Summary
Fumaric acid esters (FAEs) are licensed for the treatment of moderate‐to‐severe psoriasis in Germany but are also used off‐label in many other countries. We conducted this systematic review to synthesize the highest‐quality evidence for the benefits and risks of FAEs for psoriasis. Our primary outcomes were change in Psoriasis Area and Severity Index score and dropout rates due to adverse effects. Randomized controlled trials (RCTs) of FAEs or dimethylfumarate were included, with no restriction on age or psoriasis subtype. We searched the Cochrane Skin Group Specialised Register, CENTRAL in the Cochrane Library, Medline, Embase, LILACS and five trials registers, and hand searched six conference proceedings. Six RCTs with a total of 544 participants were included, four of which were published only as abstracts or brief reports, limiting study reporting. Five RCTs compared FAEs with placebo, and all demonstrated benefit in favour of FAEs. However, meta‐analysis was possible only for PASI 50 response after 12–16 weeks, which was achieved by 64% of participants on FAEs compared with 14% on placebo: risk ratio (RR) 4·55, 95% confidence interval (CI) 2·80–7·40; two studies; 247 participants; low‐quality evidence). There was no difference in dropout rates due to adverse effects (RR 5·36, 95% CI 0·28–102·12; one study; 27 participants; very low‐quality evidence and wide CI). More participants experienced nuisance adverse effects with FAEs (76%) than with placebo (16%) (RR 4·72, 95% CI 2·45–9·08; one study; 99 participants; moderate‐quality evidence), mainly abdominal pain, diarrhoea and flushing. One head‐to‐head study of very low‐quality evidence comparing FAEs with methotrexate reported comparable efficacy and dropout rates, although FAEs caused more flushing. The evidence in this review was limited and must be interpreted with caution; studies with better design and outcome reporting are needed.
Short abstract
What's already known about this topic?
Fumaric acid esters (FAEs) are licensed for the treatment of moderate‐to‐severe psoriasis in Germany, and are used off‐label in many other countries.
Non‐Cochrane systematic reviews previously examined the effect of FAEs in psoriasis, but have not rigorously assessed the quality of the evidence.
What does this study add?
Six randomized controlled trials with 544 participants were included, four of which were published only as abstracts or brief reports, resulting in low‐ or very low‐quality evidence.
Results suggest that FAEs are superior to placebo, but their efficacy in comparison with methotrexate is uncertain due to very low‐quality evidence.
The relative risk of nuisance adverse effects with FAEs is about five times greater than with placebo; however, there is insufficient evidence available to give an accurate figure for dropout rates due to adverse effects.
Linked Comment: Egeberg. Br J Dermatol 2016; 175:857.
Psoriasis is a chronic inflammatory skin disease with various subtypes, of which chronic plaque psoriasis is the most common.1 Fumaric acid esters (FAEs) were first used in the treatment of psoriasis in 1959 after successful self‐experimentation by Schweckendiek, a German chemist who proposed that psoriasis was caused by a disturbance in the citric acid cycle in which fumaric acid was lacking.2 FAEs contain dimethylfumarate (DMF), believed to be the active component, and salts of ethyl hydrogen fumarate.3 Fumaderm® Initial (Biogen Idec, Cambridge, MA, U.S.A.), containing 30 mg of DMF per tablet, and Fumaderm®, containing 120 mg of DMF per tablet, are commercially available and have been licensed for the treatment of psoriasis in Germany since 1994.4 They are also used for psoriasis treatment as off‐label drugs in many other countries. The aim of this Cochrane review was to provide the best available evidence for the efficacy and safety of FAEs in the treatment of psoriasis. The results are summarized in this report, and the full review is available in the Cochrane Library.5
Material and methods
This systematic review was carried out according to a prespecified protocol6 and incorporated Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.7
Search strategies
An electronic search for relevant studies was carried out up to May 2015 using the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane library, Medline via Ovid from 1946, Embase via Ovid from 1974, and the Latin American and Caribbean Health Science Information (LILACS) database from 1982. We also searched the following trial registers up to May 2015 using the search terms ‘fumaric acid’, ‘fumarate’ and ‘fumaderm’: the metaRegister of Controlled Trials (http://www.isrctn.com/page/mrct), The US National Institute of Health Ongoing Trials Register (www.clinicaltrials.gov), The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au), The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch) and the EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/).
Abstracts of proceedings not included in electronic registries from the following dermatology conferences were hand searched by two authors independently (A.A. and R.A.): American Academy of Dermatology (2008/2009), British Association of Dermatologists (2008–2010) European Academy of Dermatology and Venereology (May 2006 to May 2013), European Society for Dermatological Research (2005–2009), International Investigative Dermatology (2003 to May 2013) and Society for Investigative Dermatology (2007–2009). The reference lists of included and excluded studies were checked for further references to relevant trials. We included all relevant randomized controlled trials (RCTs), with no language restrictions.
Inclusion criteria
We included RCTs that involved participants of either sex, and any age or ethnicity, with a clinical diagnosis of psoriasis of any subtype, where FAEs, as monotherapy or in combination, were compared with placebo or any other active treatment.
Types of outcome measures
The primary outcomes were Psoriasis Area and Severity Index (PASI) score and dropout rates due to adverse effects. Other outcomes of interest were quality‐of‐life scores measured with a validated scale; the proportion of participants achieving ≥ 50%, ≥ 75% and ≥ 90% improvement in PASI (PASI 50, 75 and 90); the proportion of participants experiencing serious adverse effects and those experiencing nonserious nuisance adverse effects.
Data extraction and synthesis
The titles and abstracts of retrieved studies were screened by two authors independently (A.A. and R.A.). The full texts of potentially eligible studies were examined by the same authors who extracted data from eligible studies using a data extraction form based on the ‘checklists of items to consider in data extraction’;8 a third author (J.R.I.) adjudicated on disagreements.
Review Manager,9 the software used for Cochrane reviews, was used for statistical analysis with a fixed‐effects model. For dichotomous outcomes we pooled risk ratios (RRs) with 95% confidence intervals (CIs), while we combined the mean difference (MD) with 95% CI for continuous outcomes. We made contact with trial authors whenever possible to request relevant unreported data. Statistical heterogeneity was assessed using I 2 statistics; we took a narrative approach if the I 2 value exceeded 75%.10 The quality of evidence for each outcome was ranked using GRADEpro software, from which we produced our ‘summary of findings’ tables.7
Results
Description of the included studies
In total, 94 records were identified through the initial search: database searching (n = 80), hand searching (n = 6) and trials registers (n = 8) (Fig. 1). These included eight ongoing studies and eight duplicate reports, which were excluded, giving a total of 78 records. Of these, 11 potentially eligible studies were identified after screening the titles and abstracts. After reading the full texts, five articles were excluded due to failure to meet our prespecified inclusion criteria11, 12, 13, 14 and lack of evidence of randomization.15 As a result, six studies with a total of 544 participants were included in our review; five compared FAE with placebo16, 17, 18, 19, 20 and one used methotrexate as an active comparator.21
Figure 1.
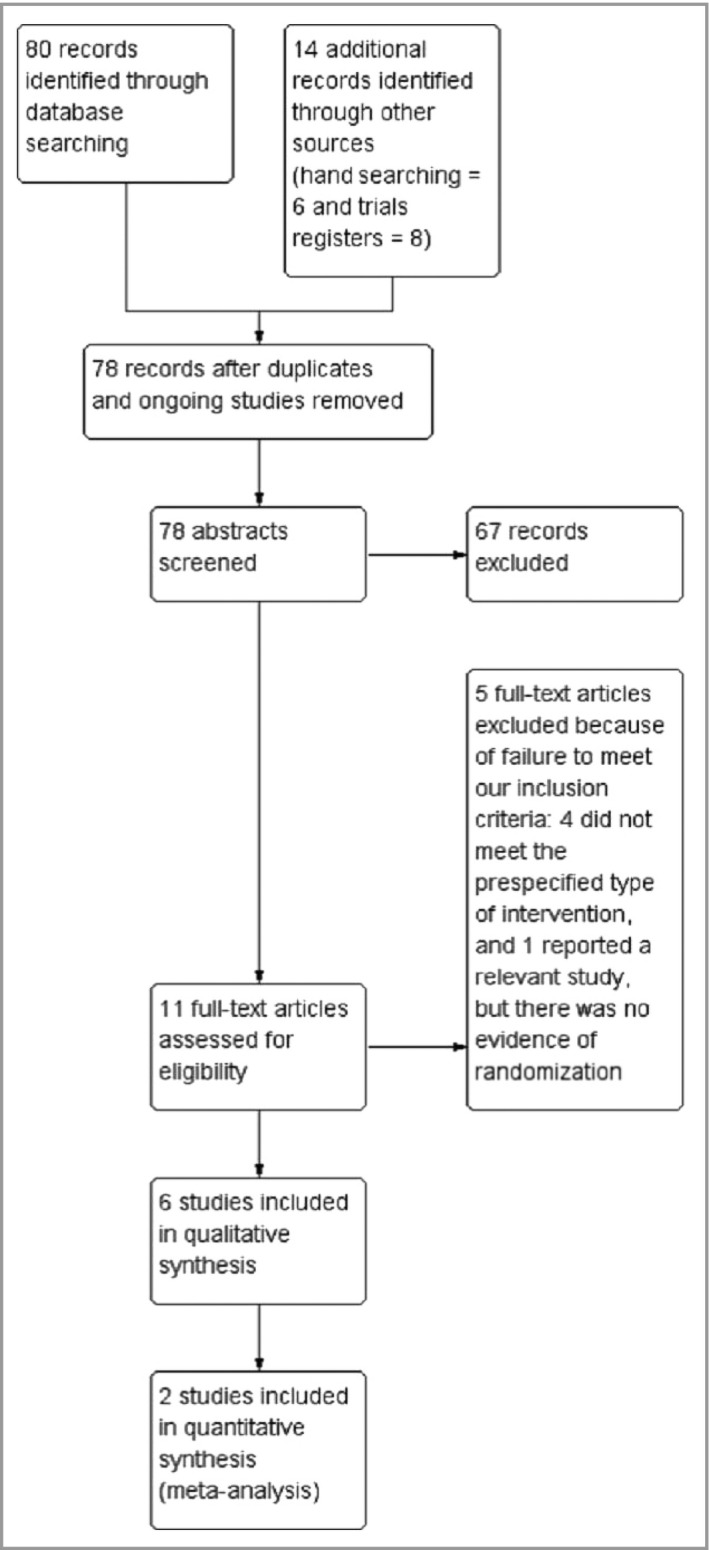
Study flow diagram.
The included studies were reported between 1990 and 2011. Only two of the six studies were published in full reports,16, 21 whereas the others were available in a brief communication,19 a letter20 and abstracts.17, 18 We were unable to obtain the full reports of published abstracts by contacting the authors. Despite the limitations of incompletely reported studies, we decided it was important to include them in our review because of the limited number of eligible RCTs.
Three of the included studies were carried out in the Netherlands,19, 20, 21 one of which was designed to measure the effect of FAEs in the treatment of psoriatic arthritis.20 However, contact with the authors confirmed that all participants had concomitant psoriasis, so we included this study to obtain safety data. All of the included studies involved adults aged > 18 years, except one study that did not report the participants’ ages.17 Participants in the included studies had chronic plaque psoriasis in two studies;18, 21 various psoriasis subtypes in two studies (chronic plaque, guttate, pustular and erythrodermic)16, 17 and unreported psoriasis subtype in two studies.19, 20
PASI score at baseline was reported in only three studies, and was required to be ≥ 10 in one study,21 ≥ 12 in one study18 and 16–24 in one study.17 Outcome reporting was at 12–16 weeks in all of the included studies, but not all of our prespecified outcomes were reported in every study. None of the included studies reported data on economic evaluations.
Risk of bias in the included studies
Three of the included studies had ‘high risk’ of bias in at least one domain.16, 17, 21 Insufficient reporting in most of the included studies, due to lack of full reports and old publications, rendered the risk of bias for most domains ‘unclear’ (Fig. 2).
Figure 2.
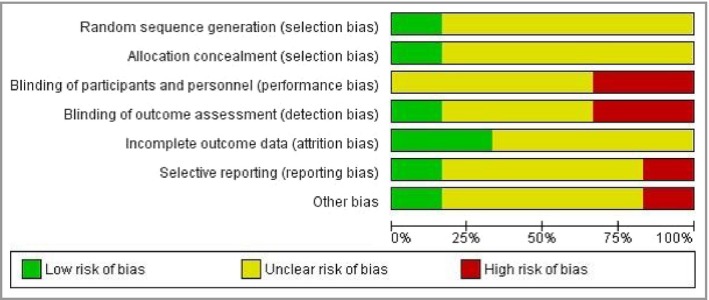
Risk‐of‐bias graph: review authors’ judgements about each risk‐of‐bias item presented as percentages across all included studies.
Effects of interventions
Due to the lack of opportunities for meta‐analyses, we used mainly a narrative approach to present the effects of FAEs in the treatment of psoriasis. The only exception was for the secondary outcome PASI 50 when FAEs were compared with placebo, where data from two studies were combined.
Comparison of fumaric acid esters with placebo
Three of the five studies comparing FAEs with placebo used a mixture of DMF plus monoethylfumarate as an intervention,16, 19, 20 whereas DMF alone was used in the other two studies.17, 18 Two of the included studies17, 18 were reported in abstracts only; contact with the lead author18 confirmed that the studies were not reported in full manuscripts and only the data contained in the abstracts are available. In view of the limited number of eligible studies, and in agreement with the Cochrane Editorial Unit, we included these abstracts in our review. The quality of evidence for each outcome is presented in Table 1.
Table 1.
Summary of findings: fumaric acid esters (FAEs) vs. placebo. Patient or population: psoriasis in adults. Setting: two reports from the Netherlands, one from Poland and two international multicentre studies. Intervention: FAE. Comparison: placebo
| Outcomes | Anticipated absolute effects (95% CI)a | Relative effect (95% CI) | No. of participants (studies) | Quality of evidence (GRADE)b | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with FAEs | |||||
| PASI score; scale range from 0 to 72 (higher score indicates more severe psoriasis) | PASI score reduced from a mean of 21·57 to 10·77 (FAE) and remained constant (placebo) (1 study, 99 participants, P < 0·001); median reduction of 71% (FAE) and 6% (placebo) (1 study, 144 participants, P < 0·001); median reduction of 67·8% (FAE) and 10·2% (placebo) (1 study, 175 participants, P < 0·001) | 418 (3 RCTs) | ⨁⨁◯◯; LOWc , d | All three studies reported significant benefit with FAEs, at week 12 (one study) and week 16 (two studies), but data could not be pooled in a meta‐analysis due to different ways of PASI score reporting | ||
| AEs leading to treatment discontinuation | Two participants withdrew from the FAE group (n = 13) compared with no dropouts in the placebo group (n = 14) (RR 5·36, 95% CI 0·28–102·12) | 27 (1 RCT) | ⨁◯◯◯; VERY LOWe , f | Outcome reported at week 16. Unclear whether any of the reported AEs were ‘serious’ | ||
| QoL assessed with Skindex‐29 (range 0–100; higher scores indicate lower level of QoL) | Mean scores reduced from 54·7 at baseline to 27·0 at week 16 in the FAE group (n = 105) and from 54·0 to 51·1 in the placebo group (n = 70) (P < 0·001) | 175 (1 RCT) | ⨁⨁◯◯; LOWc , g | The reporting abstract did not provide the statistical values needed to calculate the mean difference with 95% CI | ||
| Common nuisance AEs (not leading to treatment discontinuation) | Moderate | RR 4·72 (2·45–9·08) | 99 (1 RCT) | ⨁⨁⨁◯; MODERATEc | Most commonly stomach ache or cramps, diarrhoea and flushing | |
| 16 per 100 | 76 per 100 (39–100) | |||||
| PASI 50 | Moderate | RR 4·55 (2·80–7·40) | 247 (2 RCTs) | ⨁⨁◯◯; LOWc , g | The meta‐analysis included participants who received dimethylfumarate 720 mg | |
| 14 per 100 | 64 per 100 (39–100) | |||||
| PASI 75 | PASI 75 was attained by 39% of participants in the FAE group (n = 105) and 1% of those on placebo (n = 70) (1 study, week 16); and by 42% on FAE (n = 36) compared with 11% on placebo (n = 36) (1 study, week 12) | 247 (2 RCTs) | ⨁⨁◯◯; LOWc , g | Reported to be a statistically significant difference but data not pooled because of significant heterogeneity (I 2 = 77%) | ||
| PASI 90 (not measured) | See comment | See comment | Not estimable | – | – | Not measured in the included studies |
AE, adverse effect; CI, confidence interval; PASI, Psoriasis Area and Severity Index; PASI 50, ≥ 50% improvement in PASI; QoL, quality of Life; RCT, randomized controlled trial; RR, risk ratio. aThe risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bGRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect. cDowngraded one level due limitations in design; high risk of performance and detection bias. dDowngraded one level due to risk of publication bias; data obtained from abstract(s), full report(s) not available. eDowngraded one level due to indirectness; the study was designed for psoriatic arthritis where all participants also had psoriasis, so may not be directly applicable to those with moderate‐to‐severe psoriasis. fDowngraded two levels for imprecision; small sample size and very wide CIs that included the possibility of an effect in either direction (crosses line of no effect). gDowngraded one level due to risk of bias; insufficient reporting.
Altmeyer et al.16 reported a reduction of PASI score from a mean of 21·57 at baseline to 10·77 after 16 weeks of FAE treatment, whereas in the placebo group it remained the same (P < 0·001). Langner et al.17 compared three doses of FAE (120 mg, 360 mg, 720 mg) with placebo, and reported statistically significant reductions in PASI score after 12 weeks, compared with baseline, of 31%, 52% and 71%, respectively (P < 0·001 compared with placebo for the 360‐mg and 720‐mg doses). Similarly, Mrowietz et al.18 reported a median PASI score of 5·8 after 16 weeks of FAE treatment (n = 105), compared with a median of 14·2 in the placebo group (n = 70) (P < 0·001). This represented 67·8% and 10·2% reductions, respectively, and an effect size of 7·4 points (95% CI 5·40–9·40). It was not possible to compute the MD in these studies because of unreported mean PASI scores at baseline and follow‐up.
In a meta‐analysis from two studies17, 18 including a total of 247 participants, the number of participants who attained PASI 50 was greater with FAEs than with placebo (RR 4·55, 95% CI 2·80–7·40; P < 0·001; I 2 = 0%; low‐quality evidence) (Fig. 3). The combined PASI 50 was 64% with FAEs, compared with 14% for placebo, representing a number needed to treat to benefit (NNTB) of 2. The other studies comparing FAEs with placebo did not include a PASI score and instead measured the disease severity by estimating the body surface area involved.19, 20
Figure 3.
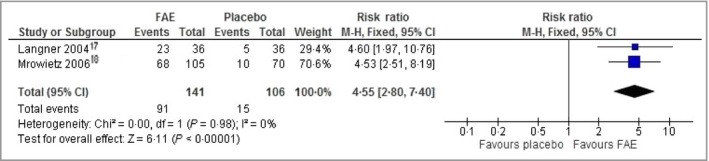
Comparison: fumaric acid esters (FAEs) vs. placebo. Outcome: ≥ 50% improvement in Psoriasis Area and Severity Index. CI, confidence interval.
The dropout rate due to FAE adverse effects was reported clearly in only one study, which was designed for psoriatic arthritis.20 In this study, two withdrawals occurred in the FAE group (n = 13) compared with no dropouts in the placebo arm (n = 14) (RR 5·36, 95% CI 0·28–102·12; 27 participants; very low‐quality evidence). However, this finding is unreliable due to indirectness and very wide CIs. The reasons for dropout in the FAE group were diarrhoea (after 6 weeks) and proteinuria with raised serum creatinine (after 12 weeks). We could not establish the RR of dropouts due to adverse effects alone in the other studies because of unclear16, 19 or lack17, 18 of reporting. None of the included studies reported whether the adverse effects that led to treatment discontinuation were serious.
One study16 reported a higher incidence of nuisance adverse effects (not leading to treatment discontinuation) with FAEs compared with placebo (RR 4·72, 95% CI 2·45–9·08; 99 participants; moderate‐quality evidence), affecting 76% of participants given FAEs (n = 49) and 16% of the placebo group (n = 50), representing a number needed to treat to harm of 2. The most common were abdominal pain, diarrhoea and flushing (percentage and RR could not be computed).
A within‐group comparison showed a statistically significant decrease of leucocytes with FAEs (P = 0·016), due to a reduction in lymphocyte count. The eosinophil count was unchanged in the placebo group, and increased in the FAE group from 2% at baseline to 3·4% at 4 weeks (P < 0·05), with a further insignificant increase to 4·7% at week 12. The maximum increase in eosinophil count was 28% (time point not stated). Another study19 with a small number of participants in the FAE group (n = 13) reported diarrhoea (100% of participants), flushing (95%) and nausea (46%) as the most common adverse effects. Increased serum creatinine to 238 μmol L−1 and reduced creatinine clearance rate by 51% were reported in one participant (8%) in the FAE group, but this was reversible (unknown whether treatment was stopped prior to improvement of the renal function).
Transient increase in liver enzymes (62%), eosinophilia (38%) and lymphopenia (31%) were also reported with FAEs, but it was not clear whether these occurrences were serious or caused treatment discontinuation. In the abstract published by Mrowietz et al.,18 gastrointestinal adverse effects were observed in 58% of participants in the FAE group (n = 105), compared with 23% of those given placebo (n = 70) (RR 2·54, 95% CI 1·60–4·03). Adverse effect severity was described as moderate in 82% of cases (unclear whether any of the remaining 18% dropped out due to severe symptoms). In this abstract, more participants experienced flushing with FAEs in comparison with placebo (42% vs. 9%) (RR 4·67, 95% CI 2·09–10·39).
Quality of life was reported in only one abstract, using Skindex‐29.18 The mean score in the FAE group decreased from 54·7 at baseline to 27·0 at week 16, in comparison with a reduction from 54·0 to 51·1 in the placebo arm, a between‐group difference of −19·3 points (P < 0·001).
Comparison of fumaric acid esters with methotrexate
Only one study, involving 60 randomized participants, compared FAEs with methotrexate in an open‐label fashion.21 Thirty participants were assigned to each group, of whom 26 of the FAE group and 25 in the methotrexate group were included in the primary analysis at week 12. The quality of evidence for each outcome is summarized in Table 2.
Table 2.
Summary of findings: fumaric acid esters (FAEs) vs. methotrexate (MTX). Patient or population: psoriasis in adults. Setting: departments of dermatology, Rotterdam and Eindhoven, the Netherlands. Intervention: FAE 720 mg (after week 9, following the standard progressive dosage regimen). Comparison: oral MTX 15 mg weekly (following gradual dose increments)
| Outcomes | Anticipated absolute effects (95% CI)a | Relative effect (95% CI) | No. of participants (studies) | Quality of evidence (GRADE)b | Comments | |
|---|---|---|---|---|---|---|
| Risk with MTX | Risk with FAE | |||||
| PASI score; scale range from 0 to 72 (higher score indicates more severe psoriasis) | Mean PASI score was 6·7 | Mean PASI score in the intervention group was 3·8 more (0·68–6·92 more) | – | 51 (1 RCT) | ⨁◯◯◯; VERY LOWc , d , e | PASI score was measured at week 12. The study reported no significant difference between FAEs and MTX based on mean change from baseline |
| AEs leading to treatment discontinuation | Moderate | RR 0·19 (0·02–1·53) | 51 (1 RCT) | ⨁◯◯◯; VERY LOWc , d , e | Based on a small sample size (FAE 26, MTX 25). The main reasons were elevated liver enzymes with MTX and diarrhoea with FAEs. No serious AEs occurred in either group | |
| 20 per 100 | 4 per 100 (0–31) | |||||
| Quality of life: not measured | See comment | See comment | Not estimable | – | – | Quality of life was not assessed |
| Common nuisance AEs (not leading to treatment discontinuation) | Moderate | RR 0·89 (0·77–1·03) | 54 (1 RCT) | ⨁◯◯◯; VERY LOWc , d , e | Only flushing was significantly more reported with FAEs. Occurrences of other AEs including laboratory findings were not significantly different | |
| 100 per 100 | 89 per 100 (77–100) | |||||
| PASI 50 | Moderate | RR 0·71 (0·41–1·22) | 51 (1 RCT) | ⨁◯◯◯; VERY LOWc , d , e | Based on a small sample size (MTX 25, FAE 26) | |
| 60 per 100 | 43 per 100 (25–73) | |||||
| PASI 75 | Moderate | RR 0·80 (0·28–2·29) | 51 (1 RCT) | ⨁◯◯◯; VERY LOWc , d , e | Based on a small sample size (MTX 25, FAE 26) | |
| 24 per 100 | 19 per 100 (7–55) | |||||
| PASI 90 | Moderate | RR 0·48 (0·05–4·98) | 51 (1 RCT) | ⨁◯◯◯; VERY LOWc , d , e | Based on a small sample size (MTX 25, FAE 26) | |
| 8 per 100 | 4 per 100 (0–40) | |||||
AE, adverse effect; CI, confidence interval; PASI, Psoriasis Area and Severity Index; PASI 50, ≥ 50% improvement in PASI; RCT, randomized controlled trial; RR, risk ratio. aThe risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bGRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect. cDowngraded one level due to limitations in design; highest dose of MTX given was 15 mg per week while the maximum dose of FAEs was given. dDowngraded one level due to limitations in design; open‐label design (high risk of performance and detection bias). eDowngraded one level due to imprecision; small sample size.
The study reported similar efficacy of FAE and methotrexate, with a mean PASI score reduction from 14·5 at baseline to 6·7 after 12 weeks in the methotrexate group (n = 25) in comparison with a reduction from 18·1 to 10·5 in the FAE group (n = 26). The reported absolute difference after adjustment for baseline values was 1·4 (95% CI −2·0 to 4·7; P = 0·42). However, when we compared the PASI scores at follow‐up (week 12), as recommended by The Cochrane Collaboration, there was a significant difference in favour of methotrexate (MD 3·80, 95% CI 0·68–6·92; very low‐quality evidence) (Fig. 4).
Figure 4.

Comparison: fumaric acid esters (FAEs) vs. methotrexate (MTX). Outcome: Psoriasis Area and Severity Index score at follow‐up. CI, confidence interval.
No significant difference was noted between the two groups in the numbers of participants who attained PASI 50 (RR 0·71, 95% CI 0·41–1·22; very low‐quality evidence), PASI 75 (RR 0·80, 95% CI 0·28–2·29; very low‐quality evidence) and PASI 90 (RR 0·48, 95% CI 0·05–4·98; very low‐quality evidence). However, the maximum dose of methotrexate used in this study (15 mg per week) may have been suboptimal, as higher doses can be prescribed in routine clinical practice. Also, the time of assessment at 12 weeks might have been too early to evaluate true efficacy. Although the study reported no significant difference in the number of participants attaining PASI 75 and PASI 90 at week 16, it must be noted that the dose of methotrexate was reduced gradually from week 12, which may have reduced the effect size.
The dropout rate due to adverse effects in both groups was not significantly different (RR 0·19, 95% CI 0·02–1·53; very low‐quality evidence) (Fig. 5). Four participants (16%) in the methotrexate group dropped out because of elevated liver enzymes; another patient dropped out due to recurrent angina unrelated to treatment. Raised liver enzymes were reported to be transient, and normalized 4–8 weeks after treatment discontinuation. Only one participant in the FAE group (4%) discontinued treatment, due to diarrhoea.
Figure 5.

Comparison: fumaric acid esters (FAEs) vs. methotrexate (MTX). Outcome: dropout rate due to adverse effects. CI, confidence interval.
Overall, the number of participants experiencing nuisance adverse effects was not significantly different between the two groups (RR 0·89, 95% CI 0·77–1·03; very low‐quality evidence). However, more participants experienced flushing in the FAE group (13 vs. two) (RR 6·50, 95% CI 1·62–26·09).
There was no significant difference in reported laboratory findings between the two groups, which may reflect the small study size. Transient increase of liver enzymes (up to double the baseline value) was observed in 11% of participants in the FAE group and 30% of participants given methotrexate (RR 0·38, 95% CI 0·11–1·26). There was transient eosinophilia (maximum measured level 1·55 × 109 cells L−1) in five participants in the FAE group, compared with none of those on methotrexate (RR 11·00, 95% CI 0·64–189·65), and transient leucocytopenia (2·1 × 109 cells L−1) in one participant in the FAE group, compared with none in the methotrexate group (RR 3·00, 95% CI 0·13–70·53). An equal number of eight participants from each group (30%) showed transient proteinuria (RR 1·00, 95% CI 0·44–2·28).
Discussion
Limited evidence suggests that FAEs are superior to placebo in the treatment of psoriasis, and there is very low‐quality evidence to determine the relative efficacy of FAEs compared with methotrexate. Commonly reported adverse effects associated with FAEs include gastrointestinal symptoms (58% of participants in one study), flushing (42%, 48% and 95% in three studies), eosinophilia (19% and 38% in two studies) and reversible proteinuria (30% in one study). However, the evidence provided by this review was limited due to a lack of full reports and inconsistencies of reporting. No long‐term studies were identified to comment on the long‐term efficacy and safety of FAEs in psoriasis.
The small number of included studies and insufficient reporting of outcomes were major limitations to address the objectives of our review. Some studies included participants with various types of psoriasis, but the outcomes reported did not indicate whether the response to FAEs varied between different subgroups. The majority of studies comparing FAEs with placebo did not report the number of participants who dropped out because of adverse effects. Variation in FAE dose increments may also have had an impact on the magnitude of treatment benefit and risk of adverse effects. More recently, the European S3 psoriasis guidelines have standardized the schedule of dose increments,22, 23 which may help to inform future FAE trial designs. We were unable to establish whether the use of DMF alone has a similar efficacy and safety profile to the mixture of DMF plus monoethylfumarate.
Other non‐Cochrane systematic reviews have also reported the superiority of FAEs over placebo in the treatment of psoriasis,24, 25, 26 and similar efficacy to methotrexate.24, 26 However, GRADEpro assessment of the level of quality of evidence in our review demonstrated that the latter conclusion is unreliable due to the very low quality of evidence. There is a relative paucity of RCTs comparing other conventional oral treatments for psoriasis with placebo.
Bansback et al.27 reported in meta‐analyses an RR of PASI 50 response of 4·74 with methotrexate 15–22·5 mg weekly (95% CI 3·52–5·73), with an NNTB of 2; and 4·06 with ciclosporin 3 mg kg−1 per day (95% CI 2·54–5·73), with an NNTB of 2. These are comparable with our findings of FAE efficacy with a PASI 50 RR of 4·55 compared with placebo (95% CI 2·80–7·40) and an NNTB of 2. However, the dropout rates and risk of adverse effects were not reported by Bansback et al. Three RCTs from the 1980s28, 29, 30 demonstrated that acitretin 50–75 mg daily was significantly better than placebo and a lower acitretin dose (10–25 mg daily) in treating psoriasis, but no PASI scores were reported and the dropout rate due to adverse effects was unclear. A Cochrane systematic review is currently underway to examine all systemic pharmacological interventions for psoriasis.31
Most of the studies included in our review were not fully reported and were performed before the requirement of trial registration. As a result we downgraded the evidence quality to low or very low. The findings in our review reinforce the conclusion of the European S3 guidelines that ‘although the use of fumarates for psoriasis has been evaluated in clinical trials, only a small number of these have followed the criteria of evidence‐based medicine’.22 Our review also highlights the inadequate reporting of adverse effects, which should be based on the Consolidated Standards of Reporting Trials (www.consort-statement.org). Application of these standards and consistency in reported outcomes based on the Core Outcome Measures in Effectiveness Trials initiative are necessary to enhance the quality and robustness of evidence in future FAE trials. There remains a need to establish the long‐term safety of FAEs, an evidence gap that is being addressed by the British Association of Dermatologists’ Biologic Interventions Register32 and other psoriasis databases.
Supporting information
Audio S1. Author audio.
Acknowledgments
The authors would like to thank Chris Taylor, who commented on the plain language summary, glossary and the readability of the first draft of the review. Also, the authors would like to thank the Cochrane Skin Group for their editorial support and guidance throughout the review process.
Funding sources
Psoriasis and Psoriatic Arthritis Alliance (PAPAA), U.K. Grant award. The National Institute for Health Research (NIHR), U.K. The NIHR, U.K., is the largest single funder of the Cochrane Skin Group.
Conflicts of interest
V.P. has received departmental support from AbbVie, Johnson & Johnson, Pfizer, GSK, Novartis and CEO. He has received honoraria from Johnson & Johnson, Novartis and AbbVie. None of these companies produces any of the interventions listed in this review. His department benefits financially from the Dermatology Life Quality Index.
This article is based on a Cochrane Review published in the Cochrane Database of Systematic Reviews 2015; 8:CD010497.
References
- 1. Lebwohl M. Psoriasis. Lancet 2003; 361:1197–204. [DOI] [PubMed] [Google Scholar]
- 2. Schweckendiek W. Treatment of psoriasis vulgaris. Med Monatsschr 1959; 13:103–4. [PubMed] [Google Scholar]
- 3. Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br J Dermatol 1999; 141:424–9. [DOI] [PubMed] [Google Scholar]
- 4. Mrowietz U, Asadullah K. Dimethylfumarate for psoriasis: more than a dietary curiosity. Trends Mol Med 2005; 11:43–8. [DOI] [PubMed] [Google Scholar]
- 5. Atwan A, Ingram JR, Abbott R et al Oral fumaric acid esters for psoriasis. Cochrane Database Syst Rev 2015; 8:CD010497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atwan A, Abbott R, Kelly M et al Oral fumaric acid esters for psoriasis. Cochrane Database Syst Rev 2013; 4:CD010497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schunemann H, Brozek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendation 2013. Available at: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html (last accessed 4 July 2016).
- 8. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Available at: http://handbook.cochrane.org/ (last accessed 4 July 2016).
- 9. Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- 10. O'Rourke K, Detsky AS. Meta‐analysis in medical research: strong encouragement for higher quality in individual research efforts. J Clin Epidemiol 1989; 42:1021–4. [DOI] [PubMed] [Google Scholar]
- 11. Balak DM, Fallah‐Arani S, Venema CM et al Addition of an oral histamine antagonist to reduce adverse events associated with fumaric acid esters in the treatment of psoriasis: a randomized double‐blind placebo‐controlled trial. Br J Dermatol 2015; 172:754–9. [DOI] [PubMed] [Google Scholar]
- 12. Friedrich M, Sterry W, Klein A et al Addition of pentoxifylline could reduce the side effects of fumaric acid esters in the treatment of psoriasis. Acta Derm Venereol 2001; 81:429–30. [DOI] [PubMed] [Google Scholar]
- 13. Gollnick H, Altmeyer P, Kaufmann R et al Topical calcipotriol plus oral fumaric acid is more effective and faster acting than oral fumaric acid monotherapy in the treatment of severe chronic plaque psoriasis vulgaris. Dermatology 2002; 205:46–53. [DOI] [PubMed] [Google Scholar]
- 14. Nieboer C, de Hoop D, Langendijk PN et al Fumaric acid therapy in psoriasis: a double‐blind comparison between fumaric acid compound therapy and monotherapy with dimethylfumaric acid ester. Dermatologica 1990; 181:33–7. [DOI] [PubMed] [Google Scholar]
- 15. Nieboer C, de Hoop D, van Loenen AC et al Systemic therapy with fumaric acid derivates: new possibilities in the treatment of psoriasis. J Am Acad Dermatol 1989; 20:601–8. [DOI] [PubMed] [Google Scholar]
- 16. Altmeyer PJ, Matthes U, Pawlak F et al Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double‐blind study in 100 patients. J Am Acad Dermatol 1994; 30:977–81. [DOI] [PubMed] [Google Scholar]
- 17. Langner A, Roszkiewicz J, Baran E, Placek W. Results of a phase II study of a novel oral fumarate, BG‐12, in the treatment of severe psoriasis (abstract P075). European Congress on Psoriasis 2004. J Eur Acad Dermatol Venereol 2004; 18:798. [Google Scholar]
- 18. Mrowietz U, Reich K, Spellman M. Efficacy, safety, and quality of life effects of a novel oral formulation of dimethyl fumarate in patients with moderate to severe plaque psoriasis: results of a phase 3 study. J Am Acad Dermatol 2006; 54 (Suppl.):AB202. [Google Scholar]
- 19. Nugteren‐Huying WM, van der Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy for psoriasis: a randomized, double‐blind, placebo‐controlled study. J Am Acad Dermatol 1990; 22:311–12. [DOI] [PubMed] [Google Scholar]
- 20. Peeters AJ, Dijkmans BA, van der Schroeff JG. Fumaric acid therapy for psoriatic arthritis. A randomized, double‐blind, placebo‐controlled study. Br J Rheumatol 1992; 31:502–4. [DOI] [PubMed] [Google Scholar]
- 21. Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trial. Br J Dermatol 2011; 164:855–61. [DOI] [PubMed] [Google Scholar]
- 22. Pathirana D, Ormerod AD, Saiag P et al European S3‐guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol 2009; 23(Suppl. 2):1–70. [DOI] [PubMed] [Google Scholar]
- 23. Nast A, Gisondi P, Ormerod AD et al European S3‐guidelines on the systemic treatment of psoriasis vulgaris – Update 2015 – Short version – EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2015; 29:2277–94. [DOI] [PubMed] [Google Scholar]
- 24. Ceglowska U, Wlodarczyk A, Slomka M. Clinical effectiveness of fumaric acid esters (Fumaderm) in psoriasis: a systematic review of literature. Presented at the ISPOR 17th Annual European Congress, Amsterdam, the Netherlands, November 2014; abstr. PSS6. [DOI] [PubMed]
- 25. Griffiths CE, Clark CM, Chalmers RJ et al A systematic review of treatments for severe psoriasis. Health Technol Assess 2000; 4:1–125. [DOI] [PubMed] [Google Scholar]
- 26. Schmitt J, Rosumeck S, Thomaschewski G et al Efficacy and safety of systemic treatments for moderate‐to‐severe psoriasis: meta‐analysis of randomized controlled trials. Br J Dermatol 2014; 170:274–303. [DOI] [PubMed] [Google Scholar]
- 27. Bansback N, Sizto S, Sun H et al Efficacy of systemic treatments for moderate to severe plaque psoriasis: systematic review and meta‐analysis. Dermatology 2009; 219:209–18. [DOI] [PubMed] [Google Scholar]
- 28. Goldfarb MT, Ellis CN, Gupta AK et al Acitretin improves psoriasis in a dose‐dependent fashion. J Am Acad Dermatol 1988; 18:655–62. [DOI] [PubMed] [Google Scholar]
- 29. Gupta AK, Goldfarb MT, Ellis CN, Voorhees JJ. Side‐effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol 1989; 20:1088–93. [DOI] [PubMed] [Google Scholar]
- 30. Olsen EA, Weed WW, Meyer CJ, Cobo LM. A double‐blind, placebo‐controlled trial of acitretin for the treatment of psoriasis. J Am Acad Dermatol 1989; 21:681–6. [DOI] [PubMed] [Google Scholar]
- 31. Sbidian E, Le Cleach L, Trinquart L et al Systemic pharmacological treatments for chronic plaque psoriasis. Cochrane Database Syst Rev 2015; 2:CD011535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burden AD, Warren RB, Kleyn CE et al The British Association of Dermatologists’ Biologic Interventions Register (BADBIR): design, methodology and objectives. Br J Dermatol 2012; 166:545–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Audio S1. Author audio.


