Abstract
Androgen deprivation therapy (ADT) for prostate cancer (PCa) increases risk of type 2 diabetes (T2DM); however the association between types and duration of ADT has not been fully elucidated. We examined how type and duration of ADT affects risk of T2DM. Using data from Prostate Cancer database Sweden (PCBaSe) we investigated risk of T2DM in a cohort of 34,031 men with PCa on ADT; i.e., anti‐androgens (AA), orchiectomy, or gonadotropin‐releasing hormone (GnRH) agonists compared to an age‐matched, PCa‐free comparison cohort (n = 167,205) using multivariate Cox proportional hazard regression. T2DM was defined as a newly filled prescription for metformin, sulphonylurea, or insulin in the Prescribed Drug Register. A total of 21,874 men with PCa received GnRH agonists, 9,143 AA and 3,014 underwent orchiectomy. Risk of T2DM was increased in men in the GnRH agonists/orchiectomy group during the first 3 years of ADT [i.e., 1 − 1.5 years HR: 1.61 (95%CI: 1.36 − 1.91)], compared to PCa‐free men. The risk decreased thereafter (e.g., 3 − 4 years HR: 1.17 (95% CI: 0.98 − 1.40)). Conversely, no increased risk was seen in men on AA (HR: 0.74 (95%CI: 0.65 − 0.84). The incidence of T2DM per 1,000 person‐years was 10 for PCa‐free men, 8 for men on AA, and 13 for men on GnRH agonists/orchiectomy. Duration of ADT has a significant impact on risk of T2DM. With the peak after three years of treatment, our data indicates that men on ADT, even for a limited period of time, such as adjuvant to radiotherapy, are at increased risk of T2DM.
Keywords: prostate cancer, type two diabetes, androgen deprivation therapy
Short abstract
What's new?
All treatments involve tradeoffs. For patients with prostate cancer, treatment with androgen deprivation therapy (ADT) can lead to an increased risk of type II diabetes. These authors set out to analyze how the duration of treatment, and the type of ADT, affect diabetes risk. They collected data on patients receiving three types of ADT: anti‐androgens, gonadotropin releasing hormone agonists, and orchiectomy, and compared them with age‐matched, cancer‐free controls. The risk of diabetes peaked after 3 years of treatment with GnRH agonists or orchiectomy. By contrast, patients receiving anti‐androgens showed no increase in diabetes risk relative to cancer‐free controls.
Androgen Deprivation Therapy (ADT) is the recommended first line treatment in all men with disseminated prostate cancer (PCa) and is also used in conjunction with radiotherapy in locally advanced disease as both neoadjuvant and adjuvant therapy.1 When men progress to castrate resistance, it is recommended that treatment with ADT continues, alongside the addition of further therapies. Given the prolonged disease trajectory of PCa, men can remain on ADT for many years,2 making any long‐term effects associated with treatment significant.
Common adverse effects of ADT include fatigue, hot flushes and impotence.3 ADT also increases the risk of cardiovascular disease,4, 5 reduces bone mineral density,6 increases risk of fractures and of type 2 diabetes (T2DM), as demonstrated by several North American cohorts.7, 8, 9, 10 This led the Food and Drug Administration (FDA) in 2010 to add a risk label on gonadotropin‐releasing hormone (GnRH) agonists for increased risk of T2DM and certain cardiovascular diseases (heart attack, sudden cardiac death, and stroke).11
ADT increases the prevalence of metabolic syndrome components such as decreased insulin sensitivity and increased body fat.12 We have previously undertaken a meta‐analysis to quantify the association between ADT and metabolic syndrome.13 The risk of metabolic syndrome for men on ADT increased almost two‐fold, relative risk (RR) 1.75 (95% CI 1.27‐2.41), as compared to men not on ADT. For T2DM this relative risk was 1.36 (95% CI 1.17‐1.58). Here, we investigate the risk of T2DM in men on ADT taking into account the impact of different types and durations of ADT (GnRH agonists, anti‐androgens (AA), and orchiectomy) on risk of T2DM.
Methods
Study population and data collection
PCBaSe Sweden 3.0 is based on the National Prostate Cancer Register (NPCR) of Sweden, which became nationwide in 1998 and covers 98% of all newly diagnosed cases of PCa, as compared to the Swedish Cancer Register.14, 15 NPCR includes information on date of diagnosis, age at diagnosis, tumour stage and differentiation, serum levels of prostate specific antigen (PSA) at time of diagnosis, and primary treatment within 6 months after date of diagnosis. Risk categories were determined according to a modified version of the National Comprehensive Cancer Network Guideline16 as follows: Low risk: T1‐2, Gleason score of 2–6 and PSA< 10 ng/ml; intermediate risk: T1‐2, Gleason score 7 and/or PSA 10–20 ng/ml; high risk: T3and/or Gleason score 8–10 and/or PSA 20—50 ng/ml; regionally metastatic/locally advanced:T4 and/or N1 and/or PSA 50–100 ng/ml in the absence of distant metastases (M0 or MX); distant metastases: M1 and/or PSA > 100 ng/ml. Using the Swedish 10‐digit personal identity number, five PCa‐free men from the general population in Sweden were randomly selected within sets of men who matched the index case on birth year and county of residence and included in the PCBaSe comparison cohort.14 Cases and the comparison cohort in PCBaSe were subsequently linked to a series of national health care registers and demographic databases, in order to obtain data on comorbidity, socioeconomic status, and cause of death. Information on filled prescriptions of anti‐androgens (AA), gonadotropin‐releasing hormone (GnRH) agonists, metformin, sulphonylurea and insulin was obtained from The National Prescribed Drug Register using ATC codes (insulin‐ ANA, metformin‐ A10BA/BD sulphonylurea‐ A10BB GnRH –L02AE AA‐ L02BB).17 The Research Ethics Board at Umeå University approved this study.
For this analysis we selected both men who received primary and secondary ADT, i.e., as a second line treatment strategy initiated after primary treatment at the time of disease progression. Primary treatment was recorded in NPCR as well as the Prescribed Drug Register, whereas secondary ADT was retrieved from the Prescribed Drug Register only.14 Co‐morbidities were measured by the Charlson Comorbidity Index (CCI), which assigns weights to a number of medical conditions, including diabetes and hypertension based on discharge diagnoses in the Patient Register.18 Each condition was assigned a score of 1, 2, 3, or 6 and the final CCI is given as the sum of these scores. Individuals were grouped into CCI categories for final scores of 0, 1, 2, or 3+. Information on age at diagnosis, primary treatment, education status, and prostate cancer risk category were also used.
Analysis
We conducted an analysis whereby PCa men on ADT and PCa‐free men were followed to identify occurrence of T2DM. The latter was defined by two filled prescriptions for insulin, metformin or sulphonylurea with a maximum time between the two prescriptions of 180 days. The date of the first filled prescription was used as the date of the event. Hazard ratios (HRs) for T2DM were calculated for men with PCa versus the comparison cohort with left truncation using a Cox proportional hazards model with age as a timescale accounting for CCI, PCa risk category and education status. Left truncation was applied because the Prescribed Drug Register started on July 1st 2005. We allowed for a run‐in period of six months and men with a filled prescription for anti‐diabetic drugs during this period were excluded from the analysis. Hence, all men with prevalent T2DM on an anti‐diabetic drug were excluded. Follow up started on 1st January 2006 and ended at date of death, date of emigration, date of T2DM prescription, or 31 December 2013, whichever came first. Men who received AA or GnRH according to NPCR and had a date of diagnosis prior to 1st January 2006 and were found to still be receiving them according to the Prescribed Drug Register during the “run in period” were considered to have been “exposed” since the date of diagnosis.15 All other exposure to AA or GNRH was defined as time from first filled prescription. In case of cross over, patients were allowed to change groups and were from then onwards considered to be exposed to the treatment in their new group. Thereby, these persons contributed person/years in each treatment category.
The association between duration of ADT and risk of T2DM was assessed using multivariate Cox proportional hazards models with left truncation in which exposure time was divided into the following intervals: 0–6 months, 6–12 months, 12–18 months, 18–24 months, 24–30 months, 30–36 months, 36–48 months, 48–60 months, 60–72 months, 72–84 months, 84–120 months, >120 months. We also calculated incidence rates per 1,000/person years for the different exposure groups. Finally, we conducted a sensitivity analysis in which incidence rates of T2DM were compared between men with PCa 2 years prior to initiation of ADT and men 2 years post initiation of ADT. This analysis included men free of T2DM who received their first ADT after 1st of August 2008 to ensure that sufficient data was available from the National Prescribed Drug Register which only start on 1st July 2005.Those men in the sensitivity analysis who developed T2DM in the period 0–2 years prior to ADT were obviously not included in the overall analysis. All data management was performed with SAS version 9.3 (SAS Institute, Cary, NC) and all data analysis was conducted with R version 2.13.2 (R Foundation for Statistical Computing, Wien, Austria).
Results
167,205 PCa‐free men and 34,031 men with PCa out of whom 21,874 (64%) received GnRH agonists, 9,143 (27%) AA and 3,014 (9%) underwent orchiectomy were included in the study (Table 1). Results for men who had undergone surgical or medical castration were analyzed together in the GnRH/Orch group.
Table 1.
Baseline characteristics of men with PCa on ADT and the matched comparison cohort of PCa‐free men in Prostate Cancer Data Base 3.0
| PCa Free Cohort | All men with PCa | AA | GnRH | Orchiectomy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | 167,205 | 34,031 | 9,143 | 21,874 | 3,014 | |||||
| Mean Follow up time (SD) | ||||||||||
| 4.2 | (2.5) | 3.5 | (2.4) | 3.6 | (2.3) | 3.5 | (2.4) | 3.1 | (2.4) | |
| Age | ||||||||||
| Mean age at diagnosis (SD) | 74.8 | (8.5) | 74.4 | (8.4) | 71.2 | (8.0) | 75.2 | (8.3) | 78.4 | (7.3) |
| <65 | 23183 | (13.9) | 4908 | (14.4) | 2119 | (23.2) | 2623 | (12.0) | 166 | (5.5) |
| 65‐74 | 55348 | (33.1) | 11806 | (34.7) | 3893 | (42.6) | 7247 | (33.1) | 666 | (22.1) |
| 75‐84 | 70809 | (42.3) | 14136 | (41.5) | 2829 | (30.9) | 9655 | (44.1) | 1652 | (54.8) |
| 85+ | 17865 | (10.7) | 3181 | (9.3) | 302 | (3.3) | 2349 | (10.7) | 530 | (17.6) |
| Age at start of ADT | ||||||||||
| <65 | ‐ | ‐ | 3592 | (10.6) | 1189 | (13.0) | 2254 | (10.3) | 149 | (4.9) |
| 65‐74 | ‐ | ‐ | 10849 | (31.9) | 3641 | (39.8) | 6596 | (30.2) | 612 | (20.3) |
| 75‐84 | ‐ | ‐ | 15493 | (45.5) | 3766 | (41.2) | 10059 | (46.0) | 1668 | (55.3) |
| 85+ | ‐ | ‐ | 4097 | (12.0) | 547 | (6.0) | 2965 | (13.6) | 585 | (19.4) |
| Entry into PCBaSe cohort/Year of PCa diagnosis | ||||||||||
| 1997‐2001 | 17889 | (10.7) | 5389 | (15.8) | 1145 | (12.5) | 3687 | (16.9) | 557 | (18.5) |
| 2002‐2005 | 49375 | (29.5) | 11281 | (33.1) | 2738 | (29.9) | 7446 | (34.0) | 1097 | (36.4) |
| 2006‐2009 | 64085 | (38.3) | 11567 | (34.0) | 3438 | (37.6) | 7161 | (32.7) | 968 | (32.1) |
| 2010‐2012 | 35856 | (21.4) | 5794 | (17.0) | 1822 | (19.9) | 3580 | (16.4) | 392 | (13.0) |
| CCI | ||||||||||
| 0 | 110713 | (66.2) | 22328 | (65.6) | 6271 | (68.6) | 14143 | (64.7) | 1914 | (63.5) |
| 1 | 29651 | (17.7) | 6247 | (18.4) | 1587 | (17.4) | 4048 | (18.5) | 612 | (20.3) |
| 2 | 15948 | (9.5) | 3309 | (9.7) | 793 | (8.7) | 2215 | (10.1) | 301 | (10.0) |
| 3+ | 10893 | (6.5) | 2147 | (6.3) | 492 | (5.4) | 1468 | (6.7) | 187 | (6.2) |
| Education Status | ||||||||||
| Low | 78732 | (47.1) | 16239 | (47.7) | 3559 | (38.9) | 10898 | (49.8) | 1782 | (59.1) |
| Middle | 56051 | (33.5) | 11579 | (34.0) | 3422 | (37.4) | 7252 | (33.2) | 905 | (30.0) |
| High | 29171 | (17.4) | 5771 | (17.0) | 2091 | (22.9) | 3403 | (15.6) | 277 | (9.2) |
| Missing | 3251 | (1.9) | 442 | (1.3) | 71 | (0.8) | 321 | (1.5) | 50 | (1.7) |
| PCa risk category | ||||||||||
| No PCa | 167205 | (100.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| 1. Low risk | ‐ | ‐ | 2334 | (6.9) | 1180 | (12.9) | 1068 | (4.9) | 86 | (2.9) |
| 2. Intermediate risk | ‐ | ‐ | 6021 | (17.7) | 2819 | (30.8) | 2929 | (13.4) | 273 | (9.1) |
| 3. High risk | ‐ | ‐ | 11775 | (34.6) | 3469 | (37.9) | 7410 | (33.9) | 896 | (29.7) |
| 4. Regionally metastatic | ‐ | ‐ | 4745 | (13.9) | 962 | (10.5) | 3354 | (15.3) | 429 | (14.2) |
| 5. Distant metastases | ‐ | ‐ | 8850 | (26.0) | 591 | (6.5) | 6956 | (31.8) | 1303 | (43.2) |
| 6. Missing data | ‐ | ‐ | 306 | (0.9) | 122 | (1.3) | 157 | (0.7) | 27 | (0.9) |
| Primary Treatment | ||||||||||
| No PCa | 167205 | (100.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| ADT | ‐ | ‐ | 24815 | (72.9) | 4113 | (45.0) | 17972 | (82.2) | 2730 | (90.6) |
| Curative treatment | ‐ | ‐ | 4352 | (12.8) | 2887 | (31.6) | 1402 | (6.4) | 63 | (2.1) |
| Deferred treatment | ‐ | ‐ | 4864 | (14.3) | 2143 | (23.4) | 2500 | (11.4) | 221 | (7.3) |
There was a five‐fold higher occurrence of metastatic disease at date of diagnosis among men in the GnRH compared to men on AA (32% vs. 6%). Conversely, five times as many men on AA had undergone primary curative treatment and subsequently received ADT, compared to men on GnRH (31% vs. 6%) (Table 1).
Table 2 shows the number of events and HRs for men receiving a new prescription for insulin, sulphonylurea or metformin on AA or GnRH/Orch over time compared to a PCa free cohort. Those in the GnRH/Orch group had an increased risk, up until 2.5–3 years of exposure, HR 1 − 1.5 years of ADT 1.61 (95% CI 1.36 − 1.91), 2 − 2.5 years of ADT 1.68 (95% CI:1.4 − 2.02), 2.5–3 years of ADT 1.42 (95% CI 1.16 − 1.76) which then reduced, 3–4 years of ADT 1.17 (95% CI0.98 − 1.40) and 7–10 years ADT 0.96 (95% CI 0.77 − 1.19). In contrast, those on AA had no increased risk of T2DM during any time period compared to PCa‐free men, HR during 0–2 years of ADT 0.73 (95% CI: 0.61 − 0.87), 2–4 years: 0.71 (95% CI 0.56 − 0.91) and >4 years 0.80 (95% CI 0.61 − 1.05).
Table 2.
Hazard ratios for Insulin, sulphonylurea or metformin, insulin, or sulphonlyurea or Metformin, in men on ADT compared to a comparison cohort of PCa‐free men
| Insulin/su/met | Insulin | Su/met | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADT years of exposure | No Events |
Crude
HR |
95% CI |
Adjusted
HR* |
95% CI | No Events | Crude HR | 95% CI |
Adjusted
HR* |
95% CI | No Events |
Crude
HR |
95%CI |
Adjusted
HR* |
95%CI |
| No ADT | 7932 | 1 | Ref. | 1 | Ref. | 1688 | 1 | Ref. | 1 | Ref. | 6320 | 1 | Ref. | 1 | Ref. |
| AA 0‐2 | 126 | 0.73 | (0.61 − 0.88) | 0.73 | (0.61 − 0.87) | 27 | 0.78 | (0.53 − 1.14) | 0.76 | (0.52 − 1.11) | 100 | 0.67 | (0.55 − 0.82) | 0.68 | (0.56 − 0.83) |
| AA 2‐4 | 71 | 0.7 | (0.54 − 0.89) | 0.71 | (0.56 − 0.91) | 16 | 0.82 | (0.50 − 1.34) | 0.83 | (0.51 − 1.36) | 55 | 0.69 | (0.53 − 0.90) | 0.71 | (0.54 − 0.92) |
| AA >4 | 61 | 0.76 | (0.58 − 0.99) | 0.8 | (0.61 − 1.05) | 9 | 0.54 | (0.28 − 1.05) | 0.59 | (0.30 − 1.13) | 52 | 0.84 | (0.64 − 1.10) | 0.88 | (0.67 − 1.16) |
| GnRH 0 −0.5 | 157 | 1.44 | (1.23 − 1.70) | 1.41 | (1.20 − 1.67) | 36 | 1.52 | (1.09 − 2.12) | 1.31 | (0.93 − 1.85) | 124 | 1.35 | (1.13 − 1.61) | 1.38 | (1.15 − 1.66) |
| GnRH 0.5 −1 | 162 | 1.54 | (1.31 − 1.80) | 1.51 | (1.28 − 1.79) | 41 | 1.82 | (1.33 − 2.47) | 1.59 | (1.15 − 2.19) | 126 | 1.45 | (1.21 − 1.73) | 1.49 | (1.24 − 1.80) |
| GnRH 1‐1.5 | 156 | 1.62 | (1.38 − 1.91) | 1.61 | (1.36 − 1.91) | 40 | 1.91 | (1.40 − 2.62) | 1.71 | (1.24 − 2.37) | 120 | 1.52 | (1.27 − 1.82) | 1.58 | (1.31 − 1.90) |
| GNRH 1.5‐2 | 132 | 1.47 | (1.23 − 1.76) | 1.48 | (1.23 − 1.78) | 35 | 1.84 | (1.32 − 2.57) | 1.67 | (1.18 − 2.36) | 100 | 1.4 | (1.15 − 1.71) | 1.46 | (1.19 − 1.79) |
| GnRH 2‐2.5 | 132 | 1.67 | (1.40 − 2.00) | 1.68 | (1.40 − 2.02) | 34 | 1.96 | (1.40 − 2.76) | 1.81 | (1.27 − 2.56) | 102 | 1.59 | (1.30 − 1.93) | 1.65 | (1.35 − 2.02) |
| GnRH 2.5‐3 | 104 | 1.41 | (1.15 − 1.73) | 1.42 | (1.16 − 1.76) | 39 | 2.49 | (1.81 − 3.42) | 2.32 | (1.67 − 3.21) | 67 | 1.17 | (0.92 − 1.49) | 1.23 | (0.96 − 1.57) |
| GnRH 3‐4 | 143 | 1.15 | (0.97 − 1.37) | 1.17 | (0.98 − 1.40) | 45 | 1.66 | (1.23 − 2.23) | 1.58 | (1.16 − 2.14) | 100 | 1.03 | (0.85 − 1.26) | 1.09 | (0.89 − 1.33) |
| GnRH 4‐5 | 127 | 1.14 | (0.94 − 1.39) | 1.19 | (0.97 − 1.45) | 50 | 2.27 | (1.71 − 3.01) | 2.22 | (1.65 − 2.97) | 78 | 1.02 | (0.82 − 1.28) | 1.09 | (0.86 − 1.36) |
| GnRH 5‐6 | 91 | 1.06 | (0.84 − 1.33) | 1.11 | (0.88 − 1.40) | 36 | 2 | (1.44 − 2.79) | 2.01 | (1.43 − 2.82) | 56 | 0.93 | (0.71 − 1.20) | 1 | (0.76 − 1.30) |
| GnRH 6‐7 | 67 | 0.95 | (0.72 − 1.24) | 1.01 | (0.77 − 1.33) | 27 | 1.88 | (1.28 − 2.74) | 1.91 | (1.30 − 2.82) | 42 | 0.88 | (0.65 − 1.20) | 0.96 | (0.71 − 1.31) |
| GnRH 7‐10 | 101 | 0.87 | (0.71 − 1.08) | 0.96 | (0.77 − 1.19) | 32 | 1.2 | (0.85 − 1.70) | 1.28 | (0.89 − 1.83) | 71 | 0.85 | (0.67 − 1.07) | 0.95 | (0.74 − 1.20) |
| GnRH >10 | 30 | 0.6 | (0.40 − 0.92) | 0.69 | (0.45 − 1.05) | 16 | 1.51 | (0.92 − 2.48) | 1.72 | (1.04 − 2.84) | 15 | 0.5 | (0.30 − 0.83) | 0.58 | (0.35 − 0.96) |
In the subgroup of men treated with insulin only (Table 2) there was a persistent increase in risk observed during all time periods for men in the GnRH/Orch group, peaking at 2.5–3 years with a HR of 2.32 (95% CI: 1.67 − 3.21). In contrast, those on AA had no increased risk of T2DM during any time period compared to PCa‐free men.
Table 2 reports the corresponding results for those receiving either metformin or sulphonylurea. Those in the GnRH/Orch group had a significantly elevated risk until 2 − 2.5 years of exposure (HR: 1.65 (95%CI: 1.35 − 2.02) before a reduction in later time periods and became nonstatistically significant. Similarly to insulin, no increased risk of T2DM was seen in those on AA at any time period. The time‐dependent results of Table 2 are also illustrated in Figure 1.
Figure 1.
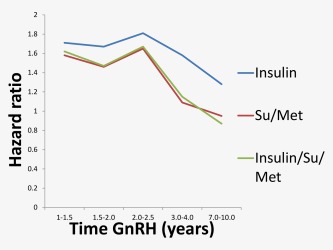
Graphical representation of risk of T2DM by time on GnRH agonists, T2DM was defined by anti‐diabetic drug prescriptions: (a) Insulin, (b) Sulphonluurea or Metaformin, (c) Insulin, Sulphonylurea or Metformin. [Color figure can be viewed at wileyonlinelibrary.com.]
The incidence of T2DM for those without ADT was 10/1,000 person‐years, for men on GnRH agonists/orchiectomy 13/1,000 person‐years and 8/1,000 person‐years for men on AA (Table 3). The results of the sensitivity analysis comparing the incidence of T2DM in men with PCa 2 years prior and after initiation of ADT are presented in Table 4. A similar increase in risk for T2DM was observed. In men treated with AA the incidence of T2DM (receiving insulin) was 1.5 vs. 1.7/1,000 person‐years 2 years before and after ADT. In those treated with GnRH/Orch, the incidence of T2DM (receiving insulin) was 2.2 vs. 5.0/1,000 person‐years, respectively, and for T2DM (receiving metformin/sulfonylurea) 11.1 vs. 11.3/1,000 person‐years.
Table 3.
Incidence of T2DM per 1,000 person‐years in PCa‐free men and men with PCa on anti‐androgens (AA) or GnRH agonists/orchiectomy (GnRH/Orch group). according to anti‐diabetic drug prescriptions.
|
Insulin/Sulphonylurea
/Metformin |
Insulin |
Metformin
/Sulphonylurea |
||||
|---|---|---|---|---|---|---|
| No of events | Incidence rate | No of events | Incidence rate | No of events | Incidence rate | |
| No ADT | 7274 | 10.45 | 1030 | 1.47 | 6244 | 8.97 |
| AA | 239 | 8.07 | 33 | 1.11 | 206 | 6.96 |
| GNRH/Orch | 1258 | 12.98 | 287 | 2.96 | 971 | 10.01 |
| Prior AA | 81 | 13.64 | 11 | 1.85 | 70 | 11.79 |
| No Prior AA | 1177 | 12.93 | 276 | 3.03 | 901 | 9.90 |
Table 4.
Event number and incidence per 1,000/person years of T2DM treated with Metformin/Sulphonylurea or Insulin in men with PCa two years before and after ADT initiation.
| Metformin/Sulphonylurea | Insulin | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 years prior to ADT initiation | 2 years after ADT initiation | 2 years prior to ADT initiation |
2 years after
ADT initiation |
|||||
| Type of ADT at initiation |
No DM
events |
Incidence of DM |
No DM
events |
Incidence of DM |
No DM
events |
Incidence of DM |
No DM
events |
Incidence of DM |
| All | 394 | 10.4 | 301 | 10.1 | 73 | 1.9 | 113 | 3.8 |
| AA | 128 | 9.1 | 91 | 8.1 | 21 | 1.5 | 19 | 1.7 |
| AA initial treatment | 50 | 9.2 | 44 | 9.5 | 10 | 1.8 | 10 | 2.2 |
| AA deferred treatment | 78 | 9.0 | 47 | 7.1 | 11 | 1.3 | 9 | 1.4 |
| GNRH | 266 | 11.1 | 210 | 11.3 | 52 | 2.2 | 94 | 5.0 |
| GNRH initial treatment | 195 | 10.9 | 159 | 11.3 | 41 | 2.3 | 70 | 5.0 |
| GNRH deferredtreatment | 71 | 11.7 | 51 | 11.2 | 11 | 1.8 | 24 | 5.2 |
Discussion
In accordance with previous studies, this large nation‐wide population‐based cohort study showed that men on ADT had an increased risk of T2DM as defined by filled prescriptions for an anti‐diabetic drug In addition, the highest risk of T2DM was reached at 3 years after the start of GnRH/Orch. In contrast, men on monotherapy anti‐androgens had no such increase.
In the first study on risk of T2DM for men on GnRH agonists, Keating et al. showed an increased risk in men aged >66 with loco‐regional PCa (HR for GnRH agonists versus no ADT: 1.44, 95% CI: 1.34‐1.55).7 However, this study had a relatively short duration of exposure, i.e., 1–4, 5–12, 13–24, >25 months. The same authors obtained similar results in a further study including men of all ages with loco‐regional PCa.10 They analyzed combined androgen blockade and AA separately and did not show an increased risk of T2DM. The effect of duration of treatment on risk of T2DM was not examined. A similar study was conducted by Alibhai et al. in a Canadian cohort of men aged ≥66 years who received at least 6 months of ADT.8 They also reported an increased risk of T2DM (HR: 1.16, 95% CI: 1.11 − 1.21), but did not examine GnRH and AA separately and combined all forms of ADT as a single exposure. There was a trend toward increased risk of diabetes with longer exposure to ADT (HR for ADT vs. no ADT: 1.09, 95% CI: 0.9 − 1.08 >24 months of exposure compared with HR: 0.99, 95% CI 0.90 − 1.08 at 6–24 months).
The longest duration of follow up to date was 25 months.7 No studies have looked at different types of ADT and the effect of duration combined. AA use in North America is substantially lower than in Europe, so there are little data from these cohorts on AA. We examined the risk of T2DM with up to ten years of exposure, which is to our knowledge the longest exposure studied to date. The highest risk associated with GnRH agonists occurred relatively early and started to decline after 3 years of treatment. For AA we did not observe an increased risk.
The observed temporal changes in risk fit with the physiological and metabolic changes previously described for GnRH agonist treatment.19 These changes include increased fat mass, reduced lean body mass and increased insulin levels, which all have been demonstrated to occur within 3 months of commencing ADT.19, 20, 21 Lee et al. measured lean body mass and fat mass in 65 men with PCa on GnRH agonists over a 12 month period. Those with longer prior exposure to treatment with GnRH agonists had less fat accumulation and less loss of lean body mass over the 12 month period.20 Similarly, GnRH agonists decrease sensitivity to insulin within 3 months of ADT start.22 Thus, the adverse metabolic effects of GnRH agonists occur within months of initiation; the consequences of these changes (i.e., developing T2DM) do not peak until 2 years later.
Strengths of our study are its large size, population‐based design of PCBaSe, and long duration of exposure to ADT. The use of an age‐matched PCa‐free comparison cohort allowed us to handle PCa heterogeneity. If we had used men with PCa receiving radical therapy or men on active surveillance/watchful waiting as the comparison group we would have introduced selection bias as these men have a different general health status than men with PCa on ADT. However, this approach does not allow us to tease out the disease effect. The sensitivity analysis comparing incidence rates of T2DM in men with PCa 2 years prior and after initiation of ADT aimed to assess this. The results remained consistent to what was seen when using the PCa free comparison cohort, with a higher incidence of T2DM in those receiving insulin observed after 2 years of GnRH treatment (2.2 vs. 5.0/1,000 person years) and not in those treated with AA.
One limitation of this study is that by using new drug prescriptions as a proxy for T2DM, we have missed all T2DM cases treated by diet alone; however, this would be similar for men with and without PCa. It would be interesting in future studies to be able to include this group of men. By only including two of the potential oral hypoglycaemic agents used for T2DM, metformin and sulphonylurea, we missed those with T2DM who were on alternative drugs. However, these only accounted for 1.32% of events. Another limitation of this study is that we did not have information about lifestyle factors including weight or family history of T2DM. However, all results were adjusted for CCI, which accounts for other comorbidities associated with lifestyle risk factors,23 as well as education status—which has also been shown to be a good indicator of baseline health status.23 Despite adjusting for several covariates, residual confounding may still be present. However, adjustment for CCI and education status reduces this possibility substantially. A further limitation is that the different risks observed between GnRH agonists and AA could potentially be explained by selection bias, rather than a real difference in the two treatments. Men treated with GnRH agonists are not only more likely to have locally advanced or distantly metastatic disease, they are also more likely to have more comorbidities than those treated with AA. Men on AA had lower PSA and T stage than those initially treated with GnRH agonists (data not shown). This reflects standard clinical practice whereby GnRH agonists are used as a primary treatment for advanced disease. Hence, men on GnRH agonists may be at higher risk of T2DM than those on AA; this however, does not diminish the clinical importance of identifying those at highest risk of T2DM during ADT.
Conclusion
Duration of GnRH agonists had a significant impact on risk of T2DM in men with PCa, with the peak risk observed after 3 years of treatment. This suggests that even men receiving adjuvant ADT, for a short time period, may be at increased risk of T2DM.
Disclaimer
The results and views expressed in the study represent those of the authors and not necessarily those of the Swedish Medical Products Agency, at which one of the authors (BZ) is employed. BZ has not received any grants or any financial support from any sponsor for the present work.
*Adjusted for CCI, PCa Stage, and Education level.
Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg, Ove Andrén, Anna Bill‐Axelsson, Ann‐Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm‐Eriksson, David Robinson, Mats Andén, Jan‐Erik Damber, Jonas Hugosson, Ingela Franck Lissbrant, Maria Nyberg, Göran Ahlgren, Ola Bratt, René Blom, Lars Egevad, Calle Walller, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandi and Karin Hellström,.
References
- 1. Horwich A, Parker C, de Reijke T, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals Oncol 2013;24:vi106–14. [DOI] [PubMed] [Google Scholar]
- 2. Lycken M, Garmo H, Adolfsson J, et al. Patterns of androgen deprivation therapies among men diagnosed with localised prostate cancer: a population‐based study. Eur J Cancer Oxford, England 2014;50:1789–98. [DOI] [PubMed] [Google Scholar]
- 3. Rhee H, Gunter JH, Heathcote P, et al. Adverse effects of androgen‐deprivation therapy in prostate cancer and their management. BJU Int 2015;115:3–13. [DOI] [PubMed] [Google Scholar]
- 4. Van Hemelrijck M, Garmo H, Holmberg L, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the population‐based PCBaSe Sweden. J Clin Oncol 2010;28:1–34778‐56. [DOI] [PubMed] [Google Scholar]
- 5. O'Farrell S, Garmo H, Holmberg L, et al. Timing and risk patterns of cardiovascular disease in men with prostate cancer on adrogen deprivation therapy. Eur J Cancer 2013;49:S16–7. [Google Scholar]
- 6. Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 2005;90:6410–7. [DOI] [PubMed] [Google Scholar]
- 7. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448–56. [DOI] [PubMed] [Google Scholar]
- 8. Alibhai SM, Duong‐Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 2009;27:3452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lage MJ, Barber BL, Markus RA. Association between androgen‐deprivation therapy and incidence of diabetes among males with prostate cancer. Urology 2007;70:1104–8. [DOI] [PubMed] [Google Scholar]
- 10. Keating NL, O'Malley AJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2010;102:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. U S Food and Drug Administration. FDA Drug Safety Communication : Update to Ongoing Safety Review of GnRH Agonists and Notification to Manufacturers of GnRH Agonists to Add New Safety Information to Labeling Regarding Increased Risk of Diabetes and Certain Cardiovascular Diseases,. Rockville, MD2011 [cited 2011 22 June]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm229986.htm.
- 12. Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 2006;91:1305–8. [DOI] [PubMed] [Google Scholar]
- 13. Bosco C, Crawley D, Adolfsson J, et al. Quantifying the evidence for the risk of metabolic syndrome and its components following androgen deprivation therapy for prostate cancer: a meta‐analysis. PloS One 2015;10:e0117344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol 2013;42:956–67. [DOI] [PubMed] [Google Scholar]
- 15. Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort profile update: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base—a refined prostate cancer trajectory. Int J Epidemiol 2016;45:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Network NCC : National Comprehensive Cancer Network Guideline 2012.
- 17. Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Safety 2007;16:726–35. [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 19. Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002;87:599–603. [DOI] [PubMed] [Google Scholar]
- 20. Lee H, McGovern K, Finkelstein JS, et al. Changes in bone mineral density and body composition during initial and long‐term gonadotropin‐releasing hormone agonist treatment for prostate carcinoma. Cancer 2005;104:1633–7. [DOI] [PubMed] [Google Scholar]
- 21. Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 2001;86:4261–7. [DOI] [PubMed] [Google Scholar]
- 22. Smith MR, Lee H, Fallon MA, et al. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology 2008;71:318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berglund A, Garmo H, Tishelman C, et al. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol 2011;185:833–9. [DOI] [PubMed] [Google Scholar]


