kojima, S. , Ueno, Y. , Soma, T. , Tanaka, H. , Semiarti, E. et al (2007) Expression of the ASYMMETRIC LEAVES2 gene in the adaxial domain of Arabidopsis leaves represses cell proliferation in this domain and is critical for the development of properly expanded leaves. Plant J. 51, 173–184. [DOI] [PubMed] [Google Scholar]
- Kanei, M. , Horiguchi, G. and Tsukaya, H. (2012) Stable establishment of cotyledon identity during embryogenesis in Arabidopsis by ANGUSTIFOLIA3 and HANABA TARANU. Development, 139, 2436–2446. [DOI] [PubMed] [Google Scholar]
- Kim, J.H. and Kende, H. (2004) A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc. Natl Acad. Sci. USA, 101, 13374–13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Choi, D. and Kende, H. (2003) The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 36, 94–104. [DOI] [PubMed] [Google Scholar]
- Kim, J.S. , Mizoi, J. , Kidokoro, S. , Maruyama, K. , Nakajima, J. , Nakashima, K. , Mitsuda, N. et al (2012) Arabidopsis growth‐regulating factor7 functions as a transcriptional repressor of abscisic acid‐ and osmotic stress‐responsive genes, including DREB2A. Plant Cell, 24, 3393–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap, E. , Kim, J.H. and Kende, H. (2000) A novel gibberellin‐induced gene from rice and its potential regulatory role in stem growth. Plant Physiol. 122, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, M.F . (2011) Stem‐loop RT‐qPCR for miRNAs In Current Protocols in Molecular Biology, Chapter 15, Unit 15 10 (Frederick M.A. et al eds), pp. 95:15.10.1–15.10.15. Hoboken: John Wiley & Sons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijt, S.J. , Greco, R. , Agalou, A. , Shao, J. , t Hoen, C.C. , Overnas, E. , Osnato, M. et al (2014) Interaction between the GROWTH‐REGULATING FACTOR and KNOTTED1‐LIKE HOMEOBOX families of transcription factors. Plant Physiol. 164, 1952–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Fan, C. , Xing, Y. , Jiang, Y. , Luo, L. , Sun, L. , Shao, D. et al (2011) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 43, 1266–1269. [DOI] [PubMed] [Google Scholar]
- Liang, G. , He, H. , Li, Y. , Wang, F. and Yu, D. (2014) Molecular mechanism of microRNA396 mediating pistil development in Arabidopsis. Plant Physiol. 164, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. , Song, Y. , Chen, Z. and Yu, D. (2009) Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol. Plant. 136, 223–236. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Hua, W. , Yang, H.L. , Zhan, G.M. , Li, R.J. , Deng, L.B. , Wang, X.F. et al (2012) The BnGRF2 gene (GRF2‐like gene from Brassica napus) enhances seed oil production through regulating cell number and plant photosynthesis. J. Exp. Bot. 63, 3727–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Guo, S. , Xu, Y. , Li, C. , Zhang, Z. , Zhang, D. , Xu, S. et al (2014) OsmiR396d‐regulated OsGRFs function in floral organogenesis in rice through binding to their targets OsJMJ706 and OsCR4. Plant Physiol. 165, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, J. , Zhang, Y.C. , Sang, Y. , Li, Q.H. and Yang, H.Q. (2005) From The Cover: A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl Acad. Sci. USA, 102, 12270–12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, H. , Sun, S. , Yao, J. , Wang, C. , Yu, S. , Xu, C. , Li, X. et al (2010) Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Natl Acad. Sci. USA, 107, 19579–19584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecchia, M.A. , Debernardi, J.M. , Rodriguez, R.E. , Schommer, C. and Palatnik, J.F. (2013) MicroRNA miR396 and RDR6 synergistically regulate leaf development. Mech. Dev. 130, 2–13. [DOI] [PubMed] [Google Scholar]
- Miao, J. , Guo, D. , Zhang, J. , Huang, Q. , Qin, G. , Zhang, X. , Wan, J. et al (2013) Targeted mutagenesis in rice using CRISPR‐Cas system. Cell Res. 23, 1233–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidbakhshfard, M.A. , Proost, S. , Fujikura, U. and Mueller‐Roeber, B. (2015) Growth‐regulating factors (GRFs): a small transcription factor family with important functions in plant biology. Mol. Plant, 8, 998–1010. [DOI] [PubMed] [Google Scholar]
- Osnato, M. , Stile, M.R. , Wang, Y. , Meynard, D. , Curiale, S. , Guiderdoni, E. , Liu, Y. et al (2010) Cross talk between the KNOX and ethylene pathways is mediated by intron‐binding transcription factors in barley. Plant Physiol. 154, 1616–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, P. , Lin, Y.S. , Song, X.J. , Shen, J.B. , Huang, W. , Shan, J.X. , Zhu, M.Z. et al (2012) The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin‐T1;3. Cell Res. 22, 1666–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, R.E. , Mecchia, M.A. , Debernardi, J.M. , Schommer, C. , Weigel, D. and Palatnik, J.F. (2010) Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development, 137, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, R.E. , Ercoli, M.F. , Debernardi, J.M. , Breakfield, N.W. , Mecchia, M.A. , Sabatini, M. , Cools, T. et al (2015) MicroRNA miR396 regulates the switch between stem cells and transit‐amplifying cells in Arabidopsis roots. Plant Cell, 27, 3354–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomura, A. , Izawa, T. , Ebana, K. , Ebitani, T. , Kanegae, H. , Konishi, S. and Yano, M. (2008) Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 40, 1023–1028. [DOI] [PubMed] [Google Scholar]
- Song, X.‐J. , Huang, W. , Shi, M. , Zhu, M.‐Z. and Lin, H.‐X. (2007) A QTL for rice grain width and weight encodes a previously unknown RING‐type E3 ubiquitin ligase. Nat. Genet. 39, 623–630. [DOI] [PubMed] [Google Scholar]
- Song, X.J. , Kuroha, T. , Ayano, M. , Furuta, T. , Nagai, K. , Komeda, N. , Segami, S. et al (2015) Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice. Proc. Natl Acad. Sci. USA, 112, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruyssen, L. , Gonzalez, N. , Werner, T. , Schmulling, T. and Inze, D. (2011) Combining enhanced root and shoot growth reveals cross talk between pathways that control plant organ size in Arabidopsis. Plant Physiol. 155, 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruyssen, L. , Verkest, A. , Gonzalez, N. , Heyndrickx, K.S. , Eeckhout, D. , Han, S.K. , Jegu, T. et al (2014) ANGUSTIFOLIA3 binds to SWI/SNF chromatin remodeling complexes to regulate transcription during Arabidopsis leaf development. Plant Cell, 26, 210–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Gu, X. , Xu, D. , Wang, W. , Wang, H. , Zeng, M. , Chang, Z. et al (2010) miR396‐targeted AtGRF transcription factors are required for coordination of cell division and differentiation during leaf development in Arabidopsis. J. Exp. Bot. 62, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Wu, K. , Yuan, Q. , Liu, X. , Liu, Z. , Lin, X. , Zeng, R. et al (2012) Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44, 950–954. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Li, S. , Liu, Q. , Wu, K. , Zhang, J. , Wang, S. , Wang, Y. et al (2015a) The OsSPL16‐GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 47, 949–954. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Xiong, G. , Hu, J. , Jiang, L. , Yu, H. , Xu, J. , Fang, Y. et al (2015b) Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 47, 944–948. [DOI] [PubMed] [Google Scholar]
- Weng, J. , Gu, S. , Wan, X. , Gao, H. , Guo, T. , Su, N. , Lei, C. et al (2008) Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 18, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Xu, L. , Xu, Y. , Dong, A. , Sun, Y. , Pi, L. , Xu, Y. and Huang, H. (2003) Novel as1 and as2 defects in leaf adaxial‐abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development, 130, 4097–4107. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Wang, J. , Huang, J. , Lan, H. , Wang, C. , Yin, C. , Wu, Y. et al (2012) Rare allele of OsPPKL1 associated with grain length causes extra‐large grain and a significant yield increase in rice. Proc. Natl Acad. Sci. USA, 109, 21534–21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.C. , Yu, Y. , Wang, C.Y. , Li, Z.Y. , Liu, Q. , Xu, J. , Liao, J.Y. et al (2013) Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat. Biotechnol. 31, 848–852. [DOI] [PubMed] [Google Scholar]
- Zuo, J. and Li, J. (2014) Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu. Rev. Genet. 48, 99–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Yield‐related trait comparisons of NILs and the recurrent parents.
Figure S2 GLW2 increases grain size and weight mainly by promoting cell expansion.
Figure S3 Map‐based cloning of GLW2.
Figure S4 GLW2 is a transcription factor.
Table S1 Yield performance of the hybrid rice generated by NILs.
Table S2 Primers used in map‐based cloning.
Table S3 Primers (or probes) used in molecular cloning, construction and gene expression analysis.


