Abstract
Aim
Limited data have been available regarding critical congenital heart disease (CHD) screening in neonatal intensive care unit (NICUs). This study evaluated the feasibility of screening for CHD by adding pulse oximetry (POX) to clinical evaluation in a NICU in Shanghai, China.
Methods
We screened 4128 eligible consecutive NICU admissions using POX plus clinical evaluation. Infants with positive screening results were then evaluated with echocardiography. Those with negative screening results were put under observation, and they also underwent echocardiography if their oxygen saturation fell below 95% on room air during hospitalisation.
Results
This enhanced procedure detected 19 critical CHD cases, and seven of these diagnoses would have been delayed if POX had not been incorporated into the screening strategy. This means that the addition of POX increased the detection rate of critical CHD from 63.2 to 100%. The false‐positive rate of critical CHD screening using POX plus clinical evaluation was higher in NICU patients with high morbidity rates.
Conclusion
When pulse oximetry screening was added to clinical evaluation, it increased the number of critical CHD cases that were detected in our NICU. This method could provide a useful screening protocol for critical CHD cases.
Keywords: Clinical evaluation, Congenital heart disease, Neonatal intensive care unit, Neonatal screening, Pulse oximetry
Abbreviations
- CHD
Congenital heart disease
- NICU
Neonatal intensive care unit
- POX
Pulse oximetry
- SpO2
Blood oxygen saturation
Key notes.
Limited data are available regarding critical congenital heart disease (CHD) screening in neonatal intensive care unit (NICUs).
Our study of more 4000 patients showed that adding pulse oximetry increased the detection rate of critical CHD from 63.2 to 100% in the NICU.
It supports the feasibility of using POX plus clinical evaluation as a screening protocol for critical CHD in this paediatric setting.
Introduction
Critical congenital heart disease (CHD) is generally defined as a congenital heart defect that leads to death or requires surgery or catheter intervention within 28 days of life 1. Early detection can improve the outcome of newborn infants, especially in the case of critical duct‐dependent lesions in which closure of the ductus arteriosus can result in acute cardiovascular collapse, acidosis and death 2. Pulse oximetry (POX) is useful in the detection of critical CHD before hospital discharge and has been successful in decreasing missed cases to 4% 3, 4. In the United States, some states have established legislation to make POX screening of CHD mandatory before newborn infants are discharged from hospital. In most of the previous studies, the screening that was performed was similar to that performed in the normal newborn nursery on the infants with a gestational age of 35 weeks and above. However, limited data are available regarding critical CHD screening in neonatal intensive care unit (NICUs).
Approximately 10–12% of newborns are admitted to a NICU. Many NICUs monitor oxygen saturation (SpO2) for all admissions 5. But POX has seldom been used in NICUs for critical CHD screening, and there has been a lack of a strategy for physicians to detect critical CHD. Lyengar et al. reported 250 patients admitted to a NICU at Northwestern University, USA, who received critical CHD screening using POX, but did not report any positive results 6. In the United States, many state authorities recommend universal screening and do not differentiate between infants cared for in NICUs and those admitted to nurseries for newborn infants 7. Patients that fall into the following categories may be missed if their physical signs are absent or overlooked by clinicians: (i) infants with critical CHD who do not undergo a foetal or postnatal echocardiogram in the NICU (ii) and/or those infants who undergo echocardiography to evaluate patent ductus arteriosus but fail to receive a complete evaluation of the heart for structural malformations (iii) and/or those whose SpO2 is only low in a limb, where a pulse oximeter probe is not applied during the entire hospital stay 8.
In the current study, we conducted a single‐centre study to evaluate the feasibility of screening for critical CHD using POX plus clinical evaluation in the NICU, with a view to providing evidence for critical CHD screening in the NICU.
Methods
Study populations
The current study focused on 4185 newborn infants who were consecutively admitted to the NICU at the Children's Hospital of Fudan University, Shanghai, China, from October 1, 2012 to September 30, 2014. The NICU, which is the largest one in East China, receives patients from the city of Shanghai and the surrounding areas who are critical, were born in an unstable condition or have complex diseases. We excluded 57 newborn infants because they were prenatally diagnosed with CHD and had received a postnatal echocardiogram prior to screening. This meant that 4128 babies were eligible for screening, including 56.2% preterm infants with a gestational age of <35 weeks. All the babies were aged more than two hours because they were transferred to our NICU from other hospitals. As shown in Table 1, there were 113 babies with an extremely low birthweight and 662 with a very low birthweight and 58.6% of them were male. The screening was completed within the first day of admission.
Table 1.
Characteristics of newborns
| Total | GA ≤35 weeks | GA >35 weeks | |
|---|---|---|---|
| Screening cases (%) | 4128 | 2319 (56.2) | 1809 (43.8) |
| Male sex (%) | 2418 (58.6) | 1338 (32.4) | 1080 (26.2) |
| Gestational age (weeks) | 34.7 ± 3.9 | 31.9 ± 2.5 | 38.4 ± 1.8 |
| Age at screening (hours) | 25 (2–506) | 24 (2–278) | 27 (2–506) |
| Birthweight (g) | 2366.6 ± 861.6 | 1781.4 ± 515.1 | 3116.7 ± 593.4 |
| BW ≤ 1000 g (%) | 113 (2.7) | ||
| 1000 < BW ≤ 1500 g (%) | 662 (16.1) | ||
| BW > 1500 g (%) | 3353 (81.2) | ||
| Test‐positive cases (%) | 2866 (69.4) | 1670 (40.5) | 1196 (29.0) |
| Pulse oximetry alone (%) | 2308 (55.9) | 1390 (33.7) | 918 (22.2) |
| Clinical evaluation alone (%) | 963 (23.3) | 486 (11.8) | 477 (11.6) |
| Pulse oximetry or clinical evaluation (%) | 2485 (60.2) | 1461 (35.4) | 1024 (24.8) |
| Echocardiography test (%) | 3092 (74.9) | 1891 (45.8) | 1201 (29.1) |
| Oxygen therapy (%) | 2402 (58.2) | 1430 (34.6) | 972 (23.6) |
| NICU stay (days) | 13.7 ± 17.7 | 25.4 ± 21.8 | 6.3 ± 8.2 |
The current study was approved by the Ethics Committee of the Children's Hospital of Fudan University. All parents were informed of the study details, and verbal informed consent was obtained from the parents.
Screening and data collection
The screening was performed when the babies were admitted to the NICU (Fig. 1). The clinical evaluations were performed by trained physicians, based on five well defined components, which were a family history of CHD, central cyanosis, heart murmur, particular facial features and extracardiac malformations. Any newborn infant with one of these abnormal findings was considered to have screened positive.
Figure 1.
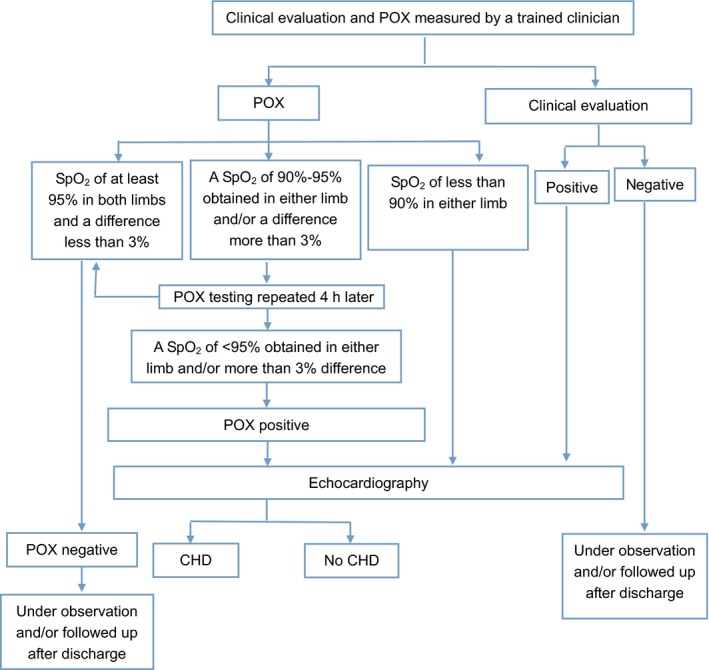
Critical CHD screening diagram in NICU.
Pulse oximetry testing was performed by the trained nurses, where the Radical‐5v (Masimo Corporation, Irvine, CA, USA) was used with a LNOP YI multisite reusable sensor (Masimo Corporation), based on the measurement criteria proposed by the United States Secretary of Health and Human Services 9. The functional saturations were measured in the right hand and either foot in a nonspecified order and the sensor secured around the palm of the baby's hand and sole of the foot with a disposable wrap. The POX testing was repeated four hours later if the first SpO2 measurement was between 90 and 95%. The screening was considered positive if: (i) an SpO2 of <95% was obtained on both the right hand and either foot during two measurements separated by four hours, (ii) the difference between the two extremities was >3% for two measurements separated by four hours or (iii) any measurement of SpO2 was <90%.
Echocardiography was undertaken within 48 hours for all babies who were classified as test positive. For those who were screened negative, but their SpO2 was less than 95% at a later stage during their hospitalisation, bedside echocardiography was undertaken before discharge. All the detected cases of CHD were registered.
Definition of critical CHD
We defined critical CHD as the following seven conditions recommended by the Secretary's Advisory Committee on Heritable Disorders in Newborn and Children 9: hypoplastic left heart syndrome, pulmonary atresia, tetralogy of Fallot, transposition of great vessels, truncus arteriosus, tricuspid atresia and total anomalous pulmonary venous return. Those significant defects that sometimes, but less consistently, cause hypoxaemia were also defined as critical CHD, for example, coarctation of the aorta, pulmonary stenosis, double outlet right ventricle, single ventricle, interrupted aortic arch and Ebstein anomaly, which needed surgery within 28 days of birth 10.
Statistical analysis
The number of critical CHD cases in the NICU and the distribution of critical CHD in different gestational ages was calculated in terms of sensitivity, specificity, positive and negative predictive value, negative and positive likelihood ratio and the 95% confidence interval (95% CI) of screening critical CHD by just POX, by just clinical evaluation and by POX plus clinical evaluation, respectively. The t test was used for the comparison between continuous variables and the chi‐square test for nonparametric statistics.
Results
Critical CHD detection
A total of 4128 newborn infants consecutively admitted to NICU without the prenatal diagnosis of CHD underwent the unit screening protocol. Of those, 69.4% of the infants screened positive, while 30.6% screened negative. A total of 19 critical CHD cases were detected (Fig. 2, Table 2). For those whose gestational age was less than 35 weeks, CHD was detected in the form of pulmonary stenosis, tetralogy of Fallot, single ventricle, transposition of great vessels, double outlet right ventricle, total anomalous pulmonary venous return, tricuspid atresia and Ebstein in addition to the most common forms of CHD, such as patent ductus arteriosus, atrial septal defect, ventricular septal defect. If POX had not been incorporated into the screening strategy, there would have been seven delayed diagnoses of critical CHD, including two tetralogy of Fallot, one single ventricle, two transposition of great vessels, one tricuspid atresia and one pulmonary atresia. Patent ductus arteriosus was observed in 1115 preterm infants (48.1%): 38 of these underwent surgical ligation and 156 received prostaglandin inhibitors to achieve closure. However, of the 1809 infants that had a gestational age of more than 35 weeks, 670 (37.0%) had patent ductus arteriosus, with only two receiving surgical ligation and another two being treated with prostaglandin inhibitors. Persistent pulmonary hypertension was observed in 1233 infants, including 1044 who also had other forms of CHD. Incorporating POX into the screening strategy prevented six persistent pulmonary hypertension cases from being delayed.
Figure 2.
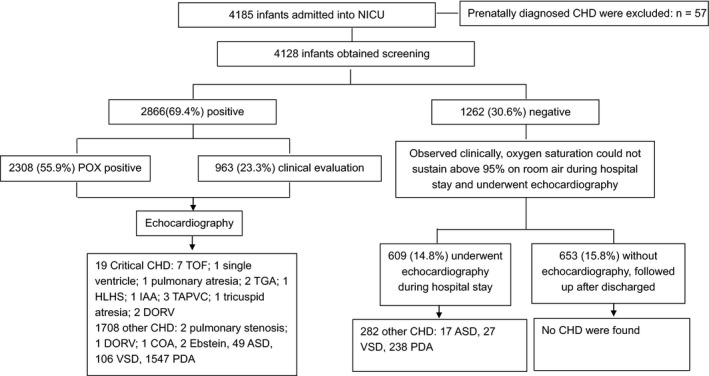
Profile of the study.
Table 2.
Spectrum of CHD diagnosed by echocardiography
| Predominant defect | Number | Gestational age ≤35 weeks | Gestational age >35 weeks | Description |
|---|---|---|---|---|
| Critical CHD | ||||
| TOF | 7 | 3 | 4 | Five of them were premature infants (two of them with SGA and one of them with malformation); the other two were omphalocele |
| Single ventricle | 1 | 1 | 0 | Premature infant (one of the twins) |
| Pulmonary atresia | 1 | 0 | 1 | Term baby with malformation |
| TGA | 2 | 1 | 1 | One was SGA, another one was premature infant with hypoglycaemia |
| HLHS | 1 | 0 | 1 | Term infant with asphyxia |
| IAA | 1 | 0 | 1 | Premature infant (infant of diabetic mother, IDM) |
| TAPVC | 3 | 1 | 2 | Term infant with aspiration pneumonia; the other two were premature infants with NRDS and hypoglycaemia |
| Tricuspid atresia | 1 | 1 | 0 | Combined with right ventricular dysplasia, premature infant |
| Other CHD | ||||
| Pulmonary stenosis | 2 | 1 | 1 | |
| DORV | 3 | 1 | 2 | 2 cases underwent surgery during neonatal period, defined as CCHD. Both of them were SGA with transient tachypnea of newborn |
| COA | 1 | 0 | 1 | |
| Ebstein | 2 | 1 | 1 | |
| ASD | 66 | 36 | 30 | Combined with PDA or PPHN |
| VSD | 133 | 71 | 62 | Combined with PDA or PPHN |
| PDA | 1785 | 1115 | 670 | 40 cases underwent surgery, 38 cases whose GA ≤35 weeks; 158 cases were treated by medication; 365 cases closed naturally; and the remaining 1222 cases were followed up after discharge |
| Total | 2009 | 1232 | 777 | |
The accuracy of different screening indicators for critical CHD
As shown in Table 3, just using POX as a screening method detected 16 of the 19 (84.2%) cases of critical CHD and just using clinical evaluation as a screening method detected 12 of the 19 (63.2%) cases of critical CHD. But when POX and clinical evaluation were both used, this detected all 19 (100%) cases of critical CHD. Using just POX as a screening indicator showed that three critical CHD cases were missed, and just using clinical evaluation indicated that seven critical CHD cases were missed. But when POX was combined with clinical evaluation, no critical CHD cases were missed.
Table 3.
Accuracy of screening method for Critical CHD in NICU
| POX | Clinical evaluation | POX + clinical evaluation | |
|---|---|---|---|
| True positives | 16 | 12 | 19 |
| False negatives | 3 | 7 | 0 |
| False positives | 2292 | 951 | 2466 |
| True negatives | 1817 | 3158 | 1643 |
| False‐positive rate (%) | 55.8 | 23.1 | 60.0 |
| Sensitivity (%) | 84.2 (62.43, 94.48) | 63.2 (41.04, 80.85) | 100 (83.18, 100) |
| Specificity (%) | 44.22 (42.71, 45.74) | 76.86 (75.54, 78.12) | 39.99 (38.50, 41.49) |
| Positive predictive value (%) | 0.69 (0.43, 1.12 ) | 1.25 (0.71, 2.17) | 0.77 (0.49, 1.19) |
| Negative predictive value (%) | 99.84 (99.52, 99.94) | 99.78 (99.54, 99.89) | 100 (99.77, 100 ) |
| Diagnostic accuracy (%) | 44.44 (42.89, 45.92) | 76.79 (75.48, 78.06) | 40.26 (38.78, 41.77) |
| Positive likelihood ratio | 1.51 (1.47–1.55) | 2.73 (2.48–3.01) | 1.666 (1.665–1.668) |
| Negative likelihood ratio | 0.36 (0.19–0. 69) | 0.48 (0.36–0.63) | 0.00 |
Data: number or percentage (95% CI).
Table 4.
False‐positive rate of screening methods for detecting Critical CHD between different gestational ages in NICU
| POX | Clinical evaluation | POX + clinical evaluation | ||||
|---|---|---|---|---|---|---|
| ≤35 weeks | >35 weeks | ≤35 weeks | >35 weeks | ≤35 weeks | >35 weeks | |
| True positives | 6 | 10 | 3 | 9 | 7 | 12 |
| False negatives | 1 | 2 | 4 | 3 | 0 | 0 |
| False positives | 1384 | 908 | 483 | 468 | 1454 | 1012 |
| True negatives | 928 | 889 | 1829 | 1329 | 858 | 785 |
| False‐positive rate (%) | 59.9 | 50.5 | 20.9 | 26.0 | 62.9 | 56.3 |
| Sensitivity (%) |
85.7 (48.7, 97.4) |
83.3 (55.2, 95.3) |
42.9 (15.8, 75.0) |
75.0 (46.8, 91.1) |
100 (64.6, 100) |
100 (75.8, 100) |
| Specificity (%) |
40.1 (38.2, 42.2) |
49.5 (47.2, 51.8) |
79.1 (77.4, 80.7) |
74.0 (71.9, 75.9) |
37.1 (35.2, 39.1) |
43.7 (41.4, 46.0) |
Data: number or percentage (95% CI).
Discussion
Before we carried out this study, there was no agreement about whether critical CHD should be screened in our NICU and no clear strategy for such screening. Many clinicians working in level three neonatal units, where high‐risk babies are cared for, are now facing the question of whether critical CHD screening using POX should be instituted in NICUs as well. This study will help individual NICUs to make decisions about, and institute policies about, carrying out critical CHD screening in their NICU. Kemper et al. 7 suggested detecting critical CHD by POX in stable late preterm infants and term infants in well infant and intermediate care nurseries. But there was no evidence‐based protocol for POX screening infants in the NICU. The importance of screening critical CHD in the NICU had been recognised, and neonatologists have voiced concern that only screening babies in well infant and intermediate care nurseries would miss detecting critical CHD babies in the NICU. One study reported that approximately 10–15% of American babies who were treated in the NICU each year who underwent multiple physical examinations and were continuously monitored for their SpO2 10. But there has been limited literature on the use of POX to screen critical CHD in the NICU. The results of our current study showed that the POX screening protocol, as employed in our previous national study 11, could also be applied in the NICU.
In our hospital, we adopted the third option recommended by Suresh 8, which was to screen all infants admitted to the NICU. The advantage of this approach was that it could detect critical CHD in preterm babies aged less than 35 weeks. As preterm infants face an equal or higher risk of having CHD than term infants, it is wise to screen them as well. As recommended by Suresh, screening all infants would allow the NICU to operate in an unambiguous manner, as it would be easier to implement a reliable system of screening if it was applied to all NICU infants instead of just a subset of NICU infants. A clear unified screening protocol can also help NICU staff to develop good screening compliance.
As no effective screening strategy for critical CHD was available for the NICU, we designed a screening protocol based on our own resources. The clear indicators and timings of screening can facilitate clinicians to make decisions in the NICU and routine screening can avoid a delayed diagnosis of critical CHD. In our current study, 19 critical CHD cases were detected in a timely manner after admission and therefore treated promptly. If we hadn't undergone simultaneous preductal and postductal SpO2 monitoring to screen critical CHD, we would have missed one case of transposition of the great vessels and six persistent pulmonary hypertension cases, resulting in delayed treatment. If there had been no screening strategy that included POX, these critical CHD could still have been detected, but it would have taken a certain amount of time to reach a clear diagnosis. If the detection of duct‐dependent lesions had been delayed, this could have led to serious consequence. And if clinical evaluation had not been included in the strategy, the detection of two tetralogy of Fallot and one double outlet right ventricle would have been delayed.
One significant difference between neonates not admitted to a NICU and our neonates was the high rate of false‐positive screening results in the NICU population. Meta‐analyses of large population‐based studies of newborn pulse oximetry screening conducted after the first 24 hours after delivery among asymptomatic newborn infants demonstrated a low false‐positive rate of 0.035–0.05% 12. In our current study, the false‐positive rate was high because it was conducted in a NICU and, for example, the population included sepsis, pneumonia and more premature babies. Actually, if there had been no screening, at least half of the admissions to our NICU would have had an echocardiogram for medical indications. In this study, we carried out 640 additional confirmatory echocardiography to ensure positive screening results. However, all the CHD cases were found in a timely manner, including patent ductus arteriosus, which was a particular issue for preterm babies. In China, one echocardiography costs 200 Chinese Yuan, which is equivalent to approximately 30 U.S. dollars, which is not expensive. This means that the results were of greater significance in terms of saving those neonatal cases of critical CHD. Other costs were not precisely defined, but it seemed that no additional burden was imposed on the clinicians because they had no trouble accepting POX monitoring in the NICU, as every infant underwent continuous POX monitoring in the NICU until discharge. Actually, only 22 infants were measured for POX again four hours after the first measurement in our study. The clinicians were well trained, and the simple test took little time, with no additional human resources and equipment costs incurred.
It is difficult to determine the optimal timings for screening critical CHD in the NICU. Because infants admitted to the NICU usually suffer from acute cardiorespiratory disease, they tend to be put on oxygen therapy. Echocardiography is cheap and the disease itself, or its therapy, such as ventilation or supplemental oxygen, would interfere with the accuracy of screening. That would lead to false‐negative screens, as in the case of oxygen therapy for lung disease, which masks low saturation from some heart lesions, or resulting in false positives, as in the case of an infant with lung disease who would have low oxygen saturation falsely attributed to heart disease 8. Therefore, the screening would have to be performed when the infants had recovered from their disease. As diseases can take different courses, this would make it difficult to decide screening timings. To address the problem, the simple rule would be to test all eligible infants 24–48 hours before hospital discharge; otherwise, the diagnosis for critical CHD would be delayed. Manja et al. 5 reported that they performed screening 24–48 hours before discharge along with other screening tests, such as hearing, and believed that such a screen could be conducted earlier. In the current study, we performed screening within 24 hours of admission. Although the false‐positive rate increased significantly, no critical case of CHD was missed and they were detected quickly. As we already known, critical CHD interventions are typically performed in the first weeks of life to optimise haemodynamics and prevent the end‐organ injury associated with delayed diagnosis. Because timely recognition of critical CHD could improve outcomes, it is important to identify and evaluate strategies to enhance early detection.
Conclusion
Pulse oximetry still plays an important role in screening critical CHD in NICUs. Based on clinical evaluation, pulse oximetry screening can significantly enhance the early detection rate of critical CHD. This suggests that POX plus clinical evaluation should be applied as a screening protocol for critical CHD in NICUs.
Funding
Our study was funded by the Shanghai Public Health Three‐Year Action Plan Project, sponsored by the Shanghai Municipal Government (G‐y H, number 2015‐82), and the National Key Research and Development Program (G‐y H, number 2016YFC1000500).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1. Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, Thangaratinam S, et al. Pulse oximetry screening for congenital heart defects in newborn infants (Pulse Ox): a test accuracy study. Lancet 2011; 378: 785–94. [DOI] [PubMed] [Google Scholar]
- 2. Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP. Pediatrics 2009; 124: 823–36. [DOI] [PubMed] [Google Scholar]
- 3. de‐Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganäs L, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 2009; 338: a3037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine—results from a prospective multicenter study. Eur J Pediatr 2010; 169: 975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manja V, Mathew B, Carrion V, Lakshminrusimha S. Critical congenital heart disease screening by pulse oximetry in a neonatal intensive care unit. J Perinatol 2015; 35: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyengar H, Kumar P, Kumar P. Pulse‐oximetry screening to detect critical congenital heart disease in the neonatal intensive care unit. Pediatr Cardiol 2013; 35: 406–10. [DOI] [PubMed] [Google Scholar]
- 7. Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics 2011; 128: e1259–67. [DOI] [PubMed] [Google Scholar]
- 8. Suresh GK. Pulse oximetry screening for critical congenital heart disease in neonatal intensive care units. J Perinatol 2013; 33: 586–8. [DOI] [PubMed] [Google Scholar]
- 9. Chang RK, Rodriguez S, Kiltzner TS. Screening newborns for congenital heart disease with pulse oximetry: survey of pediatric cardiologists. Pediatr Cardiol 2009; 30: 20–5. [DOI] [PubMed] [Google Scholar]
- 10. Lakshminrusimha S, Turkovich S, Manja V, Nair J, Kumar VH. Critical congenital heart disease screening with pulse oximetry in the neonatal intensive care unit. E‐J Neonatal Res 2012; 2: 96–101. http://www.neonatologyresearch.com/wp-content/uploads/2012/04/CHD-Screening3.pdf [Google Scholar]
- 11. Zhao QM, Ma XY, Ge XL, Liu F, Yan WL, Wu L, et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a perspective study. Lancet 2014; 384: 747–54. [DOI] [PubMed] [Google Scholar]
- 12. Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta‐analysis. Lancet 2012; 379: 2459–64. [DOI] [PubMed] [Google Scholar]


