Abstract
Long‐term survival rates for advanced ovarian cancer patients have not changed appreciably over the past four decades; therefore, development of new, effective treatment modalities remains a high priority. Tumor Treating Fields (TTFields), a clinically active anticancer modality utilize low‐intensity, intermediate frequency, alternating electric fields. The goal of this study was to evaluate the efficacy of combining TTFields with paclitaxel against ovarian cancer cells in vitro and in vivo. In vitro application of TTFields on human ovarian cancer cell lines led to a significant reduction in cell counts as compared to untreated cells. The effect was found to be frequency and intensity dependent. Further reduction in the number of viable cells was achieved when TTFields treatment was combined with paclitaxel. The in vivo effect of the combined treatment was tested in mice orthotopically implanted with MOSE‐LTICv cells. In this model, combined treatment led to a significant reduction in tumor luminescence and in tumor weight as compared to untreated mice. The feasibility of effective local delivery of TTFields to the human abdomen was examined using finite element mesh simulations performed using the Sim4life software. These simulations demonstrated that electric fields intensities inside and in the vicinity of the ovaries of a realistic human computational phantom are about 1 and 2 V/cm pk‐pk, respectively, which is within the range of intensities required for TTFields effect. These results suggest that prospective clinical investigation of the combination of TTFields and paclitaxel is warranted.
Keywords: ovarian cancer, paclitaxel, tumor treating fields, combination therapy
Short abstract
What's new?
Tumor Treating Fields (TTFields), in which tumor cell division is disrupted by exposure to alternating electric fields, are a promising therapeutic strategy against cancer. In this study, TTFields are shown to enhance the efficacy of paclitaxel in ovarian cancer, both in vitro and in vivo. The feasibility of effectively delivering TTFields across a large nonuniform volume, encompassing ovaries and potential metastatic sites, is demonstrated via electric field measurements in mice and through finite‐element mesh simulations. The results have given impetus to an open‐label pilot investigation of TTFields administered in combination with paclitaxel in patients with recurrent ovarian cancer.
Abbreviations
- 7-AAD
7-aminoactinomycin D
- ATCC
American Type Culture Collection
- C57Bl/6
C57 black 6
- CI
combination index
- DAPI
4′,6‐diamidino‐2‐phenylindole
- DMSO
dimethyl sulfoxide
- FEM
finite element method
- IP
intraperitoneal
- MOSE
mouse ovarian surface epithelium
- OS
overall survival
- pk‐pk
peak‐to‐peak
- PFS
progression‐free survival
- p/s/sr
photons per second per steradian
- TICv
tumor‐initiating variant
- TTFields
Tumor Treating Fields.
Ovarian cancer continues to be the leading cause of death among all gynecological malignancies and is the fourth cause of death in women in Western countries.1 Most ovarian tumors originate from the surface epithelium lining the ovaries or the fallopian tubes and are usually diagnosed in the advanced Stage III or IV of the disease (accounting for 75% of all ovarian cancer cases).2 The lack of obvious symptoms in early stages, when the tumor is localized to the ovary, decreases the likelihood of early detection and, thus, the probability of successful treatment. The standard of care for epithelial ovarian cancer consists of surgical debulking and combination chemotherapy with platinum and taxane (usually paclitaxel) chemotherapy agents.2 Although primary treatment is generally characterized by a high response rate, the disease reoccurs in nearly 70% of the patients.3 Second‐line treatment offers only modest improvement in survival, thus rendering the reported 5‐year relative survival rate at 27.4% for patients with metastatic disease.4, 5 Current efforts are focused on developing novel strategies that can enhance treatment efficacy and prevent disease recurrence. Most attempted treatment strategies, however, did not show a survival advantage, while some showed improvement in progression‐free survival (PFS) without improving overall survival (OS).2, 5, 6, 7 The limited effectiveness of front‐line and maintenance therapy drives a search for new strategies that enhance the effects of surgery and chemotherapy and prolong PFS and OS. Tumor‐treating fields (TTFields) are a clinically active anticancer modality delivered via continuous noninvasive application of low‐intensity, intermediate‐frequency, alternating electric fields to the region of a tumor.8 TTFields are delivered through two sets of transducer arrays so that they generate two electric fields oriented perpendicular to one another, within the patient's body.8 Previous studies have demonstrated the effectiveness of TTFields application against various cancerous cell lines and animal tumor models.8, 9, 10, 11, 12 Several pilot clinical trials and larger randomized studies in patients with solid tumors including glioblastoma and non‐small cell lung cancer, have demonstrated the safety as well as effectiveness of continuous TTFields application in patients.13, 14 Previous studies provide evidence on the direct effect of TTFields on spindle assembly in replicating cells. Specifically, TTFields were shown to destabilize microtubules consequently leading to spindle disruption and mitotic catastrophe.15
Paclitaxel chemotherapy constitutes one of the major components in the backbone for the initial therapy of ovarian cancer. Conventional first‐line chemotherapy for patients with optimally, as well as sub‐optimally debulked disease, consists of combination chemotherapy with platinum agent (carboplatin or cisplatin) plus paclitaxel, administered as described in the Gynecologic Oncology Group (GOG) protocols 158 and 111.16 Paclitaxel is also administered as standard second‐line treatment for patients who developed platinum resistance. Here, we investigated the effects of TTFields in combination with paclitaxel on ovarian cancer both in vitro and in vivo. A realistic human abdomen computational phantom was utilized in order to verify whether clinically TTFields of sufficient intensity can be delivered within the abdomen for potential therapeutic use in ovarian cancer patients.
Material and Methods
Cell culture and drugs
The human ovarian carcinoma cell line A2780 was obtained from the European Collection of Cell Cultures. The human ovarian adenocarcinoma cell lines OVCAR‐3 (HTB‐161) and Caov‐3 (HTB‐75) were obtained from the American Type Culture Collection (ATCC). Spontaneously transformed mouse ovarian surface epithelium (MOSE) were developed from C57BL/6 mice and characterized previously.17 MOSE cells were transduced with firefly luciferase lentiviral particles (GeneCopoeia) (MOSE‐LTICv) to facilitate live in vivo imaging of tumor outgrowth. Paclitaxel (Sigma Aldrich, Rehovot, Israel) stock solution was prepared in DMSO and diluted in cell culture media immediately prior to use so that final DMSO concentration did not exceed 0.1%.
TTFields application in vitro
TTFields were applied for 72 hrs using the inovitro system (Novocure, Israel) as previously described.8, 11
Cell count and cell volume
Inhibition of cell growth was analyzed by quantitatively determining cell count using a Scepter 2.0 automated cell counter (EMD Millipore). Cell volume measurements were also collected. The relative number of cells at the end of treatment was expressed as a percentage of untreated control cells. The combination index (CI) for quantification of the interaction between TTFields and paclitaxel was calculated as described18 with a correction to the sigmoidal shape of the A2780 response curve to the drug.
Flow cytometry
For detection of apoptosis, cells were double‐stained with FITC‐conjugated Annexin V (MEBCYTO 4700 Apoptosis Kit; MBL) and 7‐Aminoactinomycin D (7‐AAD; BioLegend) as per manufacturer's instructions. Data acquisition was obtained using iCyt EC800 (Sony Biotechnology) flow cytometer. For cell cycle analysis, cells were washed twice with PBS and fixed with 70% ice‐cold ethanol for 30 min. After fixation, cells were pelleted and incubated in PBS containing 10 μg/ml RNase and 7.5 μg/ml 7‐AAD (Sigma‐Aldrich) at 37°C for 30 min. Cell cycle distribution was then quantified using iCyt EC800. Fluorescence signals were collected at the wavelengths of 525/50 nm for Annexin V and 665/30 nm for 7‐AAD. The data was analyzed using the Flowjo software (TreeStar).
Microscopy
For mitotic figures analysis, cells were grown on glass cover slips and treated using the inovitro system for 72 hrs. At the end of the experiment, cells were fixed with ice‐cold methanol for 10 min. The cells were then serum‐blocked, and stained with rabbit anti‐human α‐tubulin antibodies (Abcam) for 2 hrs. Alexa Fluor 488–conjugated secondary antibody was used (Jackson ImmunoResearch). DNA was stained with the dye 4′,6‐diamidino‐2‐phenylindole (DAPI) (Sigma‐Aldrich) at 0.2 µg/ml for 20 min. Images were collected using a LSM 700 laser scanning confocal system, attached to an upright motorized microscope with ×20 and ×63/1.40 oil objective (ZeissAxio Imager Z2).
Efficacy of the combination therapy in animal models
All animal studies were approved by the Novocure Internal Animal Care Committee in accordance with the Technion‐Israel Institute of Technology guidelines for the care of laboratory animals. Female C57Bl/6 mice, 10–12‐weeks of age (Harlan Laboratories, Jerusalem, Israel), were injected (5 µl) directly into the ovarian bursa with MOSE cells transfected with the luc gene (MOSE‐LTICv) (1 × 103 cells), suspended in high concentration growth factor reduced matrigel (1:1 volume, BD Biosciences, Bedford, MA). Treatment was initiated 2 days after the tumor luminescence intensity was determined to be within the range of 200–5,000 photons per second per steradian (p/s/sr) for the whole mouse or per signal area (cm2). Mice received a single IP injection of paclitaxel (20 mg/kg body weight). Mice that did not receive chemotherapy, received an IP injection of Saline solution with DMSO (vehicle control). Application of TTFields began immediately after paclitaxel injection and was maintained for 7 days. For the application of TTFields, a set of four insulated transducers was placed around the mouse abdomen so as to generate fields in perpendicular directions. Control mice were treated by means of sham heat transducers. The transducers were connected to the NovoTTF‐100A device or the sham device. At the end of treatment, transducers were removed and the tumor luminescence was determined as described. After the luminescence measurements, mice were euthanized and the tumors were removed. The total tumor weight was determined using analytical scales.
Tumor imaging
At different time points after tumor cells injection, mice were anesthetized and injected with luciferin to a final concentration of 150 mg/kg body weight. Mice were placed inside the Biospace photon imager equipped with a highly sensitive, cooled charge coupled device camera mounted in a light‐tight specimen box (Biospace Lab, France), and monitored for 10 min for a bioluminescent reaction. Identical illumination settings were used for all images. Luminescence emission from the bioluminescent cells was normalized to p/sec/sr for the whole mouse or per signal area (cm2).
Finite element mesh simulations
The finite element method (FEM) is a commonly used technique for calculating the electric field distribution in complex geometries such as the human body.19 To calculate the distribution of TTFields in the abdomen, FEM simulations were performed using the Sim4Life 1.2 Software package (ZMT, Zurich, Switzerland). The Ella computational phantom of a healthy 26‐year‐old female was used for the simulations. The permittivity and conductivity of all tissues other than skin and fat were assigned to the phantom based on the Gabriel Model,20 which is built into the software material database. In order to match the total resistance of the model to the typical resistance measured in patients, the conductivity values for skin and fat were set manually to 0.02 S/m (instead of 0.0007 for dry skin) and 0.08 S/m (instead of 0.024), respectively. These values are at the higher range of conductivity reported for these tissue types.21, 22 To “generate” TTFields within the phantom, transducer arrays were placed on the phantoms body, and Derichlet boundary conditions of a 200 V pk‐pk voltage difference were set between the two arrays. The low frequency quasistatic electric field solver of the software package was used to perform the simulations. To simulate a possible treatment protocol, two pairs of transducer arrays were placed on the phantom body. One pair of arrays was placed on the abdomen and the back of the phantom, and the other pair of arrays was placed on the left and right sides of the body. Both pairs of arrays were placed just above the pelvic bone. The electric field distribution generated by each pair was calculated separately for each voxel. The maximal field intensity at each voxel was used for all the calculations.
Statistical analysis
Unless stated otherwise, data are expressed as mean ± SE. Multiple comparisons were analyzed by a one way ANOVA, and differences in the mean values among groups were conducted by a Dunnett post‐hoc correction. All experiments were repeated at least three times.
Results and Discussion
TTFields induce frequency and intensity dependent reduction in viability of human ovarian cancer cells in vitro
The inhibitory effect of TTFields on ovarian cancer cell growth in vitro was investigated using three human ovarian cancer cell lines (A2780, OVCAR3, and Caov‐3). Our previous observations suggest a cell type–specific optimal effective frequency for TTFields therapy.9, 10 Therefore, for in vitro studies, TTFields (4.6 V/cm pk‐pk) were applied at the frequency range of 100 to 400 kHz. Frequency titration curves demonstrated that the inhibitory effects of TTFields were maximal at 200 kHz for all tested ovarian cancer cell lines (Figs. 1 a–1c). Application of 200 kHz TTFields for 72 hrs resulted in a 77, 70 and 75% reduction in the cell count of A2780 (p < 0.001), OVCAR3 (p < 0.001), and Caov‐3 (p < 0.001) cells, respectively, compared to the control. It had been previously suggested that the cell volume is a decisive feature in determining the optimal TTFields frequency.11 This hypothesis was based on the assumption that membrane and cytoplasm permittivity and resistivity are similar between various cell lines. However, our results show that while the optimal frequency was identical for all three cell lines tested, significant differences were observed in their cell volume (A2780:2.51pL; OVCAR3: 5.4 pL; Caov‐3: 4.25 pL). This indicates that other parameters, such as cell specific membrane electrical properties or plasma contents, could influence the optimal frequency to a greater extent than the cell volume. The relationship between TTFields intensity at 200 kHz and cell counts was also evaluated. In all cell lines, treatment efficacy was a function of electric fields intensity (Figs. 1 d–1f). Significant reduction in cell counts were observed already at 1.53 V/cm [A2780 (p < 0.05), OVCAR3 (p < 0.001), and Caov‐3 (p < 0.05)].
Figure 1.
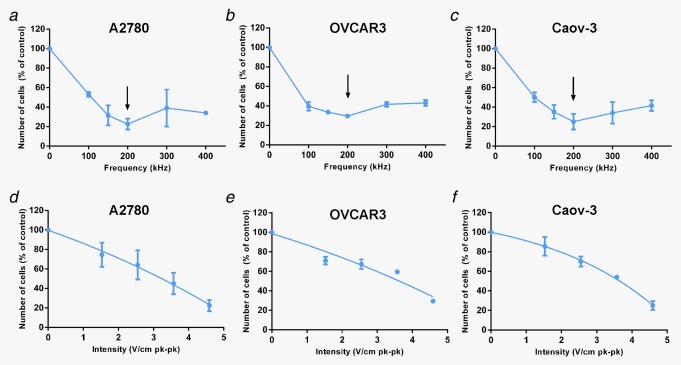
In vitro efficacy of TTFields in ovarian cancer cells. Ovarian Cancer Cells were treated for 72 hr with TTFields of different frequencies (100–400 kHz) or intensities (1.53–4.59 V/cm pk‐pk). Effect of TTFields treatment estimated using cell counts: (a, d) A2780, (b, e) OVCAR‐3, (c, f) Caov‐3. Arrow indicates optimal frequency.
Combination of TTFields and paclitaxel chemotherapy enhances the efficacy of paclitaxel in treatment of ovarian cancer cells in vitro
To assess whether adding TTFields to paclitaxel affects the responsiveness of ovarian carcinoma cells, we treated the cells with paclitaxel alone and in combination with TTFields (2.7 V/cm pk‐pk, 200 kHz) for 72 hrs. Dose‐response curves demonstrate that combining TTFields with paclitaxel enhances treatment efficacy in all tested cell lines, as expressed by a shift to the left of the combined treatment dose curve (Figs. 2 a–2c). Specifically, the combination indexes (CI) were 1.03 (A2780), 0.81 (OVCAR‐3) and 0.86 (Caov‐3), indicating additive effect in A2780 cells and synergism of the two regimens in OVCAR‐3 and Caov‐3 cell lines. We next investigated whether TTFields application increased paclitaxel‐induced apoptosis. Consistent with the cell growth inhibition, TTFields significantly increased apoptosis induced by paclitaxel in a concentration dependent manner (Figs. 2 d–2f). These preclinical data are in line with prior studies which demonstrated efficacy benefits for the combination of TTFields and paclitaxel derivatives in other cancer models including: breast, kidney, and non‐small cell lung cancers.10, 12 The enhanced effect of the combined treatment may be attributed to the fact that TTFields and paclitaxel share tubulin as their cellular target. Tubulin dimers are known to have a very large electrical dipole.23 It was postulated that the forces exerted by the electric fields on these dimers, interfere with microtubule dynamics, thus leading to the disruption of normal mitotic spindle assembly.8, 11 Paclitaxel prevents normal formation of mitotic spindle by enhancing the affinity of the interaction between individual tubulin dimers, subsequently increasing the fiber length of the spindle microtubules.24 This in turn, leads to an increase in the average fiber dipole moment, which could potentially increase TTFields‐induced forces and sensitize cells to TTFields. As both TTFields and paclitaxel exert their toxicity on mitotic cells that exhibit rapid microtubule dynamics,15, 25 we examined cell cycle progression upon treatments. Flow cytometry analysis revealed that cells exposed to paclitaxel were blocked in cell cycle progression and accumulated in G2/M phase. Similar to the induction of growth arrest and apoptosis, this accumulation in G2/M was also dose dependent, confirming previous reports demonstrating that paclitaxel acts as a phase‐specific agent.25, 26 Cells exposed to TTFields exhibited only a small increase in the accumulation in G2/M phase. Importantly, 72 hrs simultaneous treatment with low dose of paclitaxel (2, 4 and 10 nM) and TTFields, dramatically increased the number of Caov‐3 and OVCAR‐3 cells in the G2/M phase of the cell cycle (Figs. 3 a–3d). A2780 cells exposed to the combination of low dose paclitaxel and TTFields accumulated in G2/M even after short treatment duration (8 hrs). After longer treatment duration (72 hrs) G2/M accumulation was replaced by polyploid appearance of cells in G1 (data not shown). To verify these effects observed by flow cytometry, we examined the appearance of mitotic figures following the different treatments using confocal microscopy. In all tested cell lines, combination treatment with TTFields and low dose paclitaxel displayed a substantial increase in mitotic figures, indicative of mitotic arrest (Fig. 3 e). Interestingly, while abnormal spindle formation and improper attachment of chromosomes to the spindle fibers were prevalent amongst cells treated with TTFields, as previously described,15 multipolar spindles were common in the combined treatment. Taken together, these results provide further evidence for the synergy between paclitaxel and TTFields in the treatment of ovarian cancer cells. Clinical trials studying combination of spindle poisons have already shown superior antitumoral activity for combination treatments.27, 28 It is possible that TTFields therapy concomitant with paclitaxel can be an active therapeutic modality, provided that the overall toxicity is tolerable. Concomitant TTFields therapy and paclitaxel can complement paclitaxel dosing strategy in recurrent ovarian cancer.29 More efficacious low toxicity, well tolerated weekly low‐dose paclitaxel regimens, could positively impact treatment outcome.
Figure 2.
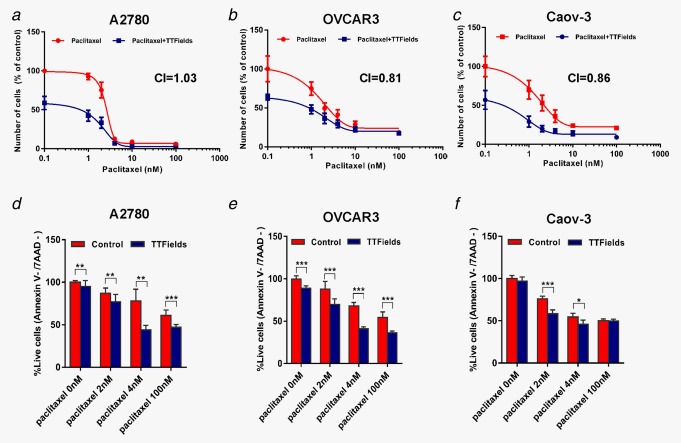
Combination of TTFields and paclitaxel chemotherapy. Ovarian Cancer Cells were treated for 72 hr with paclitaxel alone (1–100 nM) and in combination with TTFields (2.7 V/cm pk‐pk, 200 kHz). Dose–response plots of (a) A2780, (b) OVCAR‐3 and (c) Caov‐3 cells. CI: combination index. (d–f) Cellular viability of (d) A2780, (e) OVCAR‐3 and (f) Caov‐3 cells examined using flow cytometry analysis (Annexin V‐/7AAD‐). Mean ± SD 0.05 > *p > 0.01, **p < 0.01, and ***p < 0.001 from corresponding control group, student's t‐test.
Figure 3.
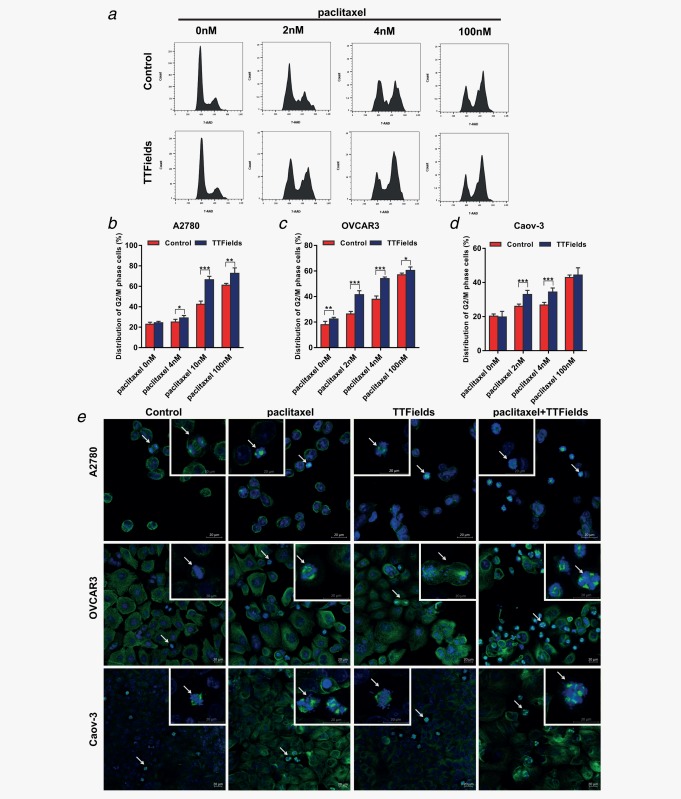
Cell cycle effects of TTFields and paclitaxel combination on ovarian cancer cells. (a–d) Cell cycle analysis performed using flow cytometry. (a) Representative plots of cell cycle distribution following the different treatments (OVCAR‐3 cells). (b–d) Changes in the percentage of cells in G2/M phase following treatment (A2780:8 hr, OVCAR‐3 and Caov‐3:72 hr). (E) Confocal fluorescence microscopy images of mitotic figures (A2780:8 hr, OVCAR‐3 and Caov‐3:72 hr). Arrows indicate cells in small micrographs (metaphase and late anaphase). Blue, DAPI‐stained DNA; green, tubulin. The scale bar represents 20 µm. Mean ± SD 0.05 > *p > 0.01, **p < 0.01 and ***p < 0.001 from corresponding control group, student's t‐test.
Application of TTFields and paclitaxel enhances treatment efficacy in murine model of ovarian cancer
Models that closely resemble human ovarian cancer were developed by several groups utilizing similar methodologies.17 In this study, we used spontaneously transformed MOSE cells transfected with the firefly luciferase gene (MOSE‐LTICv) for the establishment of a syngeneic ovarian cancer model in mice.17 This model was used for testing the efficacy of the combined treatment of TTFields and paclitaxel in vivo. For this purpose, MOSE‐LTICv cells were orthotopically implanted into the ovarian bursa of female C57BL/6 mice. Treatment was initiated 2 days after the tumor luminescence intensity was determined to be within the range of 200–5,000 p/sec/sr, and applied for 7 days (Fig. 4 a). Tumor volume (expressed as luminescence intensity) (Fig. 4 b) and tumor weight (Fig. 4 c) were compared to sham control tumors. Paclitaxel significantly reduced the tumor volume (p < 0.05); while the tumor volume in the TTFields‐treated mice was lower than control mice, this was not statistically significant. However, the combination treatment of paclitaxel and TTFields resulted in a significantly lower tumor volume compared to the untreated controls (p < 0.001), paclitaxel (p < 0.05) or TTFields (p < 0.05) treated mice. Since ascites, common in patients with advanced disease, could alter field distribution and intensity, in a separate experiment, we also performed in vivo field measurements in the ovaries of control mice that had saline infused IP to mimic accumulation of ascitic fluid. These measurements demonstrated that IP injection of 1.5 ml saline to mice with an average weight of 20 g (7.5% v/w; equivalent to ascitic fluid volume of up to 3 l in human) led to a 14% reduction in the electric fields intensities (Fig. 4 d).
Figure 4.
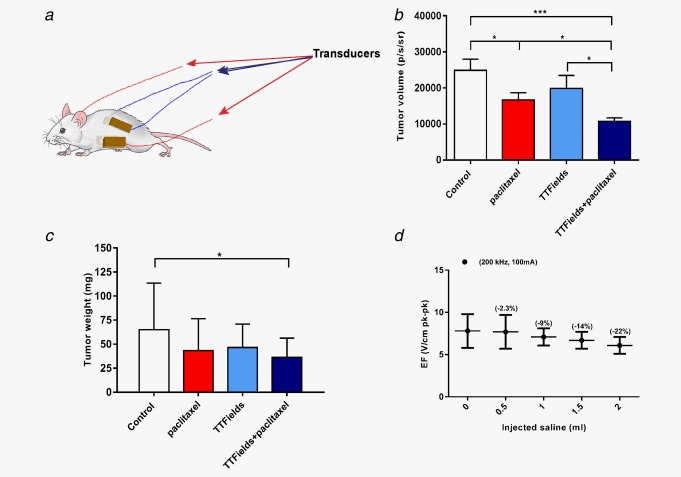
In vivo treatment effects. (a) Scheme of transducers placement around the mouse abdomen. Luminescence was measured from Mice (C57BL/6) implanted orthotopically with 1 × 103 MOSE‐LTICv cells and either left untreated (Control), treated with paclitaxel, treated with TTFields or treated with the combination of the two regimens. (b) Tumor growth as evaluated using the bioluminescent signal from tumors (Mean ± SE; student's t‐test). (c) Tumor weight (Mean ± SD; student's t‐test). N = 10 independent repeats, n = 6 to 9 mice in each group. (d) Dependence of TTFields electric field (EF) intensity (in brackets‐ % reduction in EF intensity), (as measured inside the ovaries) on ascitic fluid volume. 0.05 > *p > 0.01, **p < 0.01 and ***p < 0.001 from control group, student's t‐test.
Simulations of distribution of TTFields intensity inside and in the vicinity of ovaries
In locally advanced disease, ovarian cancer cells disseminate into the peritoneal cavity and form cell clusters in the ascitic fluid. As metastatic dissemination progresses, tumor cells encase the reproductive organs and sigmoid colon. Other common sites for distant metastatic dissemination are the omentum and peritoneum.30 Therefore, we calculated TTFields intensities in the vicinity of the ovaries to encompass possible dissemination sites. For this purpose, we performed finite‐element mesh (3‐dimensional mesh) simulations of TTFields electric field intensity and distribution inside and in the vicinity of ovaries of a realistic human computational phantom (array layouts used for simulation are illustrated in Figs. 5 a and 5 b). The simulations demonstrated effective distribution of fields in the abdomen at an average intensity of 1.85 V/cm pk‐pk, which according to our prior measurements is expected to lead to an effective response (Fig. 5 c). Specifically, 95% of the abdomen received field intensity higher than 1.53 V/cm pk‐pk, and about 60% received field intensity higher than 2.55 V/cm pk‐pk. TTFields intensities were particularly high in the peritoneal interstitial fluid, allowing for effective electric fields to be delivered to the ascitic fluid. Organ specific TTFields intensities are summarized in Figure 5 d. While targeting potential metastatic sites should improve treatment outcome, other proliferating cells in the abdomen (e.g., intestinal epithelial cells) can potentially be affected by TTFields. The intestinal mucous membrane is subjected to spatial relocation due to peristaltic movement of the intestine, which can in turn reposition the intestinal epithelial cells from the direction of the electric fields. As TTFields possess directional specificity, such repositioning can spare intestinal epithelial cells from being affected by the electric fields.8 Moreover, although other proliferating cells in treated tissue are also subject to forces exerted by the electric fields, given differences in optimal frequencies, the probability for an effect on these cells is relatively low. In summary, this study is the first preclinical demonstration that combination of paclitaxel chemotherapy with TTFields could provide a novel, potentially more effective therapeutic strategy for ovarian cancer compared with chemotherapy alone. Based on these results, an open‐label, pilot study of TTFields therapy concomitant with paclitaxel for recurrent ovarian‐carcinoma has been initiated.
Figure 5.
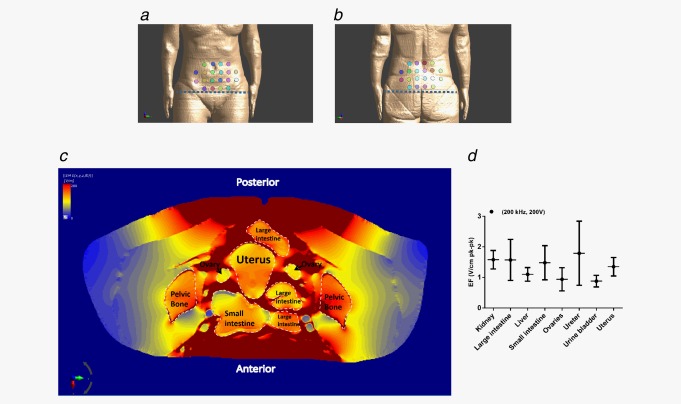
Distribution of TTFields within and in the vicinity of ovaries. Presentation of (a) anterior and (b) posterior field generating transducers. Dashed line signifies the level at which the axial slice (c) is depicted. (c) Field distribution simulation. Darker red areas represent adipose and muscle tissues. (d) Summary of organ specific distribution of TTFields intensity. Electric fields (EF) values were calculated using three dimensional modeling.
Conflicts of Interest Statement: We wish to disclose that Y.P. holds stock in Novocure Ltd. T.V., M.M., R.B., A.S., M.G., R.S.S., E.Z., Yaara Porat., Z.B., N.U., A.I., S.C., E.D.K. and U.W. are paid employees of Novocure. The other authors have no conflict of interest associated with this publication.
References
- 1. Oberaigner W, Minicozzi P, Bielska‐Lasota M, et al. Survival for ovarian cancer in Europe: the across‐country variation did not shrink in the past decade. Acta Oncolog 2012;51:441–53. [DOI] [PubMed] [Google Scholar]
- 2. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. Cancer J Clin 2011;61:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24 Suppl 6:vi24–32. [DOI] [PubMed] [Google Scholar]
- 4. Markman M, Markman J, Webster K, et al. Duration of response to second‐line, platinum‐based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J Clin Oncol 2004;22:3120–5. [DOI] [PubMed] [Google Scholar]
- 5. Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet 2009;374:1371–82. [DOI] [PubMed] [Google Scholar]
- 6. Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. [DOI] [PubMed] [Google Scholar]
- 7. Wiggans AJ, Cass GK, Bryant A, Lawrie TA, Morrison J. Poly(ADP‐ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database Syst Rev 2015;5:CD007929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res 2004;64:3288–95. [DOI] [PubMed] [Google Scholar]
- 9. Giladi M, Schneiderman RS, Porat Y, et al. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 2014;14:54–63. [DOI] [PubMed] [Google Scholar]
- 10. Giladi M, Weinberg U, Schneiderman RS, et al. Alternating electric fields (tumor‐treating fields therapy) can improve chemotherapy treatment efficacy in non‐small cell lung cancer both in vitro and in vivo. Sem Oncol 2014;41 Suppl 6:S35–41. [DOI] [PubMed] [Google Scholar]
- 11. Kirson ED, Dbaly V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA 2007;104:10152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirson ED, Schneiderman RS, Dbaly V, et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med Phys 2009;9:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pless M, Droege C, von Moos R, et al. A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non‐small cell lung cancer. Lung Cancer 2013;81:445–50. [DOI] [PubMed] [Google Scholar]
- 14. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor‐treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. Jama 2015;314:2535–43. [DOI] [PubMed] [Google Scholar]
- 15. Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep 2015;5:18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandercock J, Parmar MK, Torri V, et al. First‐line treatment for advanced ovarian cancer: paclitaxel, platinum and the evidence. Br J Cancer 2002;87:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts PC, Mottillo EP, Baxa AC, et al. Sequential molecular and cellular events during neoplastic progression: a mouse syngeneic ovarian cancer model. Neoplasia 2005;7:944–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006;58:621–81. [DOI] [PubMed] [Google Scholar]
- 19. Makarov SN, Gregory M, Noetscher GM, Nazarian A. Low Frequency Electromagnetic modelling for electrical and biological systems using Matlab, ed. Wiley, 2016. [Google Scholar]
- 20. Gabriel C. Dielectric properties of biological tissue: variation with age. Bioelectromagnetics 2005;Suppl 7:S12–8. [DOI] [PubMed] [Google Scholar]
- 21. Keller DU, Weber FM, Seemann G, et al. Ranking the influence of tissue conductivities on forward‐calculated ECGs. IEEE Trans Biomed Eng 2010;57:1568–76. [DOI] [PubMed] [Google Scholar]
- 22. Woo EJ, Hua P, Webster JG, et al. Measuring lung resistivity using electrical impedance tomography. IEEE Trans Biomed Eng 1992;39:756–60. [DOI] [PubMed] [Google Scholar]
- 23. Mershin A, Kolomenski AA, Schuessler HA, et al. Tubulin dipole moment, dielectric constant and quantum behavior: computer simulations, experimental results and suggestions. Bio Systems 2004;77:73–85. [DOI] [PubMed] [Google Scholar]
- 24. Gascoigne KE, Taylor SS. How do anti‐mitotic drugs kill cancer cells? J Cell Sci 2009;122:2579–85. [DOI] [PubMed] [Google Scholar]
- 25. Lopes NM, Adams EG, Pitts TW, et al. Cell kill kinetics and cell cycle effects of taxol on human and hamster ovarian cell lines. Cancer Chemother Pharmacol 1993;32:235–42. [DOI] [PubMed] [Google Scholar]
- 26. Demidenko ZN, Kalurupalle S, Hanko C, et al. Mechanism of G1‐like arrest by low concentrations of paclitaxel: next cell cycle p53‐dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene 2008;27:4402–10. [DOI] [PubMed] [Google Scholar]
- 27. Romero AL, Langhi M, Perez J, et al. Vinorelbine and paclitaxel as first‐line chemotherapy in metastatic breast cancer. J Clin Oncol 1999;17:74–81. [DOI] [PubMed] [Google Scholar]
- 28. Vici P, Di Lauro L, Sergi D, et al. A phase II trial of docetaxel and vinorelbine in patients with advanced breast cancer previously treated with anthracyclines. Oncology 2008;75:175–81. [DOI] [PubMed] [Google Scholar]
- 29. Markman M, Blessing J Rubin SC, et al. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel‐resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol 2006;101:436–40. [DOI] [PubMed] [Google Scholar]
- 30. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


