Abstract
Aims
Compare effects of liraglutide 1.8 mg and sulphonylurea, both combined with metformin, on glycaemic control in patients with type 2 diabetes (T2D) fasting during Ramadan.
Materials and methods
In this up to 33‐week, open‐label, active‐controlled, parallel‐group trial, adults [glycated haemoglobin (HbA1c) 7%‐10% (53‐86 mmol/mol); body mass index ≥20 kg/m2; intent to fast] were randomized (1:1) ≥10 weeks before Ramadan to either switch to once‐daily liraglutide (final dose 1.8 mg) or continue pre‐trial sulphonylurea at maximum tolerated dose, both with metformin. Primary endpoint: change in fructosamine, a validated marker of short‐term glycaemic control, during Ramadan.
Results
Similar reductions in fructosamine levels were observed for both groups during Ramadan [liraglutide (−12.8 µmol/L); sulphonylurea (−16.4 µmol/L); estimated treatment difference (ETD) 3.51 µmol/L (95% CI: −5.26; 12.28); p = 0.43], despite lower fructosamine levels in the liraglutide group at start of Ramadan. Fewer documented symptomatic hypoglycaemic episodes were reported in liraglutide‐treated (2%, three subjects) versus sulphonylurea‐treated patients (11%, 18 subjects). No severe hypoglycaemic episodes were reported by either group. Body weight decreased more during Ramadan with liraglutide (ETD: −0.54 kg; 95% CI: −0.94;−0.14; p = 0.0091). The proportion of patients reporting adverse events was similar between groups. Liraglutide led to greater HbA1c reduction [ETD: −0.59% (−6.40 mmol/mol), 95% CI: −0.79; −0.38%; −8.63; −4.17 mmol/mol; p < 0.0001].
Conclusions
Despite lower fructosamine levels and body weight at the beginning of Ramadan, use of liraglutide showed similar glycaemic improvements, fewer hypoglycaemic episodes and greater body weight reduction compared with sulphonylurea. LIRA‐Ramadan provides evidence for liraglutide being safe and efficacious for management of T2D during Ramadan fasting.
Keywords: body weight, fasting, fructosamine, GLP‐1, hypoglycaemia, liraglutide, metformin, Ramadan, sulphonylurea, type 2 diabetes
1. INTRODUCTION
The month of Ramadan is observed as a period of fasting (i.e. abstention from eating, drinking and intake of oral medications) from dawn to sunset. Approximately 100 million of the worldwide 1.6 billion Muslim population have type 2 diabetes (T2D).1, 2 Although the Quran3 exempts certain patients with T2D from fasting, the EPIDIAR study, conducted in 13 countries, reported that 79% of patients with T2D fast during Ramadan despite an increased risk of acute complications.4 The CREED study reported that 64% of patients with T2D fasted every day of Ramadan and 94.2% fasted for at least 15 days.2
American Diabetes Association (ADA) recommendations for the management of T2D during Ramadan focus on the importance of preparation by undergoing medical assessment, education regarding hypoglycaemia prevention during fasting and individual guidance 1‐2 months prior to Ramadan.5, 6 The Ramadan Education and Awareness in Diabetes (READ) programme demonstrated that individuals who attended education sessions lost more weight and experienced fewer hypoglycaemic episodes than those not attending.7 Metformin is generally considered a safe treatment during Ramadan.6 Sulphonylureas, especially glyburide and glibenclamide, should be used with caution because of the inherent risk of hypoglycaemia, increased during fasting.5, 6 Recent guidelines for T2D management during Ramadan indicate that further studies investigating the use of sulphonylureas are still required.5 Sulphonylureas and metformin represent the treatment of choice in South Asia for T2D management because of the large body of clinical evidence, as well as their availability and low cost.8 Incretin‐based therapy may be an alternative to sulphonylureas for T2D management during Ramadan because of the inherent low risk of hypoglycaemia. There have been several observational and interventional trials investigating the efficacy and safety of dipeptidyl peptidase‐4 (DPP‐4) inhibitors when used during Ramadan.9, 10, 11 By contrast, limited clinical data exist on the efficacy and safety of glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) when used during Ramadan. Therefore, studies are warranted to provide more informed decisions about choosing glucose‐lowering treatments for patients with T2D intending to fast during Ramadan.
GLP‐1RAs lower blood glucose by stimulating insulin and reducing glucagon secretion, both in a glucose‐dependent manner in relation to elevated glucose levels.12, 13, 14, 15 Liraglutide is an injectable, once‐daily GLP‐1 analogue administered subcutaneously.16, 17 Liraglutide has a well‐characterized safety profile, with the most frequently reported adverse events being gastrointestinal (i.e. nausea, vomiting and diarrhoea) during treatment initiation. Liraglutide is associated with a low risk of hypoglycaemia, which, in addition to its beneficial effect on glycaemic control and body weight, makes it potentially attractive as a glucose‐lowering treatment during Ramadan.18, 19, 20, 21
The LIRA‐Ramadan trial (ClinicalTrials.gov, NCT01917656) was conducted to compare the effect of liraglutide versus sulphonylurea, both combined with metformin, on change in glycaemic control during Ramadan fasting in patients with T2D.
2. MATERIALS AND METHODS
2.1. Study design and participants
This randomized, parallel, open‐label, active‐controlled trial was conducted at 39 sites in 7 countries (see File S1). The 33‐week trial consisted of a screening period (2 weeks) followed by dose escalation for the liraglutide group (3‐4 weeks), a treatment maintenance period (6‐19 weeks), the Ramadan treatment period (4 weeks), a post‐Ramadan treatment period (4 weeks) and follow‐up (1 week) (Figure S1, File S1). The flexible duration of the treatment maintenance period (6‐19 weeks) was to ensure recruitment of all patients prior to Ramadan. Patients had to be enrolled ≥10 weeks and ≤22 weeks prior to Ramadan.
Eligible patients were adults (18‐80 years) diagnosed with T2D on stable diabetes treatment [metformin ≥1000 mg/d and sulphonylurea (gliclazide, glipizide or glyburide/glibenclamide) at maximum tolerated dose (MTD, at least half maximal approved dose) or glimepiride (≥2 mg/d)] ≥90 days prior to screening, who had glycated haemoglobin (HbA1c) 7%‐10% (53‐86 mmol/mol), body mass index (BMI) ≥20 kg/m2, expressed intention to fast (dawn to sunset) during Ramadan 2014 after receiving counselling regarding the risk of fasting, and who were willing to give blood during Ramadan. This trial was performed in accordance with the Declaration of Helsinki22 and Good Clinical Practice23 guidelines. Prior to any trial activities, patients signed an informed consent form. The protocol was reviewed and approved by the appropriate independent ethics committees/institutional review boards.
Key exclusion criteria included treatment with glucose‐lowering agent(s) other than those stated in the inclusion criteria <90 days prior to screening (insulin was not allowed except in connection with intercurrent illness and for ≤7 days) and any contraindication for successful and sustained fasting from a medical perspective at the investigator's discretion. For additional exclusion criteria and all withdrawal criteria, see Supporting Information online.
Eligible patients were randomized using an interactive web/voice response system (IV/IWRS) in a 1:1 manner to either switch from their pre‐trial sulphonylurea to liraglutide, with dose escalation from 0.6 to 1.8 mg/d, or continue with their pre‐trial sulphonylurea at the already established MTD, both combined with metformin. No adjustments of sulphonylurea dose were allowed pre‐Ramadan. During Ramadan, the sulphonylurea dose could be adjusted according to the ADA guideline [5]. Randomization, performed by clinical supplies coordination at Novo Nordisk, was stratified, based on pre‐trial sulphonylurea (two levels: gliclazide, glipizide, glimepiride; and glyburide/glibenclamide) and menopausal state (two levels: pre‐menopausal females; and males and post‐menopausal/hysterectomized females). Liraglutide injections were to be given at any time of the day, irrespective of meals, but it was recommended to keep the injection time consistent.
2.2. Procedures
Assessments were conducted at the following timepoints:
Fructosamine: baseline, beginning and end of Ramadan.
Fasting plasma glucose (FPG): baseline, beginning and end of Ramadan and end of treatment (EoT).
HbA1c, body weight, systolic (SBP) and diastolic (DBP) blood pressure: screening, baseline, beginning and end of Ramadan and EoT.
Subjects were to record in the patient diary fasting self‐measured plasma glucose (SMPG), using the plasma glucose (PG) meter provided, on a weekly basis or more frequently at the investigator's discretion. If any fasting SMPG measurement met the limits of unacceptable hyperglycaemia (see File S1, withdrawal criteria), the subject was to contact the investigator. Treatment‐emergent adverse events (TEAEs) and hypoglycaemic episodes were assessed at all phone contacts and site visits.
2.3. Endpoints
The primary endpoint was change in fructosamine level from the beginning to the end of Ramadan. Fructosamine, which measures glycated serum proteins, is a validated marker of short‐term (2‐4 weeks) glycaemic control. These glycated proteins, primarily albumin, have a higher turnover rate compared to erythrocytes. Fructosamine therefore serves as a more relevant parameter than HbA1c, which measures long‐term (2‐3 months) blood glucose, for the assessment of glycaemic control for shorter periods of observation, such as Ramadan.24, 25 Secondary endpoints were fructosamine level at the end of Ramadan and changes from the beginning to the end of Ramadan in FPG, body weight and BP; changes from baseline to the end of Ramadan in HbA1c, FPG and body weight; and changes from baseline to EoT in HbA1c and FPG. Responder endpoints at the end of Ramadan and EoT included proportion of patients achieving: HbA1c <7.0% (53 mmol/mol); HbA1c <7.0% (53 mmol/mol) and no weight gain; HbA1c <7.0% (53 mmol/mol) and no confirmed hypoglycaemic episodes; HbA1c <7.0% (53 mmol/mol), no weight gain and no confirmed hypoglycaemic episodes.
Safety endpoints during Ramadan fasting and from baseline to EoT comprised TEAEs and hypoglycaemic episodes, including confirmed events/episodes [patient unable to self treat and/or with PG <3.1 mmol/l (56 mg/dL)] and those established by the ADA classification cut‐off26 [≤3.9 mmol/L (70 mg/dL)], with and without symptoms (e.g. severe, documented symptomatic hypoglycaemia and asymptomatic hypoglycaemia; see File S1 for definitions of hyperglycaemia).
2.4. Statistical analysis
Using a standard two‐sided t‐test, the sample size of 160 patients/arm to be randomized provided 90% power to detect a difference of 23.5 µmol in change in fructosamine level from start to end of Ramadan between treatments in patients completing Ramadan. A difference of 23.5 µmol corresponds approximately to a difference of 0.4% between treatments in HbA1c.27 In addition, the following were assumed: standard deviation (SD) of 58 µmol in change in fructosamine level from start to end of Ramadan; dropout rate of 20% and treatment difference only half the size in dropouts. Patients who withdrew prior to Ramadan are not accounted for in the primary analyses because no measurement of fructosamine was undertaken between baseline and start of Ramadan. The statistical evaluation followed the intention‐to‐treat principle, and subjects contributed to the evaluation “as randomized”. Continuous data were analyzed using a mixed effects model for repeated measures (MMRM) with visit, treatment, country and stratification groups as fixed factors and the corresponding value for the specific endpoint measured at randomization as a covariate, all nested within visit. Dichotomous endpoints were analyzed by a logistic regression model with treatment, country and stratification groups as fixed factors, HbA1c value at randomization as a covariate and weight at randomization as a covariate in the two composite endpoints including no weight gain. The results included the estimated odds ratio (OR) (liraglutide/sulphonylurea), p‐value and 95% confidence interval (CI).
The number of confirmed hypoglycaemic episodes and ADA‐classified documented symptomatic hypoglycaemic episodes were analyzed by a negative binomial regression model, where the ADA‐classified documented symptomatic hypoglycaemic episodes were analyzed post hoc. The model included treatment, country and the stratification variables as factors and HbA1c value at randomization as a covariate. Nocturnal hypoglycaemic episodes occurred with time of onset between 00:01 and 05:59 (inclusive).
TEAEs were summarized descriptively. Safety data during Ramadan refer to events/episodes that occurred from the first to the last day of individual fasting.
3. RESULTS
3.1. Subject disposition and demographics
Between January 9, 2014 and April 4, 2014, 343 subjects were randomized (liraglutide n = 172; sulphonylurea n = 171) (Figure S2, File S1). The last subject's last visit was September 4, 2014. Twenty‐six (15.1%) subjects in the liraglutide group and 24 (14.0%) in the sulphonylurea group discontinued after randomization. More subjects treated with liraglutide (20) versus those treated with sulphonylurea (8) withdrew between baseline and the beginning of Ramadan. However, more sulphonylurea‐treated subjects (11) withdrew during Ramadan than did liraglutide‐treated subjects (3). Eleven subjects on liraglutide versus none on sulphonylurea withdrew because of TEAEs (mainly gastrointestinal). Of these, 10 occurred prior to Ramadan (half of them during dose escalation) and one after Ramadan. No subjects withdrew because of TEAEs during Ramadan in any of the groups.
The demographic and baseline characteristics were well balanced between groups (Table 1).
Table 1.
Demographic and baseline characteristics
| Liraglutide 1.8 mg (n = 171) | Sulphonylurea(n = 170) | |
|---|---|---|
| Sex, n (%) | ||
| Female | 86 (50.3) | 87 (51.2) |
| Male | 85 (49.7) | 83 (48.8) |
| Age, years, mean (SD) | 54.9 (9.27) | 54.0 (9.33) |
| Duration of diabetes, years, mean (SD) | 8.0 (5.26) | 7.2 (4.39) |
| Race, n (%) | ||
| Asian | 72 (42.1) | 82 (48.2) |
| Black or African American | 2 (1.2) | 1 (0.6) |
| White | 72 (42.1) | 64 (37.6) |
| Other | 25 (14.6) | 23 (13.5) |
| Pre‐trial sulphonylurea, n (%) | ||
| Glibenclamide | 32 (18.7) | 27 (15.9) |
| Glicazide | 76 (44.4) | 74 (43.5) |
| Glimepiride | 63 (36.8) | 65 (38.2) |
| Glipizide | 0 (0) | 4 (2.4) |
| Pre‐trial sulphonylurea, mean dose, mg/d (median) | ||
| Glibenclamide | 16.7 (10) | 12.6 (10) |
| Glicazide | 164.6 (160) | 149.5 (120) |
| Glimepiride | 3.62 (4.0) | 3.62 (4.0) |
| Glipizide | 0 (0) | 15.0 (15.0) |
| Pre‐trial menopausal state | ||
| Pre‐menopausal females | 27 (15.8) | 27 (15.9) |
| Post‐menopausal females, and males | 144 (84.2) | 143 (84.1) |
| Fructosamine, µmol/L, mean (SD) | ||
| At baseline | 320.3 (53.6) | 316.0 (58.3) |
| At the beginning of Ramadan | 291.8 (54.9) | 301.6 (56.7) |
| HbA1c, mean % (SD) | ||
| At baseline | 8.3 (0.94) | 8.2 (0.91) |
| At the beginning of Ramadan | 7.2 (1.1) | 7.8 (1.1) |
| Mean mmol/mol (SD) | ||
| At baseline | 66.9 (10.2) | 66.5 (10.0) |
| At the beginning of Ramadan | 55.1 (11.6) | 61.9 (12.1) |
| FPG | ||
| Mean mmol/L (SD) | 9.3 (2.97) | 9.4 (2.94) |
| Mean mg/dL (SD) | 168.4 (53.5) | 168.4 (52.8) |
| Body weight, kg, mean (SD) | ||
| At baseline | 81.0 (17.1) | 83.1 (16.0) |
| At the beginning of Ramadan | 77.9 (16.5) | 82.2 (16.1) |
| BMI, kg/m2, mean (SD) | 30.2 (5.37) | 31.4 (5.88) |
| BP, mm Hg, mean (SD) | ||
| Systolic | 132.9 (14.39) | 133.2 (12.95) |
| Diastolic | 78.8 (9.51) | 79.4 (9.00) |
BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; SD, standard deviation.
Dosing adjustments were reported in nine subjects (6.0%) in the liraglutide group and 48 subjects (29.1%) in the sulphonylurea group during Ramadan; these are shown in File S1.
3.2. Results from the beginning to the end of Ramadan
The primary endpoint was change in fructosamine level from the beginning to end of Ramadan (Figure 1A). From a fructosamine value of 291.8 µmol/L (liraglutide) and 301.6 µmol/L (sulphonylurea) at the beginning of Ramadan, similar reductions in fructosamine level were observed during Ramadan [liraglutide (−12.8 µmol/L); sulphonylurea (−16.4 µmol/L); estimated treatment difference (ETD) 3.51 µmol/L (95% CI: −5.26; 12.28); p = 0.43]. However, from baseline to start of Ramadan, there was a significant reduction in fructosamine level with liraglutide versus sulphonylurea [ETD −13.8 µmol/L (95% CI: −22.6; −4.94); p = 0.0024; post hoc]. Sensitivity analyses confirm results from the primary MMRM analysis (Figure S3, File S1).
Figure 1.
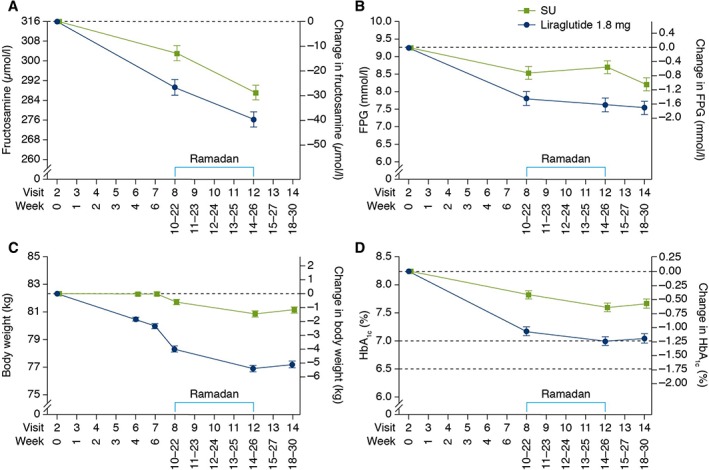
Efficacy. (A) Change in fructosamine (µmol/L) from baseline to end of Ramadan. (B) Change in FPG (mmol/L) from baseline to EoT. (C) Change in body weight from baseline to EoT. (D) Change in HbA1c from baseline to EoT. The time between visits 6 and 8 could be up to 18 weeks. EoT, end of treatment; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; SU, sulphonylurea.
During Ramadan, FPG decreased with liraglutide (−0.18 mmol/L) and increased with sulphonylurea (+0.17 mmol/L) (Figure 1B). ETD in change in FPG was −0.35 mmol/L (95% CI: −1.03; 0.33; p = 0.31).
During Ramadan fasting, fewer ADA‐classified hypoglycaemic episodes were reported with liraglutide [8.6%, 13 subjects, 1725 episodes/1000 patient‐years of exposure (PYE)] compared with sulphonylurea (17.8%, 29 subjects, 5140 episodes/1000 PYE) (Figure 2A).26 For ADA‐classified documented symptomatic hypoglycaemic episodes (liraglutide: 2.0%, three subjects, 329 episodes/1000 PYE vs. sulphonylurea: 11.0%, 18 subjects, 2336 episodes/1000 PYE)26, the estimated rate ratio (liraglutide/sulphonylurea) was 0.12 (95% CI: 0.03;0.42; p = 0.0009; post hoc). A cumulative plot of ADA‐classified documented symptomatic hypoglycaemic episodes during Ramadan is depicted in Figure 2B. Within the sulphonylurea strata, more subjects in the glibenclamide/glyburide stratum (14.8%, 4/27 subjects) experienced ADA‐classified documented hypoglycaemic episodes than in the glimepiride/gliclazide/glipizide stratum (9.8%, 14/143 subjects) (Table S1, File S1). No severe hypoglycaemic episodes were reported by either group during Ramadan. The proportion of subjects with confirmed hypoglycaemic episodes with liraglutide (2.0%, 246 episodes/1000 PYE; three subjects) also appeared lower than with sulphonylurea [4.3%, 623 episodes/1000 PYE; glimepiride (three subjects), gliclazide (two subjects), glibenclamide/glyburide (two subjects)]. Given the very low number of confirmed hypoglycaemic episodes, no proper statistical testing could be conducted. The mean cumulative plot of confirmed hypoglycaemic episodes during Ramadan is depicted in Figure S4, File S1. No confirmed nocturnal hypoglycaemic episodes were reported with liraglutide; two episodes were reported by two subjects with sulphonylurea.
Figure 2.
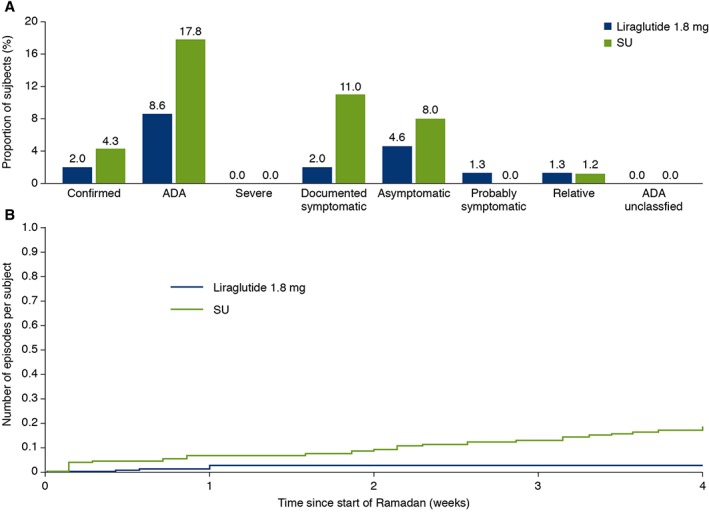
Hypoglycaemic episodes during Ramadan. (A) Proportion of subjects with confirmed and ADA‐classified hypoglycaemia. (B) Mean cumulative function of ADA‐classified documented symptomatic hypoglycaemic episodes; number of episodes per subject. Confirmed [patient unable to self treat and/or with PG <3.1 mmol/L (56 mg/dL)] and ADA‐classified hypoglycaemia [ADA: total ADA‐classified episodes; severe: patient unable to self treat; documented symptomatic: PG <3.9 mmol/L (70 mg/dL); asymptomatic: PG <3.9 mmol/L (70 mg/dL); relative: symptomatic and PG >3.9 mmol/L (70 mg/dL); probable symptomatic: episode during which symptoms of hypoglycaemia are not accompanied by plasma glucose determination (presumably caused by plasma glucose concentration ≤3.9 mmol/L {70 mg/dL}); relative: symptomatic and PG >3.9 mmol/L (70 mg/dL); unclassifiable: where hypoglycaemic episode could not be allocated to any of the above groups]. ADA, American Diabetes Association; CI, confidence interval; HbA1c, glycated haemoglobin; OR, odds ratio; PG, plasma glucose.
A statistically significantly greater reduction in mean body weight was shown with liraglutide versus sulphonylurea during Ramadan [ETD: −0.54 kg (95% CI: −0.94; −0.14); p = 0.0091] (Figure 1C).
Estimated mean change in SBP during Ramadan in the liraglutide and sulphonylurea groups was −3.45 mm Hg and +0.56 mm Hg, respectively, ETD of −4.01 mm Hg (95% CI: −6.86; −1.15; p = 0.0061). The ETD in DBP during Ramadan was not statistically significant [−1.31 mm Hg (95% CI: −3.29; 0.67); p = 0.19].
During Ramadan, the proportion of subjects reporting TEAEs was similar between the groups (liraglutide 23.7%; sulphonylurea 20.9%), although the event rate was higher with liraglutide compared to sulphonylurea (Table 2). Only liraglutide‐treated subjects reported TEAEs that were determined by the investigator to be related to trial drug (8.6%), of which all but four events were gastrointestinal disorders. Gastrointestinal TEAEs, mostly mild in severity, were reported more frequently with liraglutide (10.5%) than sulphonylurea (3.7%), with vomiting, nausea and diarrhoea most commonly reported. No TEAEs led to withdrawal during Ramadan. Two serious adverse events (SAEs) were reported with liraglutide (one gastroenteritis, one heel abscess requiring hospitalization) and none with sulphonylurea.
Table 2.
Summary of TEAEs during Ramadan
| Event | Liraglutide 1.8 mg | Sulphonylurea | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | E | R | N | % | E | R | |
| All AEs | 36 | 23.7 | 64 | 5258 | 34 | 20.9 | 43 | 3349 |
| Severe | 3 | 2.0 | 5 | 411 | 1 | 0.6 | 1 | 78 |
| Moderate | 10 | 6.6 | 12 | 986 | 9 | 5.5 | 10 | 779 |
| Mild | 27 | 17.8 | 47 | 3861 | 25 | 15.3 | 32 | 2492 |
| Serious | 2 | 1.3 | 2 | 164 | 0 | 0 | 0 | 0 |
| Fatal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Possibly or probably related to investigational medical product | 13 | 8.6 | 24 | 1972 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 13 | 8.6 | 20 | 1643 | 0 | 0 | 0 | 0 |
| Leading to withdrawal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Frequently reported AEs | ||||||||
| Gastrointestinal AEs | 16 | 10.5 | 28 | 2300 | 6 | 3.7 | 9 | 701 |
| Vomiting | 8 | 5.3 | 9 | 739 | 1 | 0.6 | 1 | 78 |
| Nausea | 7 | 4.6 | 7 | 575 | 0 | 0 | 0 | 0 |
| Diarrhoea | 4 | 2.6 | 4 | 329 | 3 | 1.8 | 3 | 234 |
| Constipation | 1 | 0.7 | 1 | 82 | 0 | 0 | 0 | 0 |
| Infection and infestations | 10 | 6.6 | 10 | 822 | 7 | 4.3 | 7 | 545 |
| Infections, pathogen unspecified | 8 | 5.3 | 8 | 657 | 6 | 3.7 | 6 | 467 |
AEs, adverse events; E, number of events; N, number of subjects; R, event rate/1000 patient‐years of exposure; TEAE, treatment‐emergent adverse event.
3.3. Results from baseline to end of Ramadan and baseline to end of trial
From baseline to end of Ramadan, glycaemic control improved significantly with liraglutide versus sulphonylurea in terms of fructosamine level [ETD −10.3 µmol/L (95% CI: −18.7; −1.89); p = 0.017], FPG [−1.05 mmol/L (95% CI: −1.58; −0.52); p = 0.0001] and HbA1c [−0.59% (−6.40 mmol/mol), (95% CI: −0.79; −0.38%; −8.63; −4.17 mmol/mol), p < 0.0001; Figure 1D]. For FPG and HbA1c, the ETDs for changes from baseline to EoT were also both statistically significantly in favour of liraglutide [−0.65 mmol/L (95% CI: −1.15; −0.15); p = 0.012 and −0.60% (−6.59 mmol/mol) (95% CI: −0.82; −0.38%; −8.98; −4.20 mmol/mol); p < 0.0001, respectively].
From baseline to EoT, fewer hypoglycaemic episodes classified according to ADA definitions were reported with liraglutide (17.0%, 29 subjects, 944 episodes/1000 PYE) than with sulphonylurea (32.4%, 55 subjects, 2622 episodes/1000 PYE) (Figure S5, File S1).26 The proportion of subjects with confirmed hypoglycaemic episodes with liraglutide (2.9%, 5 subjects, 88 episodes/1000 PYE) was lower than with sulphonylurea (9.4%, 16 subjects, 393 episodes/1000 PYE) with an estimated rate ratio (liraglutide/sulphonylurea) of confirmed hypoglycaemic episodes of 0.2 (95% CI: 0.1; 0.5; p = 0.0027). No severe hypoglycaemic episodes were reported. The estimated rate ratio (liraglutide/sulphonylurea) for ADA‐classified documented symptomatic hypoglycaemic episodes was 0.17 [95% CI: 0.08; 0.36; (p < 0.0001; post hoc)]. No confirmed nocturnal hypoglycaemic episodes were reported with liraglutide; three episodes were reported by three subjects with sulphonylurea.
The proportions of subjects meeting the ADA HbA1c and composite responder targets at end of Ramadan and EoT are presented in Figure 3. For all responder endpoints, significantly higher proportions of liraglutide‐treated subjects achieved the targets than did sulphonylurea‐treated subjects for both timeframes [OR (liraglutide/sulphonylurea) range: 2.98‐4.90; p < 0.001 for all endpoints].
Figure 3.
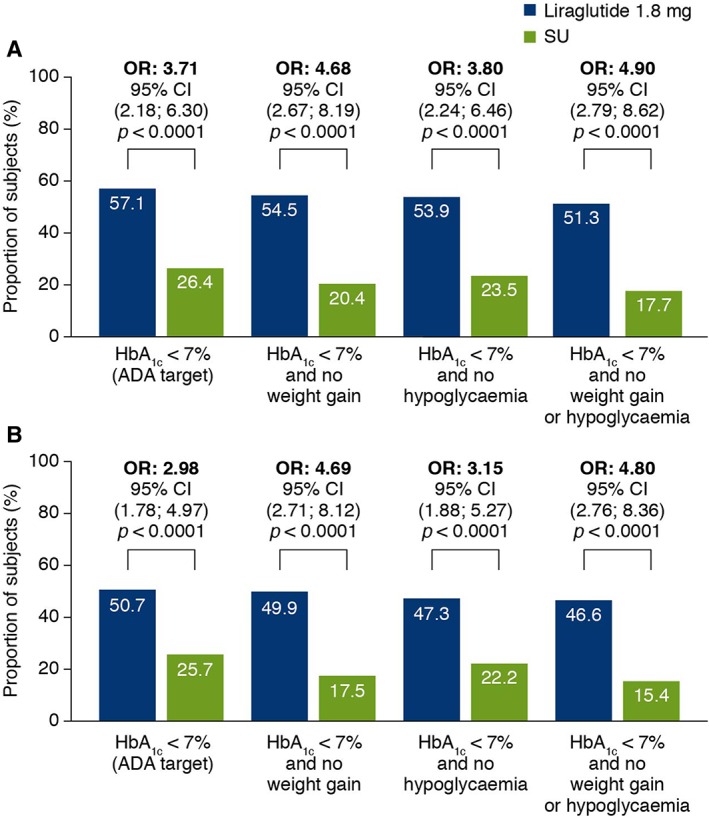
Responders for composite endpoints. (A) Proportion of subjects reaching targets at end of Ramadan (visit 12). (B) Proportion of subjects reaching targets at EoT (visit 14). Estimated proportion of subjects meeting targets (%) based on logistic regression model with treatment, country and stratification groups as fixed factors and the HbA1c value at baseline as a covariate, and baseline weight as covariate in the composite endpoints, including no weight gain. These analyses are based on subjects entering Ramadan. OR: liraglutide/SU. ADA, American Diabetes Association; EoT, end of treatment; HbA1c, glycated haemoglobin; OR, odds ratio; SU, sulphonylurea.
ETDs for body weight change from baseline to end of Ramadan [−3.94 kg (95% CI: −4.54; −3.33); p < 0.0001] and from baseline to EoT [−3.95 kg (95% CI: −4.57; −3.33); p < 0.0001] were both in favour of liraglutide.
Estimated mean change in pulse from baseline to EoT was +2.77 and +0.86 beats/min for the liraglutide and sulphonylurea groups, respectively; the ETD was statistically significant [+1.91 beats/min (95% CI: 0.17; 3.64); p = 0.032].
From baseline to EoT, the proportion of subjects reporting TEAEs and the event rate were greater with liraglutide (76.6%; 8584 events/1000 PYE) than with sulphonylurea (57.1%; 3084 events/1000 PYE) (Table S2, File S1). Most subjects had recovered by the end of the trial. There was a low incidence of SAEs (liraglutide 2.9%; sulphonylurea 1.2%). Only liraglutide‐treated subjects (11%; 6.4%) experienced TEAEs that led to withdrawal. No fatal events or cases of acute or chronic pancreatitis were reported.
4. DISCUSSION
Fasting during Ramadan is an important spiritual practice, and some patients with diabetes fast despite being at high risk of complications.2 Ramadan fasting implies major changes in dietary habits for patients with T2D and is a challenge, as it may require changes in glucose‐lowering medication.
The LIRA‐Ramadan trial is the first large, randomized, controlled trial comparing the efficacy and safety of a GLP‐1RA versus sulphonylurea during Ramadan fasting in patients with T2D. The trial duration of up to 33 weeks included assessments before, during and after Ramadan. Subjects in this trial had suboptimal glycaemic control at baseline. Previously, Brady et al. investigated the effect of liraglutide versus sulphonylurea on glycaemic control at 3 and 12 weeks post‐Ramadan, but not during Ramadan.28 Although more subjects treated with liraglutide plus metformin (26.7%) than those treated with sulphonylurea plus metformin (10.3%) achieved the primary outcome of HbA1c <7.0% (53 mmol/mol), no weight gain and no severe hypoglycaemic events 12 weeks post‐Ramadan, statistical significance was not attained (p = 0.06) because the trial was underpowered.
In LIRA‐Ramadan, despite better glycaemic control in the liraglutide group at the beginning of Ramadan, similar glycaemic improvements were achieved during Ramadan with liraglutide and sulphonylurea, with comparable reductions in fructosamine levels.
Despite lower levels of fructosamine and HbA1c during Ramadan, the risk of ADA‐documented symptomatic hypoglycaemic episodes during Ramadan was significantly lower with liraglutide versus sulphonylurea. For subjects randomized to sulphonylurea, more subjects in the glibenclamide/glyburide stratum experienced ADA‐classified hypoglycaemic episodes than those in the glimepiride/gliclazide/glipizide stratum, as previously reported.5, 29 Glibenclamide/glyburide may be associated with a higher risk of hypoglycaemia than other second‐generation sulphonylureas, specifically gliclazide, glimepiride and glipizide.29, 30 Gliclazide, previously shown to have a low hypoglycaemia rate during Ramadan, was the most frequently used sulphonylurea in this trial.10 Importantly, however, it seems the lower risk of ADA‐documented symptomatic hypoglycaemic episodes with liraglutide versus sulphonylurea applied to all the second‐generation sulphonylureas used in this trial. Risks of ADA‐classified documented symptomatic hypoglycaemia and confirmed hypoglycaemia were also significantly lower for liraglutide versus sulphonylurea in the overall trial period. Body weight and SBP decreased significantly during Ramadan and from baseline to end of Ramadan with liraglutide versus sulphonylurea. The clinical significance of these results is underlined by the significantly higher proportion of patients in the liraglutide groups achieving HbA1c <7.0% (53 mmol/mol), HbA1c <7.0% (53 mmol/mol) and no confirmed hypoglycaemia, and HbA1c <7.0% (53 mmol/mol), no weight gain and no confirmed hypoglycaemia at the end of Ramadan.
Although a similar number of patients withdrew from each group after randomization, the timing of and reasons for withdrawal were different. More patients treated with liraglutide than with sulphonylurea withdrew prior to Ramadan, primarily because of gastrointestinal AEs, with half of these occurring during dose escalation. However, during Ramadan, more patients taking sulphonylurea withdrew, mostly because of lack of efficacy. Therefore, it is recommended to complete dose escalation of liraglutide at least 6–8 weeks prior to Ramadan, as in this trial. No patients withdrew because of AEs during Ramadan.
This trial has several important design features. To ensure randomization of patients in time for the 2014 Ramadan, patients were treated for a flexible maintenance period where treatment was kept unchanged. The 6‐week minimum maintenance period was chosen to allow for patient consultation, diabetes education and lifestyle intervention, per guidelines.6 In addition, the minimum maintenance period permitted the initiation and stabilization of a new treatment prior to the associated changes in dietary habits during Ramadan, and allowed any potential gastrointestinal TEAEs associated with liraglutide to have subsided prior to Ramadan. To ensure patient safety, weekly fasting SMPG was conducted and FPG limits were in place to avoid excessive hyperglycaemia. The patient was to contact the investigator should these limits be reached. All patients were encouraged to diet and exercise as part of medical counselling prior to Ramadan.
There are some potential limitations to this trial. It was conducted open‐label as it was not feasible to produce placebo–sulphonylurea for the five sulphonylurea types allowed in this trial, of which there are multiple national/local brands. Because it was a requirement that patients had been on the MTD of sulphonylurea ≥90 days prior to randomization, the sulphonylurea group was accustomed to the treatment and less likely to have tolerability issues. This may explain the relatively low number of hypoglycaemic episodes, including no severe hypoglycaemic episodes in the sulphonylurea group during the trial. In addition, poorer glycaemic control in the sulphonylurea group, during both Ramadan and the overall trial period, may have had an effect on the number of hypoglycaemic episodes. Furthermore, because of the MTD requirement, for patients randomized to sulphonylurea, the pre‐trial sulphonylurea was to be kept stable until Ramadan. The dose of sulphonylureas in this trial was, however, consistent with or higher than doses used in other Ramadan trials, as well as in clinical practice.9, 10, 11, 31, 32, 33, 34 Generally, for sulphonylureas administered at higher than half the maximum approved dose, the glucose‐lowering effect levels off as the dose increases.35 However, theoretically, if the physician had chosen to increase the dose of sulphonylurea for patients on a dose lower than the maximum approved dose, the hypoglycaemia rate in the sulphonylurea group would probably have been higher during Ramadan. During Ramadan, reducing the dose of sulphonylurea and/or adjusting timing of administration was allowed, according to ADA recommendations.5 Most changes to the dose regimen in sulphonylurea treatment were related to dose reduction, although splitting the dose or changing the timing of the dose was also reported. There were very few changes to the dose of liraglutide during Ramadan (nine patients, of whom eight received a reduced dose), with almost all patients being maintained at the maximum dose of 1.8 mg. There were few patients reporting changes in metformin treatment; similar in both arms (nine and eight in the liraglutide and sulphonylurea arms, respectively). It should be emphasized that this was a switch trial (i.e. subjects randomized to liraglutide had pre‐trial sulphonylurea replaced by liraglutide at baseline).
In summary, despite lower fructosamine levels and lower HbA1c at the start of Ramadan, liraglutide‐treated subjects were able to fast and experienced similar glycaemic improvement compared to sulphonylurea‐treated subjects, with fewer hypoglycaemic episodes despite better glycaemic control. Thus, liraglutide is an effective, well‐tolerated and safe choice when used as glucose‐lowering therapy in patients with T2D who choose to fast during Ramadan.
Supporting information
Supplementary material
ACKNOWLEDGMENTS
The authors thank the investigators, study teams and patients who participated in this trial, and David Ørsted, MD, PhD, Rune Christensen, PhD, and Bryan Goldman, MSc, of Novo Nordisk A/S for their valuable insights into the discussion and interpretation of data. Medical writing support was provided by Michele A. Wells, PhD, of Novo Nordisk Inc. Writing, editing and submission support was provided by Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, and funded by Novo Nordisk A/S, Bagsværd, Denmark. Trial registry: ClinicalTrials.gov, NCT01917656.
Funding information
This work was funded by Novo Nordisk.
Conflict of interest
S.A. attended advisory panels for Novo Nordisk, Sanofi Aventis, Novartis, Janssen and MSD and is an employee of the American University of Beirut. AE attended advisory panels for Novo Nordisk, Sanofi, AstraZeneca, Boehringer, Novartis and MSD and participated in speakers’ bureaus for Novo Nordisk, Sanofi, AstraZeneca, Boehringer, Novartis, MSD, Lilly, Merck and Servie. W.M.W.B. received honoraria from Novo Nordisk as an investigator (patient recruitment/data collection). S.A. received research support from Novo Nordisk. A.B is an advisory board member for Novo Nordisk, Sanofi, AstraZeneca, Bayer, Pfizer, MSD and Novartis; participated in speakers’ bureaus for Novo Nordisk, Sanofi, AstraZeneca, Novartis, HIKMA, Saidal and Merck Serono; and is a consultant for Saidal and Sanofi. M.O. attended advisory panels for Novo Nordisk, Boehringer Ingelheim, Eli Lilly and Novartis and participated in speakers’ bureaus for Novo Nordisk, Boehringer Ingelheim, Novartis, Sanofi‐Aventis, Eli Lilly and Merck. A.M. received research support from Novo Nordisk. K.T. and M.S.K. are full‐time employees of Novo Nordisk. N.S received honoraria for lectures and consultation and funding for participation and travel to medical meetings from Novo Nordisk.
Author contributions
M.S.K. and K.T. were the medical specialists responsible for the trial. They and their teams designed the trial, developed the protocol and were responsible for the trial conduct. All authors had full access to all clinical findings and contributed to the data interpretation, drafting and revisions, and approved the final version to be published. S.A. takes responsibility for the report.
Azar ST, Echtay A, Wan Bebakar WM, Al Araj S, Berrah A, Omar M, Mutha A, Tornøe K, Kaltoft MS and Shehadeh N. Efficacy and safety of liraglutide compared to sulphonylurea during Ramadan in patients with type 2 diabetes (LIRA‐Ramadan): a randomized trial, Diabetes Obes Metab 2016, 18:1025–1033. DOI:10.1111/dom.12733.
REFERENCES
- 1. Pew Research Center . The future of the global muslim population. 2011. http://www.pewforum.org/2011/01/27/the‐future‐of‐the‐global‐muslim‐population/. Accessed November 3, 2015.
- 2. Babineaux SM, Toaima D, Boye KS, et al. Multi‐country retrospective observational study of the management and outcomes of patients with type 2 diabetes during Ramadan in 2010 (CREED). Diabet Med. 2015;32:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Holy Quran, Al‐Bakarah, Sura 2. Verses 183–185.
- 4. Salti I, Benard E, Detournay B, et al. A population‐based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27:2306–2311. [DOI] [PubMed] [Google Scholar]
- 5. Al‐Arouj M, Assaad‐Khalil S, Buse J, et al. Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care. 2010;33:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ibrahim M, Abu Al MM, Annabi FA, et al. Recommendations for management of diabetes during Ramadan: update 2015. BMJ Open Diabetes Res Care. 2015;3:e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bravis V, Hui E, Salih S, et al. Ramadan education and awareness in diabetes (READ) programme for Muslims with type 2 diabetes who fast during Ramadan. Diabet Med. 2010;27:327–331. [DOI] [PubMed] [Google Scholar]
- 8. Kalra S, Aamir AH, Raza A, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian J Endocrinol Metab. 2015;19:577–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassanein M, Abdallah K, Schweizer A. A double‐blind, randomized trial, including frequent patient‐physician contacts and Ramadan‐focused advice, assessing vildagliptin and gliclazide in patients with type 2 diabetes fasting during Ramadan: the STEADFAST study. Vasc Health Risk Manag. 2014;10:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al SS, Basiounny A, Echtay A, et al. The incidence of hypoglycaemia in Muslim patients with type 2 diabetes treated with sitagliptin or a sulphonylurea during Ramadan: a randomised trial. Int J Clin Pract. 2011;65:1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aravind SR, Ismail SB, Balamurugan R, et al. Hypoglycemia in patients with type 2 diabetes from India and Malaysia treated with sitagliptin or a sulfonylurea during Ramadan: a randomized, pragmatic study. Curr Med Res Opin. 2012;28:1289–1296. [DOI] [PubMed] [Google Scholar]
- 12. Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967;46:1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon‐like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–613. [DOI] [PubMed] [Google Scholar]
- 14. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon‐like peptide 1 (7‐36 amide) in type 2 (non‐insulin‐dependent) diabetic patients. Diabetologia. 1993;36:741–744. [DOI] [PubMed] [Google Scholar]
- 15. Holst JJ. Enteroglucagon. Annu Rev Physiol. 1997;59:257–271. [DOI] [PubMed] [Google Scholar]
- 16. Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon‐like peptide‐1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–1669. [DOI] [PubMed] [Google Scholar]
- 17. Novo Nordisk . Victoza (liraglutide) US Prescribing Information. April 2013.
- 18. Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B. Liraglutide, a once‐daily human glucagon‐like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet. 2009;373:473–481. [DOI] [PubMed] [Google Scholar]
- 20. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)‐2 study. Diabetes Care. 2009;32:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies MJ, Kela R, Khunti K. Liraglutide–overview of the preclinical and clinical data and its role in the treatment of type 2 diabetes. Diabetes Obes Metab. 2011;13:207–220. [DOI] [PubMed] [Google Scholar]
- 22. World Medical Association Declaration of Helsinki . Ethical principles for medical research involving human subjects. 59th WMA General Assembly; October 2008; Seoul, South Korea. [Google Scholar]
- 23. International Conference on Harmonisation . ICH harmonised tripartite guideline. Good clinical practice. 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed April 27, 2016.
- 24. European Medicines Agency . Guideline on clinical investigation of medicinal products in the treatment of diabetes mellitus. CHMP/EWP/1080/00 Rev. 1. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed April 27, 2016.
- 25. Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Workgroup on Hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. [DOI] [PubMed] [Google Scholar]
- 27. Nauck MA, Wollschlager D, Werner J, et al. Effects of subcutaneous glucagon‐like peptide 1 (GLP‐1 [7‐36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–1553. [DOI] [PubMed] [Google Scholar]
- 28. Brady EM, Davies MJ, Gray LJ, et al. A randomized controlled trial comparing the GLP‐1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during Ramadan: the treat 4 Ramadan trial. Diabetes Obes Metab. 2014;16:527–536. [DOI] [PubMed] [Google Scholar]
- 29. Schernthaner G, Grimaldi A, Di MU, et al. GUIDE study: double‐blind comparison of once‐daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest. 2004;34:535–542. [DOI] [PubMed] [Google Scholar]
- 30. Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004;64:1339–1358. [DOI] [PubMed] [Google Scholar]
- 31. Al‐Arouj M, Hassoun AA, Medlej R, et al. The effect of vildagliptin relative to sulphonylureas in Muslim patients with type 2 diabetes fasting during Ramadan: the VIRTUE study. Int J Clin Pract. 2013;67:957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Devendra D, Gohel B, Bravis V, et al. Vildagliptin therapy and hypoglycaemia in Muslim type 2 diabetes patients during Ramadan. Int J Clin Pract. 2009;63:1446–1450. [DOI] [PubMed] [Google Scholar]
- 33. Aravind SR, Al TK, Ismail SB, et al. Hypoglycaemia in sulphonylurea‐treated subjects with type 2 diabetes undergoing Ramadan fasting: a five‐country observational study. Curr Med Res Opin. 2011;27:1237–1242. [DOI] [PubMed] [Google Scholar]
- 34. Glimepiride in Ramadan (GLIRA) Study Group . The efficacy and safety of glimepiride in the management of type 2 diabetes in Muslim patients during Ramadan. Diabetes Care. 2005;28:421–422. [DOI] [PubMed] [Google Scholar]
- 35. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287:360–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


