The new compound 5-butylamino-3-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carbaldehyde has been synthesized using a microwave-assisted reaction.
Keywords: crystal structure, pharmaceutical compound, 5-aminopyrazoles, nucleophilic substitution, hydrogen bonding
Abstract
The title compound, C14H18N4O, synthesized from an unconventional microwave-assisted method using caesium carbonate as catalyst, has an approximately planar conformation with the pyridyl and pyrazole rings inclined by a dihedral angle of 7.94 (3)°, allowing the formation of an intramolecular N—H⋯N hydrogen bond. The supramolecular assembly has a three-dimensional arrangement controlled mainly by weak C—H⋯O and C—H⋯π interactions.
Chemical context
Pyrazole derivatives are compounds with notable biological activity (Peng et al., 2013 ▸) and some derivatives have the capacity to form complexes with metal ions (Budzisz et al., 2009 ▸). Currently, 5-aminopyrazoles have been found to play an important role as biologically active compounds (Zhang et al., 2014 ▸). As such, they are considered to be building blocks of high interest for pharmaceutical agents (Sakya et al., 2006 ▸) and agrochemicals (Yuan et al., 2013 ▸). Recently, our research group reported the chemoselective synthesis of 5-alkylamino-1H-pyrazole-4-carbaldehydes in which C—N bond formation in pyrazole rings were efficiently assisted by using caesium carbonate under microwave irradiation with short reaction times and excellent yields (Orrego-Hernández et al., 2015a
▸). Herein, we report the crystal structure of the new 5-(butylamino)-3-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carbaldehyde derived from 5-chloro-3-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carbaldehyde and butylamine by using the ‘caesium effect’ and microwave irradiation.
Structural commentary
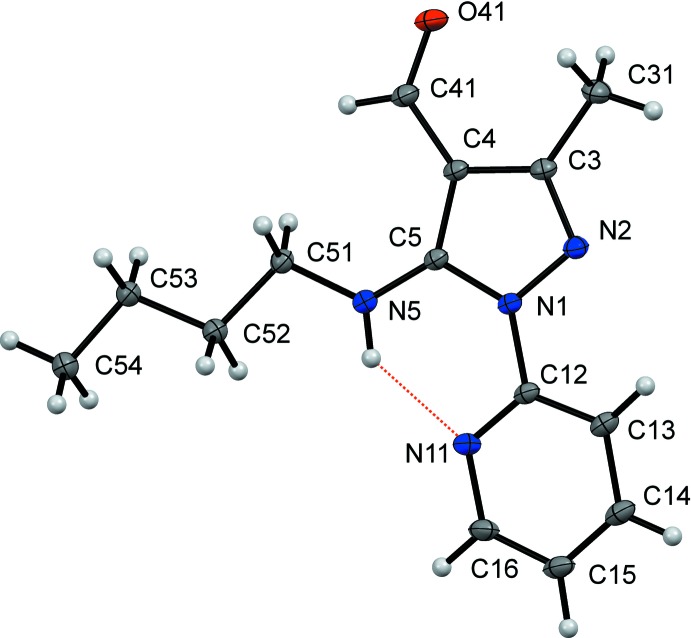
In the molecular structure of the title compound (Fig. 1 ▸), the pyridyl and pyrazole rings are nearly coplanar with a dihedral angle between their planes of 7.94 (3)°. The pyridyl ring has an orientation that allows the formation of an intramolecular N5—H1⋯N11 hydrogen bond (Fig. 1 ▸ and Table 1 ▸) to generate an S(6) motif. This structural feature is also observed in its analog 5-cyclohexylamino-3-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carbaldehyde, which even shows a smaller dihedral angle between the pyridyl and pyrazole rings [2.47 (5)°; Orrego-Hernández et al., 2015b ▸). In both molecules, the 3-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carbaldehyde nucleus presents a similar, but not identical, conformation with a maximum r.m.s. deviation of 0.0906 Å, keeping the atomic distances very similar in the pyrazole ring.
Figure 1.
The molecular structure of the title compound, showing anisotropic displacement ellipsoids drawn at the 50% probability level. The intramolecular N—H⋯N hydrogen bond is shown as a dashed line (see Table 1 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 abd Cg2 are the centroids of the C3–C5/N1/N2 and N11/C12–C16 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N5—H1⋯N11 | 0.88 (1) | 2.00 (1) | 2.7117 (7) | 137 (1) |
| C15—H15⋯O41i | 0.95 | 2.36 | 3.2906 (8) | 165 |
| C52—H52B⋯Cg1ii | 0.99 | 2.77 | 3.5141 (6) | 132 |
| C53—H53A⋯Cg2iii | 0.99 | 2.98 | 3.8761 (6) | 152 |
Symmetry codes: (i) 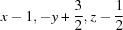 ; (ii)
; (ii) 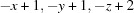 ; (iii)
; (iii) 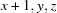 .
.
Supramolecular features
In the crystal structure, C15—H15⋯O41i [symmetry code: (i) x − 1, −y +  , z −
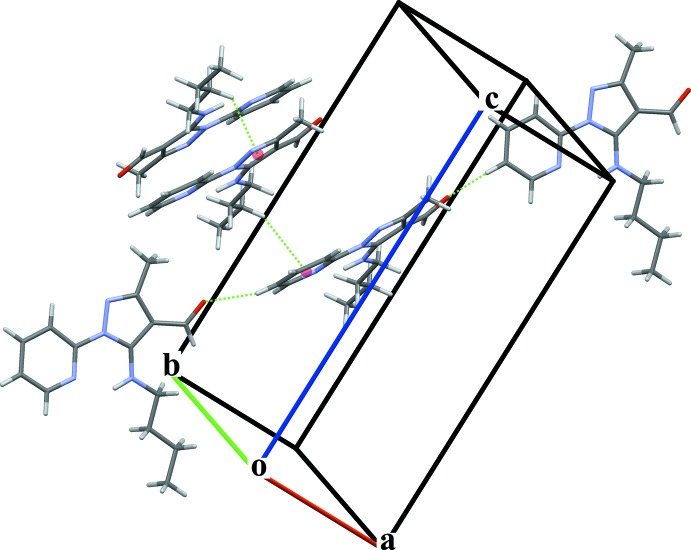
, z −  ] interactions link the molecules into C(10) chains running along [201], see Fig. 2 ▸. Parallel chains are connected by weak C52—H52B⋯Cg1ii [Cg1 is the centroid of the C3–C5/N1/N2 ring; symmetry code: (ii) −x + 1, −y + 1, −z + 2] and C53—H53A⋯Cg2iii [Cg2 is the centroid of the N11/C12–C16 ring; symmetry code: (iii) x + 1, y, z] interactions, which help to define a three-dimensional array.
] interactions link the molecules into C(10) chains running along [201], see Fig. 2 ▸. Parallel chains are connected by weak C52—H52B⋯Cg1ii [Cg1 is the centroid of the C3–C5/N1/N2 ring; symmetry code: (ii) −x + 1, −y + 1, −z + 2] and C53—H53A⋯Cg2iii [Cg2 is the centroid of the N11/C12–C16 ring; symmetry code: (iii) x + 1, y, z] interactions, which help to define a three-dimensional array.
Figure 2.
The crystal structure of the title compound, showing the C—H⋯O and C—H⋯π hydrogen-bond interactions.
Database survey
A search of the Cambridge Structural Database (CSD Version 5.37 with two updates; Groom et al., 2016 ▸) for the 1-(pyridin-2-yl)-1H-pyrazole nucleus with the possibility of any group bonded to C3, C4 or C5 gave 12 hits of which 10 correspond to organometallic compounds, one to 2-(3,5-bis(4-(n-octyloxy)phenyl)pyrazol-1-yl)pyridine and the last to 2,6-bis(pyrazolyl)pyridine. Any other search considering the presence of the butylamino or carbaldehyde groups gave no hits. However, two related compounds 5-cyclohexylamino-3-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carbaldehyde and (Z)-4-[(cyclohexylamino)methylidene]-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one have been published recently (Orrego-Hernández et al., 2015b ▸). These compounds are pyrazole derivatives which, despite the overall similarities of the molecular geometries and the potentially available donors and acceptors for hydrogen-bonding interactions, present different supramolecular assemblies.
Synthesis and crystallization
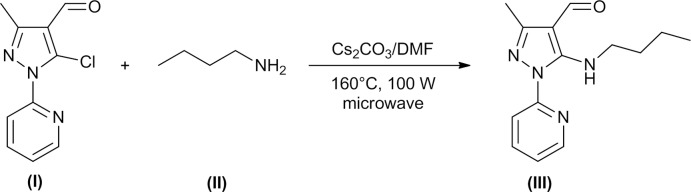
All reactive and solvents, including caesium carbonate (99%, Aldrich), were purchased from commercial sources and used as received. A mixture of 5-chloro-3-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carbaldehyde [(I) in Fig. 3 ▸; 0.100 g, 0.45 mmol, 1 equiv.], butylamine [(II) in Fig. 3 ▸; 0.56 mmol, 1.3 equiv.], caesium carbonate (0.029 g, 20% mmol, 0.2 equiv.) and 2 mL of dimethylformamide (DMF) were placed in a reaction tube of a CEM DiscoverTM, containing a magnetic stirring bar. The tube was sealed with a plastic microwave septum and was irradiated at 433 K for 25 min at 100 W. The resulting crude product was partitioned between dichloromethane and water. The organic layer was washed with water, then brine, and dried over anhydrous sodium sulfate. Subsequently, the solvent was removed under vacuum and the residue was purified by silica gel flash chromatography (DCM) to afford 5-(butylamino)-3-methyl-1-(pyridin-2-yl)-1H-pyrazole-4-carbaldehyde [(III) in Fig. 3 ▸]. Yellow crystals of (III) suitable for single-crystal X-ray diffraction were grown in DMF by slow evaporation, at ambient temperature and in air, [94% yield, m.p. 354 K]. HRMS (ESI+): [M + H]+ calculated for C14H19N4O+ 259.1553, found 259.1546. Yield 0.109 g, 94%; m.p. 348–350 K; IR νmax (KBr): 3448, 3211, 3096, 2924, 2858, 1643, 1596, 1563, 1436, 1002 cm−1; 1H NMR (CDCl3): 0.95 (t, J = 7.4, 3H), 1.44 (m, 2H), 1.68 (m, 2H), 2.44 (s, 3H), 3.60 (t, J = 7.1 Hz, 2H), 7.10 (t, J = 5.2 Hz, 1H), 7.78 (t, J = 7.0 Hz, 1H), 7.93 (d, J = 8.4 Hz, 1H), 8.28 (d, J = 4.8 Hz, 1H), 9.82 (s, 1H); 13C NMR (CDCl3): 13.7 (CH3), 14.5 (CH3), 19.9 (CH2), 32.0 (CH2), 46.4 (CH2), 106.6 (C), 114.0 (CH), 119.8 (CH), 138.8 (CH), 145.8 (CH), 152.8 (C), 153.0 (C), 154.3 (C), 182.0 (CH); MS (EI) m/z 258 (M +, 26%), 215 (67), 187 (59), 134 (32), 93 (47), 78 (76), 51 (24), 32 (100); HRMS m/z (ESI) calculated for [C14H18N4O+H]+: 259.1553; found 259.1546 [(M + H)+].
Figure 3.
Schematic representation of the microwave-assisted reaction using caesium carbonate as catalyst.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. H atoms were placed in calculated positions (C—H = 0.95–0.99 Å) and included as riding with isotropic displacement parameters set at 1.2–1.5 times the U eq value of the parent atom. H atoms belonging to NH groups were located in difference density maps and were freely refined.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C14H18N4O |
| M r | 258.32 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 100 |
| a, b, c (Å) | 9.2854 (2), 7.59144 (18), 19.4452 (5) |
| β (°) | 102.818 (3) |
| V (Å3) | 1336.52 (6) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.09 |
| Crystal size (mm) | 0.10 × 0.10 × 0.05 |
| Data collection | |
| Diffractometer | Rigaku MicroMax-007HF |
| Absorption correction | Multi-scan [SADABS (Bruker, 2008 ▸) and Blessing (1995 ▸)] |
| T min, T max | 0.766, 0.996 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 14709, 6368, 5580 |
| R int | 0.016 |
| (sin θ/λ)max (Å−1) | 0.848 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.037, 0.110, 1.05 |
| No. of reflections | 6368 |
| No. of parameters | 178 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.51, −0.23 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989016017187/bg2597sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016017187/bg2597Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016017187/bg2597Isup3.cml
CCDC reference: 1511522
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors are grateful for financial support from the Universidad de los Andes and the Colombian Institute for Science and Research (COLCIENCIAS).
supplementary crystallographic information
Crystal data
| C14H18N4O | F(000) = 552 |
| Mr = 258.32 | Dx = 1.284 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.2854 (2) Å | Cell parameters from 5580 reflections |
| b = 7.59144 (18) Å | θ = 2.2–37.1° |
| c = 19.4452 (5) Å | µ = 0.09 mm−1 |
| β = 102.818 (3)° | T = 100 K |
| V = 1336.52 (6) Å3 | Block, yellow |
| Z = 4 | 0.10 × 0.10 × 0.05 mm |
Data collection
| Rigaku MicroMax-007HF diffractometer | 6368 independent reflections |
| Radiation source: Microfocus rotating anode X-ray tube, Rigaku MicroMax-007HF | 5580 reflections with I > 2σ(I) |
| Confocal Max Flux optic monochromator | Rint = 0.016 |
| Detector resolution: 512 pixels mm-1 | θmax = 37.1°, θmin = 2.2° |
| Fullsphere data collection, phi and ω scans | h = −15→11 |
| Absorption correction: multi-scan [SADABS (Bruker, 2008) and Blessing (1995)] | k = −12→9 |
| Tmin = 0.766, Tmax = 0.996 | l = −26→32 |
| 14709 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: mixed |
| wR(F2) = 0.110 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0645P)2 + 0.1608P] where P = (Fo2 + 2Fc2)/3 |
| 6368 reflections | (Δ/σ)max = 0.002 |
| 178 parameters | Δρmax = 0.51 e Å−3 |
| 0 restraints | Δρmin = −0.23 e Å−3 |
Special details
| Experimental. It should be noted that the esd's of the cell dimensions are probably too low; they should be multiplied by a factor of 2 to 10 |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.36539 (5) | 0.84047 (6) | 1.01082 (2) | 0.01366 (8) | |
| H1 | 0.4865 (12) | 0.6805 (13) | 0.9303 (5) | 0.026 (2)* | |
| N2 | 0.33841 (5) | 0.91944 (6) | 1.07161 (2) | 0.01495 (9) | |
| C4 | 0.56919 (6) | 0.79751 (7) | 1.09414 (3) | 0.01398 (9) | |
| C3 | 0.45948 (6) | 0.89383 (7) | 1.12030 (3) | 0.01481 (9) | |
| N5 | 0.55256 (5) | 0.68697 (7) | 0.97014 (3) | 0.01577 (9) | |
| C5 | 0.50458 (6) | 0.76672 (7) | 1.02231 (3) | 0.01291 (9) | |
| C51 | 0.70133 (6) | 0.62013 (7) | 0.97538 (3) | 0.01519 (9) | |
| H51A | 0.7209 | 0.5222 | 1.0098 | 0.018* | |
| H51B | 0.7741 | 0.7147 | 0.9921 | 0.018* | |
| C41 | 0.70862 (6) | 0.73665 (8) | 1.13548 (3) | 0.01890 (11) | |
| H41 | 0.7729 | 0.6763 | 1.1117 | 0.023* | |
| O41 | 0.74985 (6) | 0.75754 (8) | 1.19936 (3) | 0.02881 (12) | |
| C31 | 0.47057 (7) | 0.96306 (9) | 1.19315 (3) | 0.02071 (11) | |
| H31A | 0.5549 | 1.0435 | 1.2054 | 0.031* | |
| H31B | 0.4843 | 0.8647 | 1.2266 | 0.031* | |
| H31C | 0.3797 | 1.0264 | 1.1952 | 0.031* | |
| C16 | 0.16351 (7) | 0.74104 (9) | 0.83692 (3) | 0.02033 (11) | |
| H16 | 0.1776 | 0.6732 | 0.7979 | 0.024* | |
| C15 | 0.03122 (7) | 0.83021 (9) | 0.83065 (3) | 0.02048 (11) | |
| H15 | −0.0437 | 0.8234 | 0.7886 | 0.025* | |
| C14 | 0.01147 (6) | 0.93004 (8) | 0.88782 (3) | 0.01946 (11) | |
| H14 | −0.0779 | 0.9931 | 0.8853 | 0.023* | |
| C13 | 0.12286 (6) | 0.93706 (8) | 0.94845 (3) | 0.01672 (10) | |
| H13 | 0.1119 | 1.0050 | 0.9880 | 0.020* | |
| C12 | 0.25184 (6) | 0.84098 (7) | 0.94950 (3) | 0.01387 (9) | |
| N11 | 0.27354 (6) | 0.74497 (7) | 0.89524 (3) | 0.01754 (9) | |
| C52 | 0.71751 (6) | 0.55527 (8) | 0.90348 (3) | 0.01585 (10) | |
| H52A | 0.7075 | 0.6566 | 0.8707 | 0.019* | |
| H52B | 0.6366 | 0.4715 | 0.8847 | 0.019* | |
| C53 | 0.86547 (6) | 0.46467 (8) | 0.90607 (3) | 0.01637 (10) | |
| H53A | 0.9465 | 0.5504 | 0.9214 | 0.020* | |
| H53B | 0.8789 | 0.3679 | 0.9410 | 0.020* | |
| C54 | 0.87324 (7) | 0.39047 (9) | 0.83406 (3) | 0.01989 (11) | |
| H54A | 0.9708 | 0.3383 | 0.8367 | 0.030* | |
| H54B | 0.8568 | 0.4855 | 0.7991 | 0.030* | |
| H54C | 0.7971 | 0.3000 | 0.8202 | 0.030* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.01143 (17) | 0.01598 (19) | 0.01227 (17) | 0.00075 (14) | −0.00017 (14) | 0.00018 (14) |
| N2 | 0.01383 (19) | 0.01611 (19) | 0.01371 (18) | 0.00040 (14) | 0.00052 (14) | −0.00137 (14) |
| C4 | 0.01133 (19) | 0.0156 (2) | 0.0135 (2) | −0.00096 (15) | −0.00037 (16) | 0.00017 (16) |
| C3 | 0.0136 (2) | 0.0155 (2) | 0.0140 (2) | −0.00133 (16) | 0.00010 (16) | −0.00071 (16) |
| N5 | 0.01264 (18) | 0.0202 (2) | 0.01381 (18) | 0.00099 (15) | 0.00157 (14) | −0.00068 (15) |
| C5 | 0.01084 (19) | 0.0134 (2) | 0.01358 (19) | −0.00073 (15) | 0.00066 (15) | 0.00134 (15) |
| C51 | 0.0126 (2) | 0.0166 (2) | 0.0161 (2) | −0.00029 (16) | 0.00243 (16) | −0.00001 (16) |
| C41 | 0.0137 (2) | 0.0238 (3) | 0.0168 (2) | 0.00154 (18) | −0.00179 (18) | −0.00114 (19) |
| O41 | 0.0213 (2) | 0.0437 (3) | 0.0168 (2) | 0.0072 (2) | −0.00562 (17) | −0.00410 (19) |
| C31 | 0.0202 (2) | 0.0248 (3) | 0.0153 (2) | 0.0004 (2) | 0.00020 (19) | −0.00476 (19) |
| C16 | 0.0190 (2) | 0.0280 (3) | 0.0119 (2) | −0.0014 (2) | −0.00112 (18) | 0.00153 (19) |
| C15 | 0.0163 (2) | 0.0270 (3) | 0.0152 (2) | −0.0032 (2) | −0.00273 (18) | 0.00527 (19) |
| C14 | 0.0135 (2) | 0.0217 (3) | 0.0204 (2) | −0.00041 (18) | −0.00236 (18) | 0.00482 (19) |
| C13 | 0.0127 (2) | 0.0171 (2) | 0.0183 (2) | 0.00055 (16) | −0.00094 (17) | 0.00179 (17) |
| C12 | 0.01192 (19) | 0.0151 (2) | 0.0130 (2) | −0.00119 (15) | −0.00053 (15) | 0.00273 (15) |
| N11 | 0.0159 (2) | 0.0230 (2) | 0.01223 (18) | 0.00039 (16) | −0.00005 (15) | 0.00079 (15) |
| C52 | 0.0141 (2) | 0.0184 (2) | 0.0148 (2) | 0.00027 (17) | 0.00264 (16) | 0.00057 (17) |
| C53 | 0.0143 (2) | 0.0196 (2) | 0.0152 (2) | 0.00035 (17) | 0.00317 (17) | 0.00079 (17) |
| C54 | 0.0181 (2) | 0.0246 (3) | 0.0172 (2) | 0.0018 (2) | 0.00455 (19) | −0.00125 (19) |
Geometric parameters (Å, º)
| N1—C5 | 1.3804 (7) | C16—N11 | 1.3478 (7) |
| N1—N2 | 1.3968 (7) | C16—C15 | 1.3841 (9) |
| N1—C12 | 1.4056 (7) | C16—H16 | 0.9500 |
| N2—C3 | 1.3137 (7) | C15—C14 | 1.3909 (9) |
| C4—C5 | 1.4119 (7) | C15—H15 | 0.9500 |
| C4—C3 | 1.4357 (8) | C14—C13 | 1.3862 (8) |
| C4—C41 | 1.4403 (8) | C14—H14 | 0.9500 |
| C3—C31 | 1.4928 (8) | C13—C12 | 1.3986 (8) |
| N5—C5 | 1.3396 (7) | C13—H13 | 0.9500 |
| N5—C51 | 1.4539 (7) | C12—N11 | 1.3340 (8) |
| N5—H1 | 0.876 (10) | C52—C53 | 1.5272 (8) |
| C51—C52 | 1.5208 (8) | C52—H52A | 0.9900 |
| C51—H51A | 0.9900 | C52—H52B | 0.9900 |
| C51—H51B | 0.9900 | C53—C54 | 1.5258 (8) |
| C41—O41 | 1.2261 (7) | C53—H53A | 0.9900 |
| C41—H41 | 0.9500 | C53—H53B | 0.9900 |
| C31—H31A | 0.9800 | C54—H54A | 0.9800 |
| C31—H31B | 0.9800 | C54—H54B | 0.9800 |
| C31—H31C | 0.9800 | C54—H54C | 0.9800 |
| C5—N1—N2 | 111.99 (4) | C15—C16—H16 | 118.1 |
| C5—N1—C12 | 129.61 (5) | C16—C15—C14 | 117.90 (5) |
| N2—N1—C12 | 118.37 (4) | C16—C15—H15 | 121.1 |
| C3—N2—N1 | 105.12 (4) | C14—C15—H15 | 121.1 |
| C5—C4—C3 | 104.82 (5) | C13—C14—C15 | 119.67 (6) |
| C5—C4—C41 | 129.14 (5) | C13—C14—H14 | 120.2 |
| C3—C4—C41 | 125.89 (5) | C15—C14—H14 | 120.2 |
| N2—C3—C4 | 112.39 (5) | C14—C13—C12 | 117.86 (6) |
| N2—C3—C31 | 119.97 (5) | C14—C13—H13 | 121.1 |
| C4—C3—C31 | 127.63 (5) | C12—C13—H13 | 121.1 |
| C5—N5—C51 | 125.11 (5) | N11—C12—C13 | 123.54 (5) |
| C5—N5—H1 | 114.2 (7) | N11—C12—N1 | 116.94 (5) |
| C51—N5—H1 | 120.7 (7) | C13—C12—N1 | 119.51 (5) |
| N5—C5—N1 | 121.17 (5) | C12—N11—C16 | 117.28 (5) |
| N5—C5—C4 | 133.17 (5) | C51—C52—C53 | 112.78 (5) |
| N1—C5—C4 | 105.66 (5) | C51—C52—H52A | 109.0 |
| N5—C51—C52 | 109.55 (4) | C53—C52—H52A | 109.0 |
| N5—C51—H51A | 109.8 | C51—C52—H52B | 109.0 |
| C52—C51—H51A | 109.8 | C53—C52—H52B | 109.0 |
| N5—C51—H51B | 109.8 | H52A—C52—H52B | 107.8 |
| C52—C51—H51B | 109.8 | C54—C53—C52 | 111.19 (5) |
| H51A—C51—H51B | 108.2 | C54—C53—H53A | 109.4 |
| O41—C41—C4 | 124.33 (6) | C52—C53—H53A | 109.4 |
| O41—C41—H41 | 117.8 | C54—C53—H53B | 109.4 |
| C4—C41—H41 | 117.8 | C52—C53—H53B | 109.4 |
| C3—C31—H31A | 109.5 | H53A—C53—H53B | 108.0 |
| C3—C31—H31B | 109.5 | C53—C54—H54A | 109.5 |
| H31A—C31—H31B | 109.5 | C53—C54—H54B | 109.5 |
| C3—C31—H31C | 109.5 | H54A—C54—H54B | 109.5 |
| H31A—C31—H31C | 109.5 | C53—C54—H54C | 109.5 |
| H31B—C31—H31C | 109.5 | H54A—C54—H54C | 109.5 |
| N11—C16—C15 | 123.75 (6) | H54B—C54—H54C | 109.5 |
| N11—C16—H16 | 118.1 | ||
| C5—N1—N2—C3 | −0.35 (6) | C5—N5—C51—C52 | −174.43 (5) |
| C12—N1—N2—C3 | 177.72 (5) | C5—C4—C41—O41 | −173.30 (7) |
| N1—N2—C3—C4 | −0.34 (6) | C3—C4—C41—O41 | 1.38 (10) |
| N1—N2—C3—C31 | 179.52 (5) | N11—C16—C15—C14 | 0.30 (10) |
| C5—C4—C3—N2 | 0.88 (6) | C16—C15—C14—C13 | −0.09 (9) |
| C41—C4—C3—N2 | −174.85 (5) | C15—C14—C13—C12 | −0.37 (9) |
| C5—C4—C3—C31 | −178.97 (6) | C14—C13—C12—N11 | 0.69 (9) |
| C41—C4—C3—C31 | 5.29 (10) | C14—C13—C12—N1 | −178.05 (5) |
| C51—N5—C5—N1 | 174.02 (5) | C5—N1—C12—N11 | 6.28 (8) |
| C51—N5—C5—C4 | −5.74 (10) | N2—N1—C12—N11 | −171.40 (5) |
| N2—N1—C5—N5 | −178.93 (5) | C5—N1—C12—C13 | −174.90 (5) |
| C12—N1—C5—N5 | 3.28 (9) | N2—N1—C12—C13 | 7.42 (7) |
| N2—N1—C5—C4 | 0.89 (6) | C13—C12—N11—C16 | −0.49 (9) |
| C12—N1—C5—C4 | −176.91 (5) | N1—C12—N11—C16 | 178.28 (5) |
| C3—C4—C5—N5 | 178.77 (6) | C15—C16—N11—C12 | −0.02 (9) |
| C41—C4—C5—N5 | −5.69 (10) | N5—C51—C52—C53 | −173.79 (5) |
| C3—C4—C5—N1 | −1.02 (6) | C51—C52—C53—C54 | 176.10 (5) |
| C41—C4—C5—N1 | 174.53 (6) |
Hydrogen-bond geometry (Å, º)
Cg1 abd Cg2 are the centroids of the C3–C5/N1/N2 and N11/C12–C16 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N5—H1···N11 | 0.876 (10) | 2.004 (11) | 2.7117 (7) | 137.0 (9) |
| C15—H15···O41i | 0.95 | 2.36 | 3.2906 (8) | 165 |
| C52—H52B···Cg1ii | 0.99 | 2.77 | 3.5141 (6) | 132 |
| C53—H53A···Cg2iii | 0.99 | 2.98 | 3.8761 (6) | 152 |
Symmetry codes: (i) x−1, −y+3/2, z−1/2; (ii) −x+1, −y+1, −z+2; (iii) x+1, y, z.
References
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed]
- Bruker (2008). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2011). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Budzisz, E., Miernicka, M., Lorenz, I.-P., Mayer, P., Krajewska, U. & Rozalski, M. (2009). Polyhedron, 28, 637–645.
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., Giacovazzo, C., Mallamo, M., Mazzone, A., Polidori, G. & Spagna, R. (2012). J. Appl. Cryst. 45, 357–361.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Orrego-Hernández, J., Cobo, J. & Portilla, J. (2015a). Eur. J. Org. Chem. pp. 5064–5069.
- Orrego Hernández, J., Portilla, J., Cobo, J. & Glidewell, C. (2015b). Acta Cryst. C71, 363–368. [DOI] [PubMed]
- Peng, X.-M., Cai, G.-X. & Zhou, C.-H. (2013). Curr. Top. Med. Chem. 13, 1963–2010. [DOI] [PubMed]
- Sakya, S. M., Lundy DeMello, K. M., Minich, M. L., Rast, B., Shavnya, A., Rafka, R. J., Koss, D. A., Cheng, H., Li, J., Jaynes, B. H., Ziegler, C. B., Mann, D. W., Petras, C. F., Seibel, S. B., Silvia, A. M., George, D. M., Lund, L. A., Denis, S. S., Hickman, A., Haven, M. L. & Lynch, M. P. (2006). Bioorg. Med. Chem. Lett. 16, 288–292. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Yuan, J.-G., Wu, H.-X., Lu, M.-L., Song, G.-P. & Xu, H.-H. (2013). J. Agric. Food Chem. 61, 4236–4241. [DOI] [PubMed]
- Zhang, Z., Ojo, K. K., Vidadala, R., Huang, W., Geiger, J. A., Scheele, S., Choi, R., Reid, M. C., Keyloun, K. R., Rivas, K., Siddaramaiah, L. K., Comess, K. M., Robinson, K. P., Merta, P. J., Kifle, L., Hol, W. G. J., Parsons, M., Merritt, E. A., Maly, D. J., Verlinde, C. L. M. J., Van Voorhis, W. C. & Fan, E. (2014). ACS Med. Chem. Lett. 5, 40–44. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989016017187/bg2597sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016017187/bg2597Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016017187/bg2597Isup3.cml
CCDC reference: 1511522
Additional supporting information: crystallographic information; 3D view; checkCIF report