Abstract
The kinetochore is the macromolecular protein complex that drives chromosome segregation in eukaryotes. Its most fundamental function is to connect centromeric DNA to dynamic spindle microtubules. Studies in popular model eukaryotes have shown that centromere protein (CENP)-A is critical for DNA-binding, whereas the Ndc80 complex is essential for microtubule-binding. Given their conservation in diverse eukaryotes, it was widely believed that all eukaryotes would utilize these components to make up a core of the kinetochore. However, a recent study identified an unconventional type of kinetochore in evolutionarily distant kinetoplastid species, showing that chromosome segregation can be achieved using a distinct set of proteins. Here, I review the discovery of the two kinetochore systems and discuss how their studies contribute to a better understanding of the eukaryotic chromosome segregation machinery.
Keywords: chromosomes, kinetochores, trypanosomes
Introduction
Faithful transmission of genetic information from generation to generation is essential for the survival of all organisms. In eukaryotes, chromosome replication occurs during S phase and duplicated chromosomes are physically connected by the cohesin complex [1] (Figure 1). Chromosome segregation is directed by the kinetochore, the proteinaceous structure that assembles onto the centromeric DNA and interacts with spindle microtubules during mitosis and meiosis [2–5]. In many species, centromeres consist of repetitive sequences that span several kilobases up to megabases [6]. Kinetochores assemble onto a small portion of these sequences and their positional information is epigenetically inherited from generation to generation [7–9]. Microtubules are dynamic polymers that grow and shrink by incorporating or dissociating α-/β-tubulin subunits at their tips [10]. By interacting with microtubules, kinetochores enable duplicated chromosomes to align at the center of the spindle. Accurate segregation requires sister kinetochores to make bi-oriented attachments to microtubules emanating from opposite poles. Attachment errors must be recognized and corrected prior to anaphase [11]. The spindle checkpoint monitors attachment status and delays the onset of anaphase until all chromosomes form correct bi-oriented attachments [12,13]. Once all chromosomes have achieved bi-orientation, the anaphase promoting complex (APC/C) is activated, leading to the cleavage of cohesin complexes and separation of sister chromatids [14–16].
Figure 1. Chromosome segregation.
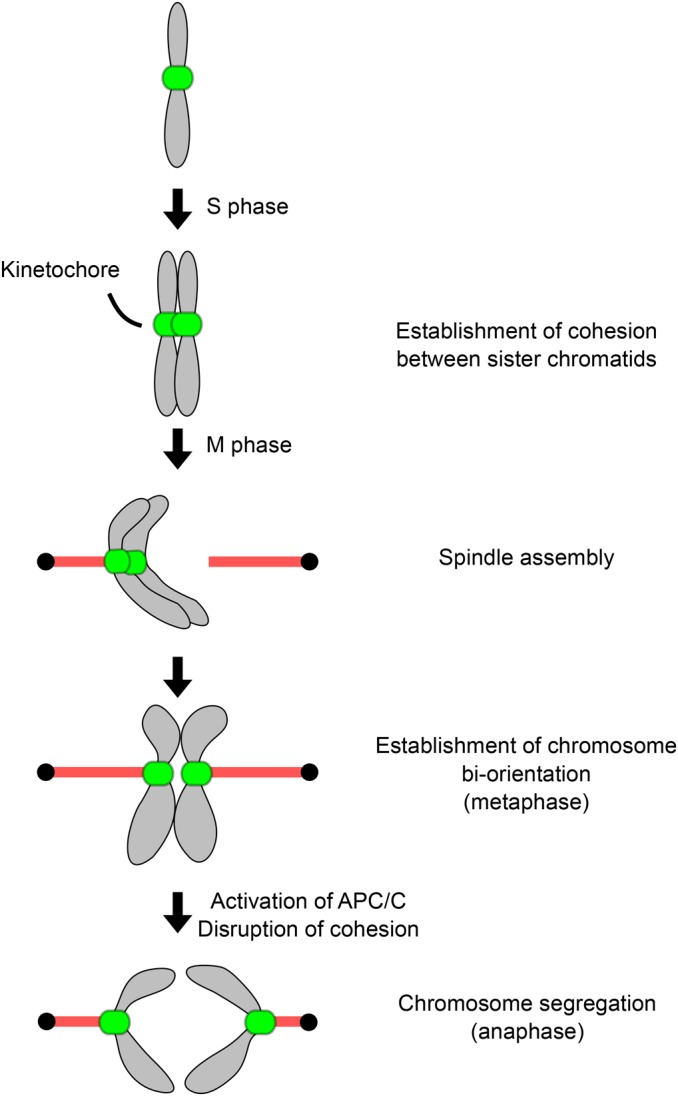
Chromosome duplication occurs during S phase, linking sister chromatids by cohesion. When cells enter mitosis, a bipolar spindle is assembled and kinetochores start to interact with dynamic microtubules. Once all chromosomes achieve bi-oriented attachments, the spindle checkpoint is satisfied. Subsequent activation of APC/C leads to the destruction of cohesion, segregating sister chromatids away from each other in anaphase.
Given the central role of kinetochores in co-ordinating chromosome segregation, elucidating their molecular mechanism of action is a fundamental issue in cell biology. The most basic kinetochore functions are to bind DNA and dynamic microtubules. What kind of proteins carry out these functions? How did they evolve? Identifying and characterizing the constituent proteins are clearly necessary as the first step to address these questions. Here, I review the discovery of two different kinetochore systems and discuss how their studies will contribute to a better understanding of chromosome segregation machines in eukaryotes. I place emphasis on the unconventional kinetoplastid kinetochore and refer readers to comprehensive reviews on the conventional kinetochore [3,4,17–19].
Conventional kinetochores
Discovery
Centromeres/kinetochores were first recognized cytologically as primary constrictions on each chromosome where spindle microtubules attach [20]. Kinetochore structures were visualized by electron microscopy [21–23], revealing a trilaminar structure at the periphery of the centromere. Identification of kinetochore components was made possible by the discovery of autoimmune sera from patients with scleroderma spectrum disease that stained the centromere regions of chromosomes [24]. Using the sera, the first kinetochore proteins (centromere proteins, CENP-A, B, C) were recognized [25,26]. Their cDNA clones were subsequently isolated and protein sequences revealed [27–29].
Although the first kinetochore proteins were discovered in humans, many additional kinetochore proteins were subsequently identified in budding yeast owing to the power of genetics and its simple centromere structure [30]. For example, biochemical purifications of centromere DNA-binding proteins [31], genetic screens for mutants that have increased rates of chromosome loss [32,33], one-hybrid [34] and two-hybrid screens [35] all led to the identification of many kinetochore proteins. More recently, affinity purification and mass spectrometry analysis accelerated the identification [36,37].
Increased sensitivity in homology detection algorithms has allowed the identification of homologous proteins in more and more species as the number of sequenced genomes has expanded [38–41]. Genome-wide RNAi screens [42,43] and proteomic analyses of isolated chromosomes [44,45] also identified kinetochore proteins. To date, more than 80 proteins have been identified that at least transiently localize to kinetochore regions, including structural kinetochore proteins (e.g. CENP-A, CCAN, KNL1, Mis12 and Ndc80), regulatory proteins [e.g. Aurora B/chromosomal passenger complex (CPC), Mps1, Plk1, PP1 and PP2A], and spindle checkpoint proteins (e.g. Bub1, Bub3, BubR1/Mad3, Mad1 and Mad2). After identifying proteins that localize at kinetochores, the next crucial step is to reveal their function. Most notably, which proteins bind DNA or microtubules?
Identification of DNA-binding kinetochore proteins
Cloning and sequencing of CENP-A cDNA revealed that it is a histone H3 variant [29,46]. This was a very exciting finding because it not only revealed that CENP-A is a DNA-binding protein but also suggested a possible mechanism for how kinetochore positions could be epigenetically inherited [47,48]. Subsequent studies have shown that CENP-A is essential for kinetochore assembly in various eukaryotes [42,49,50]. Given its importance in kinetochore biology, extensive studies have been performed to better understand the nature and function of CENP-A ([51–54]).
Other proteins that can bind DNA, at least in vitro, include CENP-CMif2 [55,56], CENP-UAme1/CENP-QOkp1 [57], and CENP-T/W/S/X [58–60]. Minor groove DNA-binding motifs are found in some of these proteins: the AT-hook [61] in CENP-CMif2 and CENP-UAme1/CENP-QOkp1 [56,57,62], and the SPKK motif [63] in CENP-A [64]. However, these short motifs are often poorly conserved even among closely related species, suggesting that they are probably not essential for their functions but may contribute to enhancing their DNA-binding affinity [56]. Regardless, the presence of these short motifs could be indicative of DNA-binding activities for a given protein.
Identification of microtubule-binding kinetochore proteins
Human Ndc80HEC was originally identified in a two-hybrid screen with retinoblastoma protein as bait [65], whereas its yeast homolog was identified in a spindle pole body preparation [66]. Ndc80 interacts with Nuf2, Spc24, and Spc25, which together form a rod-shaped complex [67,68]. All four subunits contain globular domains and extensive coiled-coil regions. Although depletion studies showed that the Ndc80 complex is essential for kinetochore–microtubule attachment [36,69,70], it was not clear whether the complex directly interacts with microtubules or recruits other kinetochore proteins that bind microtubules. It was an in vitro microtubule co-sedimentation assay that revealed that the former was the case [71,72]. However, it took nearly a decade since the identification of Ndc80 to assign it a function at the kinetochore. Given its now recognized importance in microtubule-binding, extensive studies have been carried out to mechanistically understand how the Ndc80 complex binds to microtubules ([73–78]).
Kinetochore subcomplexes and hierarchical assembly
Another important aspect of kinetochore research is to understand its design principles. Electron microscopy studies have visualized the overall architecture of kinetochores within cells [79,80] as well as isolated kinetochores [81]. At the molecular level, kinetochores are composed of biochemically stable/isolatable subcomplexes, such as the Ndc80 subcomplex, Mis12 subcomplex, and CCAN [37,82–86]. It is thought that these subcomplexes assemble in a hierarchical manner at the kinetochore [82]. CENP-A is at the base of this hierarchy and recruits CENP-C [52,87], which in turn recruits the Mis12 subcomplex [88,89] as well as CCAN components [90,91]. The KNL1, Mis12, and Ndc80 subcomplexes can form a larger complex called the KMN network that has microtubule-binding activities [71,92]. The relative positions of these subcomplexes within kinetochores have been determined by super-resolution microscopy, revealing the overall protein architecture of kinetochores [93–95].
Structural features
The majority of kinetochore proteins lack obvious functional domains or similarity to other proteins at the primary sequence level. However, structural analyses have revealed common domains in proteins that do not have significant sequence similarity. For example, the RWD domain was found in Spc24, Spc25, CENP-PCtf19, CENP-OMcm21, Csm1, Mad1, and KNL1 proteins [67,68,92,96,97], calponin homology domain in Ndc80 and Nuf2 proteins [72,73], and tetratricopeptide repeat domain in Bub1, BubR1, and Mps1 proteins [98,99]. Furthermore, CENP-LIml3 has similarity to the bacterial recombination-associated protein RdgC and TATA-binding protein TBP [100,101], CENP-M folds like a small GTPase [102], the C-terminal dimerization domain of CENP-CMif2 has similarity to the bacterial transcription factor Hth-3 [56], and point centromere-specific Ndc10 has similarity to a bacterial tyrosine recombinase [103,104]. These studies highlight the importance of structural analysis in kinetochore research to reveal distant homology. They also suggest that the highly complicated kinetochores likely originated from a few protein modules aided by gene duplication and functional diversification [105].
How widely are these kinetochore proteins conserved?
Microtubules appear to be utilized for chromosome segregation in all eukaryotes studied thus far, and α-/β-tubulins are one of the most highly conserved proteins [106–108]. Kinetochore proteins, in contrast, vary to a great extent in primary sequences [109]. Yet, thanks to increased sensitivity in homology search algorithms, we now know that many of the above-mentioned kinetochore proteins are conserved in various eukaryotes [40,62]. CENP-A, CENP-C, and components of the Ndc80 complex are widely found in diverse eukaryotes, suggesting that most eukaryotes utilize these kinetochore proteins to bind DNA or microtubules. However, although CENP-A was thought to be conserved in all eukaryotes, a recent study showed that CENP-A is absent in some holocentric insects [110]. Furthermore, two popular model eukaryotes (Drosophila melanogaster and Caenorhabditis elegans) appear to have lost the majority of CCAN components [111–114]. Therefore, it has become clear that the repertoire of kinetochore proteins can be dramatically different among eukaryotes despite functional conservation [115].
More extreme cases were found in kinetoplastids, a group of unicellular eukaryotes defined by the presence of kinetoplast which is a large structure in the mitochondrion that contains the mitochondrial DNA [116]. When the genomes of three kinetoplastid species were sequenced (Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major), none of the core structural kinetochore proteins were identified [117–119]. In contrast, components of basic cell cycle machinery were readily identified, including the CDK/cyclin system, the cohesin complex, separase, the condensin complex, Aurora B kinase, Polo-like kinase, MAD2, APC/C, and proteasomes [120]. However, chromosome segregation depends on spindle microtubules [121], and electron microscopy has visualized kinetochore-like electron-dense plaques that appear to form end-on attachments to spindle microtubules in mitotic cells [122–124]. Yet, nothing was known about the identity of kinetochore components in kinetoplastids. Do they have conventional kinetochore proteins that simply diverged too far in primary sequences? Or do they have previously unforeseen types of kinetochore proteins?
Unconventional kinetochores in kinetoplastids
Discovery: personal reflections
My scientific career started in 2003 when I joined Dr Yoshinori Watanabe's group in Tokyo to study how Polo-like kinase modulates kinetochore function to promote meiosis-specific chromosome segregation in fission yeast [125]. I then moved to Dr Sue Biggins' laboratory in Seattle to study mitotic kinetochores using budding yeast. There, I achieved the isolation of native kinetochores for the first time [44,126,127] and obtained a PhD. When I was thinking about projects for my post-doctoral training, I became interested in finding out whether basic kinetochore proteins are conserved in all eukaryotes. After I learned that kinetoplastids do not have any apparent canonical kinetochore components including CENP-A [128,129], I joined the laboratory of Dr Keith Gull in Oxford in November 2010 to identify kinetochore proteins in T. brucei. There were no magical antibodies that recognized the centromere regions of its chromosomes, so how could I achieve this goal from scratch? With my expertise in protein purification, I first immunoprecipitated conserved mitotic proteins and performed mass spectrometry to identify interacting proteins [the immunoprecipitation (IP)/mass spectrometry (MS) approach]. Because spindle checkpoint components co-purify with kinetochore proteins in other eukaryotes [130], I first made a YFP fusion for MAD2, the only spindle checkpoint protein homolog identified in T. brucei. However, this protein turned out to localize at basal bodies, having no detectable signal at kinetochores [120]. Consistent with this observation, IP/MS of YFP-MAD2 only led to the identification of another protein that localized to basal bodies (B. Akiyoshi, unpublished data). I then made YFP fusions for various proteins, including AUK1 (Aurora B kinase homolog [131]), CDC20 (activator of the APC/C [132]), and XMAP215 (microtubule-associated protein [133,134]). Again, although IP/MS of these proteins worked and identified interacting proteins, this approach failed to identify any protein that localized at kinetochores (B. Akiyoshi, unpublished data).
Another idea I had in mind was to carry out a YFP-tagging screen. The T. brucei genome has approximately 9000 proteins, which means that I should identify kinetochore proteins if I study all of them. This was a simple and straightforward idea, but I did not know where to start. It was before the development of PCR-based tagging methods in the laboratory [135], meaning that I had to make targeting plasmids for each gene [136]. Coincidentally, Dr Christine Clayton's group published a cell cycle transcriptome analysis of different cell cycle stages in March 2011 in PLoS ONE [137]. Impressed by the quality of data, I immediately started tagging uncharacterized genes whose transcript levels were up-regulated in either S phase or mitosis. This approach paid off. Examination of only 30 genes identified one corresponding protein that had a characteristic kinetochore localization pattern: formation of dots in the nucleus during S phase, their alignment on mitotic spindles in metaphase, and close association with spindle poles during late anaphase. This was my first ‘Eureka!’ moment and happened in June 2011. Later I named the protein KKT1 for kinetoplastid kinetochore protein 1. Using KKT1 as bait, I aimed to identify more kinetochore proteins. However, my first IP of YFP-KKT1 completely failed using the same protocol that had worked for those conserved proteins mentioned above (cryolysis using mortar and pestle). It turned out that the YFP-KKT1 signal remained in the detergent-insoluble fraction, so I tried various methods to release it. I eventually found that sonication efficiently solubilized KKT1, and immunoprecipitation from this sample led to the identification of 12 proteins as possible KKT1 interaction partners. YFP-tagging revealed typical kinetochore localization patterns for all of them, which was another moment of big excitement. By continuing the hunt and repeating the IP/MS of these newly identified proteins, I identified 19 proteins by March 2012 (KKT1–19). These proteins all had kinetochore-like localization and their RNAi-mediated knockdowns resulted in chromosome mis-segregation. However, there was a fairly valid criticism I had to face. ‘Are they really kinetochore proteins?’
In other eukaryotes, people have used various approaches to examine kinetochore localization such as immunoelectron microscopy [138], chromatin immunoprecipitation (ChIP) [139], chromosome spreads [140], and co-localization with other known kinetochore proteins [36]. I first tried to perform immunoelectron microscopy to examine whether KKT proteins localize at the kinetochore-like electron-dense plaques [124], but it turned out that the fixation condition completely killed the YFP epitope. Then, I switched my effort to a ChIP approach, although it had a different problem. That is, the resolution of previously mapped centromere positions [141] did not allow me to determine which specific DNA fragments should be examined by PCR or Southern blot. I struggled >1 year at this stage. Eventually, deep sequencing of ChIP samples revealed that KKT2 and KKT3 were specifically enriched in the centromere regions, thereby showing that these KKT proteins are bona fide kinetochore proteins. We first submitted the paper to Nature, but it was rejected without review as being uninteresting, which happened to be the same reaction to Bill Earnshaw's paper on the discovery of CENP-A, B, C [26,142]. We next submitted it to Cell and it was accepted within 1 month [143].
I recently identified one more kinetochore protein, KKT20 [144], so 20 KKT proteins have been identified to date (Figure 2). It is important to mention that there may be more kinetochore proteins still waiting to be identified. For example, proteins that weakly or very transiently interact with KKT proteins may well have been missed in the IP/MS approach. Other methods, such as BioID [145] and genome-wide GFP tagging screen, may identify the rest of kinetochore proteins (if any). Another caveat is that any gene not predicted to encode a protein in the T. brucei genome database will remain unidentified. With these caveats in mind, I decided to study these KKT proteins in depth.
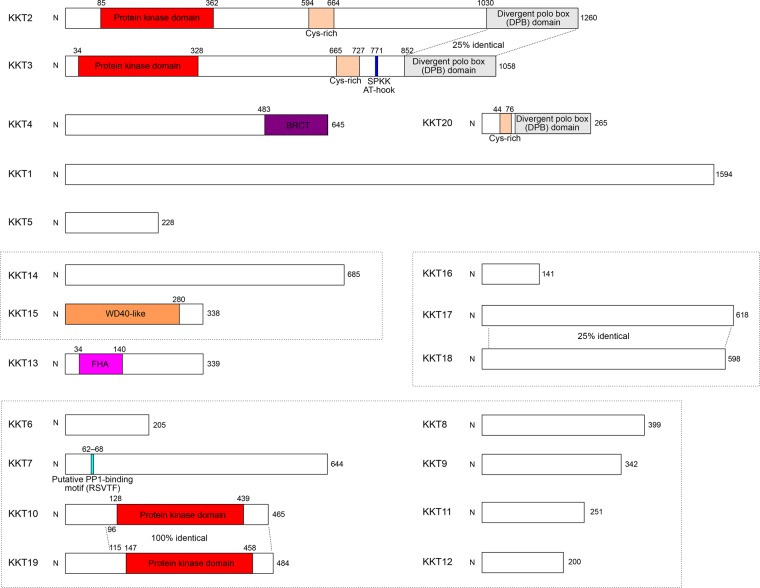
Figure 2. Diagram of T. brucei KKT proteins.
Identified domains and motifs are shown. Putative subcomplexes are grouped in dotted boxes. Adapted from [143].
Sequence analysis
Initial BLAST and HMMER searches of KKT proteins identified their homologous proteins in both parasitic and free-living kinetoplastids [143]. For most KKT proteins, these searches did not reveal any significant hit outside kinetoplastids. Some KKT proteins had similarity to various proteins due to the presence of the following conserved domains (Figure 2): a BRCT (BRCA1 C terminus) domain in KKT4, an FHA (Forkhead-associated) domain in KKT13, a WD40-like domain in KKT15, and a protein kinase domain in KKT2, KKT3, KKT10, and KKT19. BRCT and FHA are typically protein–protein interaction domains and are often found in proteins involved in the DNA damage response [146]. WD40 is also a protein–protein interaction domain that is one of the most abundant domains in eukaryotic genomes [146]. T. brucei has 190 predicted eukaryotic protein kinases, and comparative kinome analysis suggested that KKT2 and KKT3, despite having characteristic features of active protein kinases, do not have a clear affiliation to any known group or family among eukaryotic kinases [147]. Although KKT10 and KKT19 have been classified as members of the CLK/Lammer subfamily in the CMGC family, they may have adapted to carry out kinetochore functions in kinetoplastids as judged by the significant differences between KKT10/KKT19 and the human or Arabidopsis CLK/Lammer kinases [143].
I have also been using various sequence analysis software to try to reveal the function and nature of KKT proteins. This includes multiple sequence alignments to determine conserved regions [148], coiled-coil predictions [149], disordered region predictions [150], motif scans [151,152], secondary structure predictions [153,154], and HHpred [155]. Based on these analyses, I recently identified a highly divergent polo box domain (DPB) in the C-terminal region of KKT2, KKT3, and KKT20 [144]. KKT20 does not have a kinase domain, but has similarity to KKT2 and KKT3 in the central domain, suggesting that these proteins likely share common ancestry and that the ancestor might be PLK. In fact, a simple BLAST search showed that the kinase domains of KKT2 and KKT3 are more similar to that of PLK than other kinases. KKT10 and KKT19 exhibit a high degree of similarity at the protein level, as do KKT17 and KKT18. These results suggest that gene duplication played an important role in the evolution of kinetoplastid kinetochores, as seen for conventional kinetochores.
Predictions of functions
Similar to conventional kinetochores, kinetoplastid kinetochores appear to consist of subcomplexes as judged by the IP/MS results and differential localization patterns [143]. On the basis of IP/MS data, I assigned several putative subcomplexes that could represent functional modules: the KKT14/KKT15 subcomplex, the KKT16/KKT17/KKT18 subcomplex, and the KKT6/KKT7/KKT8/KKT9/KKT10/KKT11/KKT12/KKT19 subcomplex (Figure 2).
Studies of conventional kinetochore proteins have shown that their functions are often manifested in localization patterns [17]. For example, DNA-binding kinetochore components (e.g. CENP-A and CENP-C) are often constitutively localized at centromeres, whereas microtubule-binding components (e.g. Ndc80 and KNL1) localize to kinetochores specifically during M phase. Although the unconventional kinetoplastid kinetochore proteins may not follow the same principle, there is no evidence to believe otherwise. In fact, the observation that components of the putative subcomplexes mentioned above largely follow the same localization pattern supports this possibility (Figure 3). In this sense, the most promising candidates for DNA-binding proteins are KKT2, KKT3, and KKT4.
Figure 3. Differential localization timings of KKT proteins.
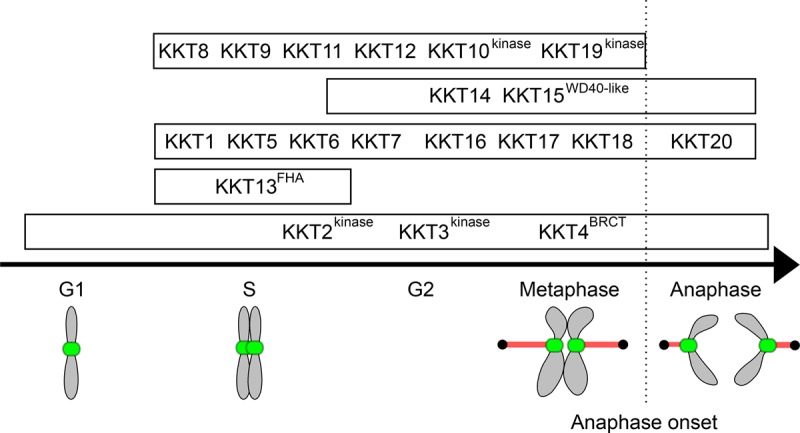
Adapted from [143].
Which KKT proteins bind DNA?
KKT2 and KKT3 have putative DNA-binding motifs (AT-hook and SPKK) in some, but not all, kinetoplastids. This is reminiscent of conventional DNA-binding kinetochore proteins such as CENP-A and CENP-C (see above), supporting the possibility that KKT2 and KKT3 may bind DNA. Besides these motifs, KKT2 and KKT3 have three domains conserved among kinetoplastids: an N-terminal protein kinase domain, a central domain that has conserved Cys residues, and the C-terminal DPB. Expression of truncated proteins in vivo can determine which region(s) is important for kinetochore/centromere localization, which might correspond to their DNA-binding domains (e.g. see [55,156,157] for CENP-C studies). It will be necessary to directly test whether KKT2 and KKT3 have any DNA-binding activity in vitro using pure proteins. Isolation of native KKT proteins or subcomplexes from trypanosomes in good quantity or stoichiometry turned out to be difficult, at least in my hands. Therefore, recombinant KKT proteins (full length or truncations) will need to be expressed and purified to carry out in vitro assays.
While in vitro assays are important to dissect their DNA-binding activities, in vivo analyses will be essential to determine their relevance. RNAi-mediated knockdown showed that KKT2 is important for faithful chromosome segregation [143], and a kinome-wide RNAi screen showed that KKT3 is essential for growth [158], suggesting that these homologous proteins likely have non-redundant functions. It will be necessary to examine whether other KKT proteins fail to localize to kinetochores in the absence of KKT2 or KKT3 to determine the localization hierarchy. Although inducible RNAi-mediated knockdown experiments can be easily performed in T. brucei [159,160], other methods such as conditional knockdowns [161], conditional knockouts [162], or a protein degradation system [163] may be necessary to achieve better depletion efficiency and observe defects.
Besides putative DNA-binding domains, KKT2 and KKT3 also have a protein kinase domain classified as unique among eukaryotic kinases [147]. What are the substrates of these protein kinases? Considering the absence of centromere-specific histones in kinetoplastids [128,164], KKT2/KKT3 might phosphorylate histones specifically at the centromere and this phosphorylation mark might provide centromere identity. Or they might phosphorylate and recruit other KKT proteins onto the kinetochore. Understanding the functions of KKT2 and KKT3 will be key to elucidating the assembly of kinetoplastid kinetochores.
Which KKT proteins bind microtubules?
Unlike the DNA side, sequence analysis did not reveal any obvious domain implicated in microtubule-binding. In the light of conventional kinetochore research, it will be important to invest efforts to obtain recombinant KKT proteins and perform microtubule co-sedimentation assays. Although tubulins purified from bovine brain are commonly used in sedimentation assays, it may be important to use trypanosome tubulins to detect binding [165,166].
The primary candidates for microtubule-binding kinetochore proteins are the KKT14 and KKT15 proteins whose localization pattern resembles that of Ndc80. It will be crucial to test these and other KKT proteins in a sedimentation assay. Once microtubule-binding proteins are identified, more detailed analyses will need to be carried out to determine whether the mechanism of microtubule-binding is distinct from conventional kinetochore proteins.
Design principles and regulations
It is also important to reveal the interaction network of KKT proteins. For example, how does the KKT10 kinase localize at kinetochores from S phase and dissociate at the metaphase–anaphase transition? What happens if KKT10 does not dissociate properly? To answer these questions, it will be necessary to reveal which proteins interact with KKT10 and how their interactions are regulated. Numerous phosphorylation sites have been identified on the 20 KKT proteins in my IP/MS analyses (B. Akiyoshi, unpublished data), so it is likely that phosphorylation plays important roles in regulating interactions as in other eukaryotes [167–171]. Although it is challenging to understand the interaction network of 20 proteins, this will be key to gaining insights into the architectural design of kinetoplastid kinetochores.
Aurora B is a highly conserved protein kinase found in all sequenced eukaryotes, including kinetoplastids [172]. It is the catalytic subunit of the CPC that displays dynamic localization patterns during mitosis and regulates various mitotic functions, including kinetochore regulations [173]. As in other eukaryotes, Aurora B localizes to kinetochore regions during prometaphase/metaphase in T. brucei ([131] and my unpublished data). Therefore, its kinetochore function might be conserved in kinetoplastids. In line with this possibility, KKT7 has putative-binding motifs for the PP1 phosphatase (RSVTF conserved among kinetoplastids and SILK found in Leishmania) that is known to counteract Aurora B activities [174,175].
Aurora B is critical for the destabilization of improper microtubule attachments by phosphorylating kinetochores. It has been proposed that the extent of phosphorylation is dependent on the distance from its substrates, thereby explaining why improper tension-less attachments can be specifically destabilized [176–180]. A key element in this hypothesis is the enrichment of Aurora B at the inner centromere regions, which become physically separated from bi-oriented kinetochores due to microtubule-pulling forces. Indeed, distinct space (∼1 µm) has been observed in between metaphase sister kinetochores in various eukaryotes [181], including yeasts [182], humans [183], plants [184], Cyanidioschyzon merolae [185], and Plasmodium [186]. In contrast, there is no evidence for such space in kinetoplastids. Electron microscopy studies showed that when sister kinetochore pairs interact with microtubules from opposite poles, they have a back-to-back configuration without distinct space between the two structures in T. brucei, T. cruzi, and Leishmania [123,124,187]. The analysis of YFP-tagged KKT proteins in T. brucei supports these observations [143], so Aurora B may never be significantly separated from sister kinetochores in kinetoplastids. Understanding their Aurora B regulation might provide novel insights into the mechanism of chromosome bi-orientation. Interestingly, a recent study in budding yeast showed that its chromosomes can bi-orient without the inner centromere localization of Aurora B [188]. These observations imply that there is still a lot to learn about the molecular mechanism of chromosome segregation in eukaryotes.
The spindle checkpoint is a surveillance mechanism that ensures high fidelity chromosome segregation in eukaryotes [13,189]. Although widely conserved among diverse eukaryotes [190,191], the spindle checkpoint is dispensable for the survival of yeasts, flies, and even human cells under certain conditions [192–196]. Indeed, checkpoint-independent mechanisms are also important for the regulation of the APC/C activities [197–201]. Interestingly, there is no evidence of functional spindle checkpoint or feedback control in kinetoplastids. For example, disruption of mitotic spindles or inhibition of DNA replication do not noticeably delay the onset of anaphase or cytokinesis in T. brucei [120,202,203]. Nonetheless, KKT4 and KKT20 co-purified with significant amounts of APC/C components [143,144], and the BRCT domain of a human microcephalin (MCPH1) protein has been shown to interact with the APC/C, at least in vitro [204]. Therefore, KKT4 and KKT20 might directly affect the activation/inactivation of the APC/C, regulating anaphase onset in a feedforward manner.
Which organisms have unconventional kinetochores?
Kinetoplastids are a widespread group of unicellular eukaryotes found in diverse environmental conditions, including hydrothermal vents [205–208]. They are defined by the presence of kinetoplast, a large structure in the mitochondrion that contains the mitochondrial DNA [116]. Kinetoplastids can be divided into two subclasses, the early diverging Prokinetoplastina and the Metakinetoplastina [209]. The latter can be further divided into three orders for the bodonid species (Eubodonida, Parabodonida, and Neobodonida) and the trypanosomatids [210]. KKT proteins are found in all sequenced bodonids (Bodo saltans and Trypanoplasma borreli) [211] and trypanosomatids (e.g. T. brucei, T. cruzi, Leishmania, and Phytomonas spp.) [117–119,212]. Furthermore, at least some KKT proteins are present in an early diverging Prokinetoplastina, Perkinsela sp. [213], suggesting that KKT proteins are likely a conserved feature of all kinetoplastids (my unpublished data).
Kinetoplastids belong to Euglenozoa, which is a large and diverse group of flagellates [214]. Four lineages are currently known: Kinetoplastids, Euglenids, Diplonemids, and Symbiontids [215]. Genome sequences are available only for kinetoplastids, and it was not known whether KKT proteins are present specifically in kinetoplastids or common among Euglenozoa [143]. Interestingly, examination of Euglena gracilis transcriptome [216] revealed clear homologs of conventional kinetochore proteins, including Ndc80, Nuf2, Spc25, and several candidates for CENP-A (based on the presence of longer loop 1 [217]), as well as spindle checkpoint proteins (Mad1, Mad2, and Mad3) (my unpublished data). In contrast, I failed to identify any obvious KKT proteins in this organism. Although its genome sequence will need to confirm it, these data suggest that E. gracilis likely has conventional kinetochore proteins, not KKT proteins. If true, it may be appropriate to re-classify Euglenozoa into two separate groups (see below). It will be interesting to examine whether other Euglenozoa species (e.g. Diplonema papillatum [218–220] and Calkinsia aureus [221]) utilize conventional or unconventional kinetochore proteins.
Perspectives
Despite the long research on chromosome segregation machinery in eukaryotes, we know little about its evolutionary origins or history. For example, what kind of segregation machinery was used in the last eukaryotic common ancestor (LECA)? The notions that microtubules are used in all eukaryotes studied thus far and that eukaryotes and tubulins likely evolved from Archaea [222–224] suggest that the LECA probably used tubulin-based polymers to drive chromosome segregation. In contrast, there is little trace of prokaryotic traits in kinetochore proteins, making it difficult to reveal what kind of proteins might have been used to bridge between DNA and tubulin-based polymers in this hypothetical eukaryote.
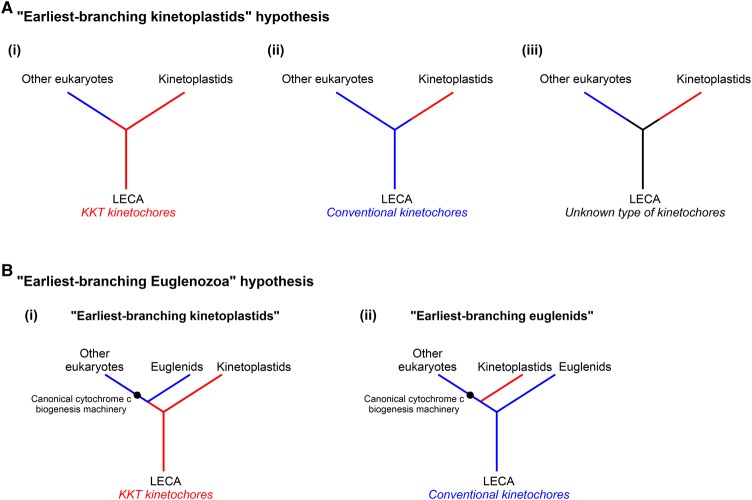
Another difficulty stems from the uncertainty of the position of the root of the eukaryotic tree of life, meaning that we still do not have concrete views about which organisms are the earliest-branching eukaryotes [214,225]. Therefore, it remains unclear whether the unique kinetoplastid kinetochore represents an ancient feature or a derived one. However, among several competing hypotheses [226–228], it has been proposed that Euglenozoa (or species within Euglenozoa) may represent the earliest-branching eukaryotes [229] based on their unique mitochondrial cytochromes c/c1 and the absence of a recognizable biogenesis apparatus for these proteins [230]. The discovery of unconventional kinetochore proteins in kinetoplastids supports this hypothesis. If kinetoplastids are among the earliest-branching eukaryotes (Figure 4A), then the LECA might have possessed the KKT-based kinetochore (Figure 4Ai). In this scenario, only kinetoplastids have retained them while other eukaryotes have lost them and invented conventional kinetochores. Alternatively, it is equally possible that LECA had conventional kinetochores that have been lost in kinetoplastids (Figure 4Aii). It is also possible that LECA had a hitherto unknown type of kinetochores (Figure 4Aiii). Furthermore, the finding that Euglena has conventional kinetochore proteins raises the possibility that kinetoplastids, not Euglena, could be the earliest-branching eukaryotes, although other interpretations are also possible (Figure 4B). Genome sequences of other Euglenozoa species might provide further insights into this question.
Figure 4. Hypothetical models on early eukaryotic history and origin of kinetoplastid kinetochores.
(A) The earliest-branching kinetoplastid hypothesis. In this scenario, LECA may have had KKT kinetochores (i), conventional kinetochores (ii), or unknown type of kinetochores (iii). Conventional and KKT-based kinetochores are shown in blue and red, respectively. Note that this is a highly simplified and non-comprehensive set of possibilities of early eukaryotic history and origins of kinetoplastid kinetochores. (B) The earliest-branching Euglenozoa hypothesis based on their unique cytochrome c biogenesis machinery. In this scenario, either kinetoplastids (i) or euglenids (ii) could be the earliest-branching eukaryotes.
Regardless of the evolutionary origins, understanding the nature of unconventional kinetochore proteins will likely provide important insights into the fundamental principles of eukaryotic segregation machines. Furthermore, the unique kinetochore proteins represent an ideal drug target against parasitic kinetoplastids [231,232]. Understanding the molecular functions of KKT proteins should therefore contribute to developing specific drugs. Finally, it is worth mentioning that there is no organism known so far that contains both types of kinetochores. Can they coexist in a given organism? Is the conventional kinetochore system more accurate than the unconventional one? Are there as-yet different types of kinetochores to be found? Surely a lot of exciting discoveries will be made in the next few decades in the field of chromosome segregation research.
Abbreviations
APC/C, anaphase promoting complex/cyclosome; CENP, centromere protein; ChIP, chromatin immunoprecipitation; CPC, chromosomal passenger complex; DPB, divergent polo box domain; IP, immunoprecipitation; KKT, kinetoplastid kinetochore; LECA, last eukaryotic common ancestor; MCPH1, microcephalin; MS, mass spectrometry.
Funding
I am supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society [grant number 098403/Z/12/Z] as well as a Wellcome-Beit Prize Fellowship [grant number 098403/Z/12/A].
Acknowledgements
I thank John Archibald and Julius Lukeš for sharing unpublished Perkinsela genome data, and Ellis O'Neill and Rob Field for the predicted protein sequence dataset of Euglena gracilis. I also thank Sue Biggins and Gabriele Marcianò for comments on the manuscript.
Competing Interests
The Author declares that there are no competing interests associated with this manuscript.
References
- 1.Nasmyth K. and Haering C.H. (2009) Cohesin: its roles and mechanisms. Annu. Rev. Genet. 43, 525–558 doi: 10.1146/annurev-genet-102108-134233 [DOI] [PubMed] [Google Scholar]
- 2.McIntosh J.R., Molodtsov M.I. and Ataullakhanov F.I. (2012) Biophysics of mitosis. Q. Rev. Biophys. 45, 147–207 doi: 10.1017/S0033583512000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biggins S. (2013) The composition, functions, and regulation of the budding yeast kinetochore. Genetics 194, 817–846 doi: 10.1534/genetics.112.145276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheeseman I.M. (2014) The kinetochore. Cold Spring Harb. Perspect. Biol. 6, a015826 doi: 10.1101/cshperspect.a015826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pesenti M.E., Weir J.R. and Musacchio A. (2016) Progress in the structural and functional characterization of kinetochores. Curr. Opin. Struct. Biol. 37, 152–163 doi: 10.1016/j.sbi.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Allshire R.C. and Karpen G.H. (2008) Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat. Rev. Genet. 9, 923–937 doi: 10.1038/nrg2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black B.E. and Cleveland D.W. (2011) Epigenetic centromere propagation and the nature of CENP-A nucleosomes. Cell 144, 471–479 doi: 10.1016/j.cell.2011.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukagawa T. and Earnshaw W.C. (2014) The centromere: chromatin foundation for the kinetochore machinery. Dev. Cell 30, 496–508 doi: 10.1016/j.devcel.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westhorpe F.G. and Straight A.F. (2015) The centromere: epigenetic control of chromosome segregation during mitosis. Cold Spring Harb. Perspect. Biol. 7, a015818 doi: 10.1101/cshperspect.a015818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai A. and Mitchison T.J. (1997) Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117 doi: 10.1146/annurev.cellbio.13.1.83 [DOI] [PubMed] [Google Scholar]
- 11.Nicklas R.B. (1997) How cells get the right chromosomes. Science 275, 632–637 doi: 10.1126/science.275.5300.632 [DOI] [PubMed] [Google Scholar]
- 12.Murray A.W. (2011) A brief history of error. Nat. Cell Biol. 13, 1178–1182 doi: 10.1038/ncb2348 [DOI] [PubMed] [Google Scholar]
- 13.Musacchio A. (2015) The molecular biology of spindle assembly checkpoint signaling dynamics. Curr. Biol. 25, R1002–R1018 doi: 10.1016/j.cub.2015.08.051 [DOI] [PubMed] [Google Scholar]
- 14.Pines J. (2011) Cubism and the cell cycle: the many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 12, 427–438 doi: 10.1038/nrm3132 [DOI] [PubMed] [Google Scholar]
- 15.Primorac I. and Musacchio A. (2013) Panta rhei: the APC/C at steady state. J. Cell Biol. 201, 177–189 doi: 10.1083/jcb.201301130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang L. and Barford D. (2014) Insights into the anaphase-promoting complex: a molecular machine that regulates mitosis. Curr. Opin. Struct. Biol. 29, 1–9 doi: 10.1016/j.sbi.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 17.Cheeseman I.M. and Desai A. (2008) Molecular architecture of the kinetochore-microtubule interface. Nat. Rev. Mol. Cell Biol. 9, 33–46 doi: 10.1038/nrm2310 [DOI] [PubMed] [Google Scholar]
- 18.Santaguida S. and Musacchio A. (2009) The life and miracles of kinetochores. EMBO J. 28, 2511–2531 doi: 10.1038/emboj.2009.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdaasdonk J.S. and Bloom K. (2011) Centromeres: unique chromatin structures that drive chromosome segregation. Nat. Rev. Mol. Cell Biol. 12, 320–332 doi: 10.1038/nrm3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flemming W. (1882) Zellsubstanz, kern und zelltheilung, F.C.W. Vogel Leipzig [Google Scholar]
- 21.Luykx P. (1965) The structure of the kinetochore in meiosis and mitosis in Urechis eggs. Exp. Cell Res. 39, 643–657 doi: 10.1016/0014-4827(65)90068-6 [DOI] [PubMed] [Google Scholar]
- 22.Brinkley B.R. and Stubblefield E. (1966) The fine structure of the kinetochore of a mammalian cell in vitro. Chromosoma 19, 28–43 doi: 10.1007/BF00332792 [DOI] [PubMed] [Google Scholar]
- 23.Jokelainen P.T. (1967) The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J. Ultrastruct. Res. 19, 19–44 doi: 10.1016/S0022-5320(67)80058-3 [DOI] [PubMed] [Google Scholar]
- 24.Moroi Y., Peebles C., Fritzler M.J., Steigerwald J. and Tan E.M. (1980) Autoantibody to centromere (kinetochore) in scleroderma sera. Proc. Natl Acad. Sci. USA 77, 1627–1631 doi: 10.1073/pnas.77.3.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guldner H.H., Lakomek H.J. and Bautz F.A. (1984) Human anti-centromere sera recognise a 19.5 kD non-histone chromosomal protein from HeLa cells. Clin. Exp. Immunol. 58, 13–20 PMID: [PMC free article] [PubMed] [Google Scholar]
- 26.Earnshaw W.C. and Rothfield N. (1985) Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91, 313–321 doi: 10.1007/BF00328227 [DOI] [PubMed] [Google Scholar]
- 27.Earnshaw W.C., Sullivan K.F., Machlin P.S., Cooke C.A., Kaiser D.A., Pollard T.D. et al. (1987) Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J. Cell Biol. 104, 817–829 doi: 10.1083/jcb.104.4.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saitoh H., Tomkiel J., Cooke C.A., Ratrie H., Maurer M., Rothfield N.F. et al. (1992) CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 70, 115–125 doi: 10.1016/0092-8674(92)90538-N [DOI] [PubMed] [Google Scholar]
- 29.Sullivan K.F., Hechenberger M. and Masri K. (1994) Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J. Cell Biol. 127, 581–592 doi: 10.1083/jcb.127.3.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke L. and Carbon J. (1980) Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature 287, 504–509 doi: 10.1038/287504a0 [DOI] [PubMed] [Google Scholar]
- 31.Lechner J. and Carbon J. (1991) A 240 kD multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell 64, 717–725 doi: 10.1016/0092-8674(91)90501-O [DOI] [PubMed] [Google Scholar]
- 32.Maine G.T., Sinha P. and Tye B.K. (1984) Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics 106, 365–385 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer F., Gerring S.L., Connelly C. and Hieter P. (1990) Mitotic chromosome transmission fidelity mutants in Saccharomyces cerevisiae. Genetics 124, 237–249 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortiz J., Stemmann O., Rank S. and Lechner J. (1999) A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev. 13, 1140–1155 doi: 10.1101/gad.13.9.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann C., Cheeseman I.M., Goode B.L., McDonald K.L., Barnes G. and Drubin D.G. (1998) Saccharomyces cerevisiae Duo1p and Dam1p, novel proteins involved in mitotic spindle function. J. Cell Biol. 143, 1029–1040 doi: 10.1083/jcb.143.4.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wigge P.A. and Kilmartin J.V. (2001) The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol. 152, 349–360 doi: 10.1083/jcb.152.2.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheeseman I.M., Anderson S., Jwa M., Green E.M., Kang J.-s., Yates J.R. III et al. (2002) Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111, 163–172 doi: 10.1016/S0092-8674(02)00973-X [DOI] [PubMed] [Google Scholar]
- 38.Buchwitz B.J., Ahmad K., Moore L.L., Roth M.B. and Henikoff S. (1999) Cell division: a histone-H3-like protein in C. elegans. Nature 401, 547–548 doi: 10.1038/44062 [DOI] [PubMed] [Google Scholar]
- 39.Goshima G., Kiyomitsu T., Yoda K. and Yanagida M. (2003) Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J. Cell Biol. 160, 25–39 doi: 10.1083/jcb.200210005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meraldi P., McAinsh A.D., Rheinbay E. and Sorger P.K. (2006) Phylogenetic and structural analysis of centromeric DNA and kinetochore proteins. Genome Biol. 7, R23 doi: 10.1186/gb-2006-7-3-r23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schleiffer A., Maier M., Litos G., Lampert F., Hornung P., Mechtler K. et al. (2012) CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat. Cell Biol. 14, 604–613 doi: 10.1038/ncb2493 [DOI] [PubMed] [Google Scholar]
- 42.Oegema K., Desai A., Rybina S., Kirkham M. and Hyman A.A. (2001) Functional analysis of kinetochore assembly in Caenorhabditis elegans. J. Cell Biol. 153, 1209–1226 doi: 10.1083/jcb.153.6.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goshima G., Wollman R., Goodwin S.S., Zhang N., Scholey J.M., Vale R.D. et al. (2007) Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316, 417–421 doi: 10.1126/science.1141314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akiyoshi B., Nelson C.R., Ranish J.A. and Biggins S. (2009) Quantitative proteomic analysis of purified yeast kinetochores identifies a PP1 regulatory subunit. Genes Dev. 23, 2887–2899 doi: 10.1101/gad.1865909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohta S., Bukowski-Wills J.-C., Sanchez-Pulido L., Alves Fde L., Wood L., Chen Z.A. et al. (2010) The protein composition of mitotic chromosomes determined using multiclassifier combinatorial proteomics. Cell 142, 810–821 doi: 10.1016/j.cell.2010.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer D.K., O'Day K., Trong H.L., Charbonneau H. and Margolis R.L. (1991) Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc. Natl Acad. Sci. USA 88, 3734–3738 doi: 10.1073/pnas.88.9.3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Earnshaw W.C. and Migeon B.R. (1985) Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma 92, 290–296 doi: 10.1007/BF00329812 [DOI] [PubMed] [Google Scholar]
- 48.Voullaire L.E., Slater H.R., Petrovic V. and Choo K.H. (1993) A functional marker centromere with no detectable alpha-satellite, satellite III, or CENP-B protein: activation of a latent centromere? Am. J. Hum. Genet. 52, 1153–1163 PMID: [PMC free article] [PubMed] [Google Scholar]
- 49.Collins K.A., Castillo A.R., Tatsutani S.Y. and Biggins S. (2005) De novo kinetochore assembly requires the centromeric histone H3 variant. Mol. Biol. Cell 16, 5649–5660 doi: 10.1091/mbc.E05-08-0771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S.-T., Rattner J.B., Jablonski S.A. and Yen T.J. (2006) Mapping the assembly pathways that specify formation of the trilaminar kinetochore plates in human cells. J. Cell Biol. 175, 41–53 doi: 10.1083/jcb.200606020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tachiwana H., Kagawa W., Shiga T., Osakabe A., Miya Y., Saito K. et al. (2011) Crystal structure of the human centromeric nucleosome containing CENP-A. Nature 476, 232–235 doi: 10.1038/nature10258 [DOI] [PubMed] [Google Scholar]
- 52.Kato H., Jiang J., Zhou B.-R., Rozendaal M., Feng H., Ghirlando R. et al. (2013) A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP-C. Science 340, 1110–1113 doi: 10.1126/science.1235532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falk S.J., Guo L.Y., Sekulic N., Smoak E.M., Mani T., Logsdon G.A. et al. (2015) CENP-C reshapes and stabilizes CENP-A nucleosomes at the centromere. Science 348, 699–703 doi: 10.1126/science.1259308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westhorpe F.G., Fuller C.J. and Straight A.F. (2015) A cell-free CENP-A assembly system defines the chromatin requirements for centromere maintenance. J. Cell Biol. 209, 789–801 doi: 10.1083/jcb.201503132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugimoto K., Yata H., Muro Y. and Himeno M. (1994) Human centromere protein C (CENP-C) is a DNA-binding protein which possesses a novel DNA-binding motif. J. Biochem. 116, 877–881 PMID: [DOI] [PubMed] [Google Scholar]
- 56.Cohen R.L., Espelin C.W., De Wulf P., Sorger P.K., Harrison S.C. and Simons K.T. (2008) Structural and functional dissection of Mif2p, a conserved DNA-binding kinetochore protein. Mol. Biol. Cell 19, 4480–4491 doi: 10.1091/mbc.E08-03-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hornung P., Troc P., Malvezzi F., Maier M., Demianova Z., Zimniak T. et al. (2014) A cooperative mechanism drives budding yeast kinetochore assembly downstream of CENP-A. J. Cell Biol. 206, 509–524 doi: 10.1083/jcb.201403081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hori T., Amano M., Suzuki A., Backer C.B., Welburn J.P., Dong Y. et al. (2008) CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell 135, 1039–1052 doi: 10.1016/j.cell.2008.10.019 [DOI] [PubMed] [Google Scholar]
- 59.Nishino T., Takeuchi K., Gascoigne K.E., Suzuki A., Hori T., Oyama T. et al. (2012) CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell 148, 487–501 doi: 10.1016/j.cell.2011.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeuchi K., Nishino T., Mayanagi K., Horikoshi N., Osakabe A., Tachiwana H. et al. (2014) The centromeric nucleosome-like CENP-T-W-S-X complex induces positive supercoils into DNA. Nucleic Acids Res. 42, 1644–1655 doi: 10.1093/nar/gkt1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aravind L. and Landsman D. (1998) AT-hook motifs identified in a wide variety of DNA-binding proteins. Nucleic Acids Res. 26, 4413–4421 doi: 10.1093/nar/26.19.4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westermann S. and Schleiffer A. (2013) Family matters: structural and functional conservation of centromere-associated proteins from yeast to humans. Trends Cell Biol. 23, 260–269 doi: 10.1016/j.tcb.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 63.Suzuki M. (1989) SPKK, a new nucleic acid-binding unit of protein found in histone. EMBO J. 8, 797–804 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malik H.S., Vermaak D. and Henikoff S. (2002) Recurrent evolution of DNA-binding motifs in the Drosophila centromeric histone. Proc. Natl Acad. Sci. USA 99, 1449–1454 doi: 10.1073/pnas.032664299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y., Riley D.J., Chen P.L. and Lee W.H. (1997) HEC, a novel nuclear protein rich in leucine heptad repeats specifically involved in mitosis. Mol. Cell. Biol. 17, 6049–6056 doi: 10.1128/MCB.17.10.6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wigge P.A., Jensen O.N., Holmes S., Souès S., Mann M. and Kilmartin J.V. (1998) Analysis of the Saccharomyces spindle pole by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. J. Cell Biol. 141, 967–977 doi: 10.1083/jcb.141.4.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciferri C., De Luca J., Monzani S., Ferrari K.J., Ristic D., Wyman C. et al. (2005) Architecture of the human ndc80-hec1 complex, a critical constituent of the outer kinetochore. J. Biol. Chem. 280, 29088–29095 doi: 10.1074/jbc.M504070200 [DOI] [PubMed] [Google Scholar]
- 68.Wei R.R., Sorger P.K. and Harrison S.C. (2005) Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc. Natl Acad. Sci. USA 102, 5363–5367 doi: 10.1073/pnas.0501168102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCleland M.L., Kallio M.J., Barrett-Wilt G.A., Kestner C.A., Shabanowitz J., Hunt D.F. et al. (2004) The vertebrate Ndc80 complex contains Spc24 and Spc25 homologs, which are required to establish and maintain kinetochore-microtubule attachment. Curr. Biol. 14, 131–137 doi: 10.1016/j.cub.2003.12.058 [DOI] [PubMed] [Google Scholar]
- 70.DeLuca J.G., Dong Y., Hergert P., Strauss J., Hickey J.M., Salmon E.D. et al. (2005) Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell 16, 519–531 doi: 10.1091/mbc.E04-09-0852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheeseman I.M., Chappie J.S., Wilson-Kubalek E.M. and Desai A. (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 doi: 10.1016/j.cell.2006.09.039 [DOI] [PubMed] [Google Scholar]
- 72.Wei R.R., Al-Bassam J. and Harrison S.C. (2007) The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat. Struct. Mol. Biol. 14, 54–59 doi: 10.1038/nsmb1186 [DOI] [PubMed] [Google Scholar]
- 73.Ciferri C., Pasqualato S., Screpanti E., Varetti G., Santaguida S., Dos Reis G. et al. (2008) Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133, 427–439 doi: 10.1016/j.cell.2008.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Powers A.F., Franck A.D., Gestaut D.R., Cooper J., Gracyzk B., Wei R.R. et al. (2009) The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 136, 865–875 doi: 10.1016/j.cell.2008.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alushin G.M., Ramey V.H., Pasqualato S., Ball D.A., Grigorieff N., Musacchio A. et al. (2010) The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature 467, 805–810 doi: 10.1038/nature09423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarangapani K.K., Akiyoshi B., Duggan N.M., Biggins S. and Asbury C.L. (2013) Phosphoregulation promotes release of kinetochores from dynamic microtubules via multiple mechanisms. Proc. Natl Acad. Sci. USA 110, 7282–7287 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ji Z., Gao H. and Yu H. (2015) Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 348, 1260–1264 doi: 10.1126/science.aaa4029 [DOI] [PubMed] [Google Scholar]
- 78.Hiruma Y., Sacristan C., Pachis S.T., Adamopoulos A., Kuijt T., Ubbink M. et al. (2015) Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 348, 1264–1267 doi: 10.1126/science.aaa4055 [DOI] [PubMed] [Google Scholar]
- 79.Dong Y., Vanden Beldt K.J., Meng X., Khodjakov A. and McEwen B.F. (2007) The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions. Nat. Cell Biol. 9, 516–522 doi: 10.1038/ncb1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McIntosh J.R., Grishchuk E.L., Morphew M.K., Efremov A.K., Zhudenkov K., Volkov V.A. et al. (2008) Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell 135, 322–333 doi: 10.1016/j.cell.2008.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gonen S., Akiyoshi B., Iadanza M.G., Shi D., Duggan N., Biggins S. et al. (2012) The structure of purified kinetochores reveals multiple microtubule-attachment sites. Nat. Struct. Mol. Biol. 19, 925–929 doi: 10.1038/nsmb.2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Wulf P., McAinsh A.D. and Sorger P.K. (2003) Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev. 17, 2902–2921 doi: 10.1101/gad.1144403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pinsky B.A., Tatsutani S.Y., Collins K.A. and Biggins S. (2003) An Mtw1 complex promotes kinetochore biorientation that is monitored by the Ipl1/Aurora protein kinase. Dev. Cell 5, 735–745 doi: 10.1016/S1534-5807(03)00322-8 [DOI] [PubMed] [Google Scholar]
- 84.Obuse C., Iwasaki O., Kiyomitsu T., Goshima G., Toyoda Y. and Yanagida M. (2004) A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat. Cell Biol. 6, 1135–1141 doi: 10.1038/ncb1187 [DOI] [PubMed] [Google Scholar]
- 85.Foltz D.R., Jansen L.E.T., Black B.E., Bailey A.O., Yates J.R. and Cleveland D.W. (2006) The human CENP-A centromeric nucleosome-associated complex. Nat. Cell Biol. 8, 458–469 doi: 10.1038/ncb1397 [DOI] [PubMed] [Google Scholar]
- 86.Okada M., Cheeseman I.M., Hori T., Okawa K., McLeod I.X., Yates J.R. III et al. (2006) The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat. Cell Biol. 8, 446–457 doi: 10.1038/ncb1396 [DOI] [PubMed] [Google Scholar]
- 87.Carroll C.W., Milks K.J. and Straight A.F. (2010) Dual recognition of CENP-A nucleosomes is required for centromere assembly. J. Cell Biol. 189, 1143–1155 doi: 10.1083/jcb.201001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Przewloka M.R., Venkei Z., Bolanos-Garcia V.M., Debski J., Dadlez M. and Glover D.M. (2011) CENP-C is a structural platform for kinetochore assembly. Curr. Biol. 21, 399–405 doi: 10.1016/j.cub.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 89.Screpanti E., De Antoni A., Alushin G.M., Petrovic A., Melis T., Nogales E. et al. (2011) Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr. Biol. 21, 391–398 doi: 10.1016/j.cub.2010.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klare K., Weir J.R., Basilico F., Zimniak T., Massimiliano L., Ludwigs N. et al. (2015) CENP-C is a blueprint for constitutive centromere-associated network assembly within human kinetochores. J. Cell Biol. 210, 11–22 doi: 10.1083/jcb.201412028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKinley K.L., Sekulic N., Guo L.Y., Tsinman T., Black B.E. and Cheeseman I.M. (2015) The CENP-L-N complex forms a critical node in an integrated meshwork of interactions at the centromere-kinetochore interface. Mol. Cell 60, 886–898 doi: 10.1016/j.molcel.2015.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petrovic A., Mosalaganti S., Keller J., Mattiuzzo M., Overlack K., Krenn V. et al. (2014) Modular assembly of RWD domains on the Mis12 complex underlies outer kinetochore organization. Mol. Cell 53, 591–605 doi: 10.1016/j.molcel.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 93.Joglekar A.P., Bloom K. and Salmon E.D. (2009) In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr. Biol. 19, 694–699 doi: 10.1016/j.cub.2009.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wan X., O'Quinn R.P., Pierce H.L., Joglekar A.P., Gall W.E., DeLuca J.G. et al. (2009) Protein architecture of the human kinetochore microtubule attachment site. Cell 137, 672–684 doi: 10.1016/j.cell.2009.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magidson V., He J., Ault J.G., O'Connell C.B., Yang N., Tikhonenko I. et al. (2016) Unattached kinetochores rather than intrakinetochore tension arrest mitosis in taxol-treated cells. J. Cell Biol. 212, 307–319 doi: 10.1083/jcb.201412139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmitzberger F. and Harrison S.C. (2012) RWD domain: a recurring module in kinetochore architecture shown by a Ctf19–Mcm21 complex structure. EMBO Rep. 13, 216–222 doi: 10.1038/embor.2012.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim S., Sun H., Tomchick D.R., Yu H. and Luo X. (2012) Structure of human Mad1 C-terminal domain reveals its involvement in kinetochore targeting. Proc. Natl Acad. Sci. USA 109, 6549–6554 doi: 10.1073/pnas.1118210109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bolanos-Garcia V.M., Kiyomitsu T., D'Arcy S., Chirgadze D.Y., Grossmann J.G., Matak-Vinkovic D. et al. (2009) The crystal structure of the N-terminal region of BUB1 provides insight into the mechanism of BUB1 recruitment to kinetochores. Structure 17, 105–116 doi: 10.1016/j.str.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nijenhuis W., von Castelmur E., Littler D., De Marco V., Tromer E., Vleugel M. et al. (2013) A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J. Cell Biol. 201, 217–231 doi: 10.1083/jcb.201210033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo Q., Tao Y., Liu H., Teng M. and Li X. (2013) Structural insights into the role of the Chl4-Iml3 complex in kinetochore assembly. Acta Crystallogr. D Biol. Crystallogr. 69, 2412–2419 doi: 10.1107/S0907444913022397 [DOI] [PubMed] [Google Scholar]
- 101.Hinshaw S.M. and Harrison S.C. (2013) An Iml3-Chl4 heterodimer links the core centromere to factors required for accurate chromosome segregation. Cell Rep. 5, 29–36 doi: 10.1016/j.celrep.2013.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Basilico F., Maffini S., Weir J.R., Prumbaum D., Rojas A.M., Zimniak T. et al. (2014) The pseudo GTPase CENP-M drives human kinetochore assembly. eLife 3, e02978 doi: 10.7554/eLife.02978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cho U.-S. and Harrison S.C. (2012) Ndc10 is a platform for inner kinetochore assembly in budding yeast. Nat. Struct. Mol. Biol. 19, 48–55 doi: 10.1038/nsmb.2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perriches T. and Singleton M.R. (2012) Structure of yeast kinetochore Ndc10 DNA-binding domain reveals unexpected evolutionary relationship to tyrosine recombinases. J. Biol. Chem. 287, 5173–5179 doi: 10.1074/jbc.C111.318501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohno S. (1970) Evolution by gene duplication, Springer-Verlag, New York [Google Scholar]
- 106.Wickstead B. and Gull K. (2011) The evolution of the cytoskeleton. J. Cell Biol. 194, 513–525 doi: 10.1083/jcb.201102065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drechsler H. and McAinsh A.D. (2012) Exotic mitotic mechanisms. Open Biol. 2, 120140 doi: 10.1098/rsob.120140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Findeisen P., Mühlhausen S., Dempewolf S., Hertzog J., Zietlow A., Carlomagno T. et al. (2014) Six subgroups and extensive recent duplications characterize the evolution of the eukaryotic tubulin protein family. Genome Biol. Evol. 6, 2274–2288 doi: 10.1093/gbe/evu187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Henikoff S., Ahmad K. and Malik H.S. (2001) The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293, 1098–1102 doi: 10.1126/science.1062939 [DOI] [PubMed] [Google Scholar]
- 110.Drinnenberg I.A., deYoung D., Henikoff S. and Malik H.S. (2014) Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. eLife 3, e03676 doi: 10.7554/eLife.03676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheeseman I.M., Niessen S., Anderson S., Hyndman F., Yates J.R. III, Oegema K. et al. (2004) A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 18, 2255–2268 doi: 10.1101/gad.1234104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Przewloka M.R., Zhang W., Costa P., Archambault V., D'Avino P.P., Lilley K.S. et al. (2007) Molecular analysis of core kinetochore composition and assembly in Drosophila melanogaster. PLoS ONE 2, e478 doi: 10.1371/journal.pone.0000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y., Petrovic A., Rombaut P., Mosalaganti S., Keller J., Raunser S. et al. (2016) Insights from the reconstitution of the divergent outer kinetochore of Drosophila melanogaster. Open Biol. 6, 150236 doi: 10.1098/rsob.150236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richter M.M., Poznanski J., Zdziarska A., Czarnocki-Cieciura M., Lipinszki Z., Dadlez M. et al. (2016) Network of protein interactions within the Drosophila inner kinetochore. Open Biol. 6, 150238 doi: 10.1098/rsob.150238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drinnenberg I.A., Henikoff S. and Malik H.S. (2016) Evolutionary turnover of kinetochore proteins: a Ship of Theseus? Trends Cell Biol. 26, 498–510 doi: 10.1016/j.tcb.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vickerman K. (1962) The mechanism of cyclical development in trypanosomes of the Trypanosoma brucei sub-group: an hypothesis based on ultrastructural observations. Trans. R. Soc. Trop. Med. Hyg. 56, 487–495 doi: 10.1016/0035-9203(62)90072-X [DOI] [PubMed] [Google Scholar]
- 117.Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D.C. et al. (2005) The genome of the African trypanosome Trypanosoma brucei. Science 309, 416–422 doi: 10.1126/science.1112642 [DOI] [PubMed] [Google Scholar]
- 118.El-Sayed N.M., Myler P.J., Bartholomeu D.C., Nilsson D., Aggarwal G., Tran A.-N. et al. (2005) The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309, 409–415 doi: 10.1126/science.1112631 [DOI] [PubMed] [Google Scholar]
- 119.Ivens A.C., Peacock C.S., Worthey E.A., Murphy L., Aggarwal G., Berriman M. et al. (2005) The genome of the kinetoplastid parasite, Leishmania major. Science 309, 436–442 doi: 10.1126/science.1112680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akiyoshi B. and Gull K. (2013) Evolutionary cell biology of chromosome segregation: insights from trypanosomes. Open Biol. 3, 130023 doi: 10.1098/rsob.130023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ersfeld K. and Gull K. (1997) Partitioning of large and minichromosomes in Trypanosoma brucei. Science 276, 611–614 doi: 10.1126/science.276.5312.611 [DOI] [PubMed] [Google Scholar]
- 122.Vickerman K. and Preston T.M. (1970) Spindle microtubules in the dividing nuclei of trypanosomes. J. Cell. Sci. 6, 365–383 PMID: [DOI] [PubMed] [Google Scholar]
- 123.Solari A.J. (1980) The 3-dimensional fine structure of the mitotic spindle in Trypanosoma cruzi. Chromosoma 78, 239–255 doi: 10.1007/BF00328395 [DOI] [PubMed] [Google Scholar]
- 124.Ogbadoyi E., Ersfeld K., Robinson D., Sherwin T. and Gull K. (2000) Architecture of the Trypanosoma brucei nucleus during interphase and mitosis. Chromosoma 108, 501–513 doi: 10.1007/s004120050402 [DOI] [PubMed] [Google Scholar]
- 125.Kim J., Ishiguro K., Nambu A., Akiyoshi B., Yokobayashi S., Kagami A. et al. (2015) Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature 517, 466–471 doi: 10.1038/nature14097 [DOI] [PubMed] [Google Scholar]
- 126.Akiyoshi B., Sarangapani K.K., Powers A.F., Nelson C.R., Reichow S.L., Arellano-Santoyo H. et al. (2010) Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468, 576–579 doi: 10.1038/nature09594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Akiyoshi B. and Biggins S. (2012) Reconstituting the kinetochore–microtubule interface: what, why, and how. Chromosoma 121, 235–250 doi: 10.1007/s00412-012-0362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lowell J.E. and Cross G.A.M. (2004) A variant histone H3 is enriched at telomeres in Trypanosoma brucei. J. Cell Sci. 117, 5937–5947 doi: 10.1242/jcs.01515 [DOI] [PubMed] [Google Scholar]
- 129.Talbert P.B., Bayes J.J. and Henikoff S. (2009) Evolution of centromeres and kinetochores: a two-part fugue. In The kinetochore (De Wulf P. and. Earnshaw W.C., eds), pp. 1–37, Springer, New York [Google Scholar]
- 130.Kiyomitsu T., Obuse C. and Yanagida M. (2007) Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev. Cell 13, 663–676 doi: 10.1016/j.devcel.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 131.Li Z., Lee J.H., Chu F., Burlingame A.L., Günzl A. and Wang C.C. (2008) Identification of a novel chromosomal passenger complex and its unique localization during cytokinesis in Trypanosoma brucei. PLoS ONE 3, e2354 doi: 10.1371/journal.pone.0002354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Herzog F., Primorac I., Dube P., Lenart P., Sander B., Mechtler K. et al. (2009) Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science 323, 1477–1481 doi: 10.1126/science.1163300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hsu K.-S. and Toda T. (2011) Ndc80 internal loop interacts with Dis1/TOG to ensure proper kinetochore-spindle attachment in fission yeast. Curr. Biol. 21, 214–220 doi: 10.1016/j.cub.2010.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller M.P., Asbury C.L. and Biggins S. (2016) A TOG protein confers tension sensitivity to kinetochore-microtubule attachments. Cell 165, 1428–1439 doi: 10.1016/j.cell.2016.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dean S., Sunter J., Wheeler R.J., Hodkinson I., Gluenz E. and Gull K. (2015) A toolkit enabling efficient, scalable and reproducible gene tagging in trypanosomatids. Open Biol. 5, 140197 doi: 10.1098/rsob.140197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kelly S., Reed J., Kramer S., Ellis L., Webb H., Sunter J. et al. (2007) Functional genomics in Trypanosoma brucei: a collection of vectors for the expression of tagged proteins from endogenous and ectopic gene loci. Mol. Biochem. Parasitol. 154, 103–109 doi: 10.1016/j.molbiopara.2007.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Archer S.K., Inchaustegui D., Queiroz R. and Clayton C. (2011) The cell cycle regulated transcriptome of Trypanosoma brucei. PLoS ONE 6, e18425 doi: 10.1371/journal.pone.0018425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brenner S., Pepper D., Berns M.W., Tan E. and Brinkley B.R. (1981) Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J. Cell Biol. 91, 95–102 doi: 10.1083/jcb.91.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meluh P.B. and Koshland D. (1997) Budding yeast centromere composition and assembly as revealed by in vivo cross-linking. Genes Dev. 11, 3401–3412 doi: 10.1101/gad.11.24.3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hayashi A., Ogawa H., Kohno K., Gasser S.M. and Hiraoka Y. (1998) Meiotic behaviours of chromosomes and microtubules in budding yeast: relocalization of centromeres and telomeres during meiotic prophase. Genes Cells 3, 587–601 doi: 10.1046/j.1365-2443.1998.00215.x [DOI] [PubMed] [Google Scholar]
- 141.Obado S.O., Bot C., Nilsson D., Andersson B. and Kelly J.M. (2007) Repetitive DNA is associated with centromeric domains in Trypanosoma brucei but not Trypanosoma cruzi. Genome Biol. 8, R37 doi: 10.1186/gb-2007-8-3-r37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Earnshaw W.C. (2015) Discovering centromere proteins: from cold White hands to the A, B, C of CENPs. Nat. Rev. Mol. Cell Biol. 16, 443–449 doi: 10.1038/nrm4001 [DOI] [PubMed] [Google Scholar]
- 143.Akiyoshi B. and Gull K. (2014) Discovery of unconventional kinetochores in kinetoplastids. Cell 156, 1247–1258 doi: 10.1016/j.cell.2014.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nerusheva O.O. and Akiyoshi B. (2016) Divergent polo box domains underpin the unique kinetoplastid kinetochore. Open Biol. 6, 150206 doi: 10.1098/rsob.150206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morriswood B., Havlicek K., Demmel L., Yavuz S., Sealey-Cardona M., Vidilaseris K. et al. (2013) Novel bilobe components in Trypanosoma brucei identified using proximity-dependent biotinylation. Eukaryotic Cell 12, 356–367 doi: 10.1128/EC.00326-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reinhardt H.C. and Yaffe M.B. (2013) Phospho-Ser/Thr-binding domains: navigating the cell cycle and DNA damage response. Nat. Rev. Mol. Cell Biol. 14, 563–580 doi: 10.1038/nrm3640 [DOI] [PubMed] [Google Scholar]
- 147.Parsons M., Worthey E.A., Ward P.N. and Mottram J.C. (2005) Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics 6, 127 doi: 10.1186/1471-2164-6-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Katoh K. and Standley D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lupas A., Van Dyke M. and Stock J. (1991) Predicting coiled coils from protein sequences. Science 252, 1162–1164 doi: 10.1126/science.252.5009.1162 [DOI] [PubMed] [Google Scholar]
- 150.Bryson K., McGuffin L.J., Marsden R.L., Ward J.J., Sodhi J.S. and Jones D.T. (2005) Protein structure prediction servers at University College London. Nucleic Acids Res. 33, W36–W38 doi: 10.1093/nar/gki410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L. et al. (2009) MEME suite: tools for motif discovery and searching. Nucl. Acids Res. doi: 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Finn R.D., Coggill P., Eberhardt R.Y., Eddy S.R., Mistry J., Mitchell A.L. et al. (2016) The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44, D279–D285 doi: 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buchan D.W.A., Minneci F., Nugent T.C.O., Bryson K. and Jones D.T. (2013) Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 41, W349–W357 doi: 10.1093/nar/gkt381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Drozdetskiy A., Cole C., Procter J. and Barton G.J. (2015) JPred4: a protein secondary structure prediction server. Nucl. Acids Res. 43, W389–W394 doi: 10.1093/nar/gkv332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Söding J., Biegert A. and Lupas A.N. (2005) The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–W248 doi: 10.1093/nar/gki408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang C.H., Tomkiel J., Saitoh H., Johnson D.H. and Earnshaw W.C. (1996) Identification of overlapping DNA-binding and centromere-targeting domains in the human kinetochore protein CENP-C. Mol. Cell. Biol. 16, 3576–3586 doi: 10.1128/MCB.16.7.3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Milks K.J., Moree B. and Straight A.F. (2009) Dissection of CENP-C-directed centromere and kinetochore assembly. Mol. Biol. Cell 20, 4246–4255 doi: 10.1091/mbc.E09-05-0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jones N.G., Thomas E.B., Brown E., Dickens N.J., Hammarton T.C. and Mottram J.C. (2014) Regulators of Trypanosoma brucei cell cycle progression and differentiation identified using a kinome-wide RNAi screen. PLoS Pathog. 10, e1003886 doi: 10.1371/journal.ppat.1003886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ngô H., Tschudi C., Gull K. and Ullu E. (1998) Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc. Natl Acad. Sci. USA 95, 14687–14692 doi: 10.1073/pnas.95.25.14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Z., Morris J.C., Drew M.E. and Englund P.T. (2000) Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J. Biol. Chem. 275, 40174–40179 doi: 10.1074/jbc.M008405200 [DOI] [PubMed] [Google Scholar]
- 161.Merritt C. and Stuart K. (2013) Identification of essential and non-essential protein kinases by a fusion PCR method for efficient production of transgenic Trypanosoma brucei. Mol. Biochem. Parasitol. 190, 44–49 doi: 10.1016/j.molbiopara.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim H.-S., Li Z., Boothroyd C. and Cross G.A.M. (2013) Strategies to construct null and conditional null Trypanosoma brucei mutants using Cre-recombinase and loxP. Mol. Biochem. Parasitol. 191, 16–19 doi: 10.1016/j.molbiopara.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nishimura K., Fukagawa T., Takisawa H., Kakimoto T. and Kanemaki M. (2009) An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Meth. 6, 917–922 doi: 10.1038/nmeth.1401 [DOI] [PubMed] [Google Scholar]
- 164.Siegel T.N., Hekstra D.R., Kemp L.E., Figueiredo L.M., Lowell J.E., Fenyo D. et al. (2009) Four histone variants mark the boundaries of polycistronic transcription units in Trypanosoma brucei. Genes Dev. 23, 1063–1076 doi: 10.1101/gad.1790409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.MacRae T.H. and Gull K. (1990) Purification and assembly in vitro of tubulin from Trypanosoma brucei brucei. Biochem. J. 265, 87–93 doi: 10.1042/bj2650087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Widlund P.O., Podolski M., Reber S., Alper J., Storch M., Hyman A.A. et al. (2012) One-step purification of assembly-competent tubulin from diverse eukaryotic sources. Mol. Biol. Cell 23, 4393–4401 doi: 10.1091/mbc.E12-06-0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gascoigne K.E., Takeuchi K., Suzuki A., Hori T., Fukagawa T. and Cheeseman I.M. (2011) Induced ectopic kinetochore assembly bypasses the requirement for CENP-A nucleosomes. Cell 145, 410–422 doi: 10.1016/j.cell.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Akiyoshi B., Nelson C.R. and Biggins S. (2013) The Aurora B kinase promotes inner and outer kinetochore interactions in budding yeast. Genetics 194, 785–789 doi: 10.1534/genetics.113.150839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gascoigne K.E. and Cheeseman I.M. (2013) CDK-dependent phosphorylation and nuclear exclusion coordinately control kinetochore assembly state. J. Cell Biol. 201, 23–32 doi: 10.1083/jcb.201301006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim S. and Yu H. (2015) Multiple assembly mechanisms anchor the KMN spindle checkpoint platform at human mitotic kinetochores. J. Cell Biol. 208, 181–196 doi: 10.1083/jcb.201407074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rago F., Gascoigne K.E. and Cheeseman I.M. (2015) Distinct organization and regulation of the outer kinetochore KMN network downstream of CENP-C and CENP-T. Curr. Biol. 25, 671–677 doi: 10.1016/j.cub.2015.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hochegger H., Hégarat N. and Pereira-Leal J.B. (2013) Aurora at the pole and equator: overlapping functions of Aurora kinases in the mitotic spindle. Open Biol. 3, 120185 doi: 10.1098/rsob.120185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Carmena M., Wheelock M., Funabiki H. and Earnshaw W.C. (2012) The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 13, 789–803 doi: 10.1038/nrm3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Francisco L., Wang W. and Chan C.S. (1994) Type 1 protein phosphatase acts in opposition to Ipl1 protein kinase in regulating yeast chromosome segregation. Mol. Cell. Biol. 14, 4731–4740 doi: 10.1128/MCB.14.7.4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu D., Vleugel M., Backer C.B., Hori T., Fukagawa T., Cheeseman I.M. et al. (2010) Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 188, 809–820 doi: 10.1083/jcb.201001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tanaka T.U., Rachidi N., Janke C., Pereira G., Galova M., Schiebel E. et al. (2002) Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108, 317–329 doi: 10.1016/S0092-8674(02)00633-5 [DOI] [PubMed] [Google Scholar]
- 177.Andrews P.D., Ovechkina Y., Morrice N., Wagenbach M., Duncan K., Wordeman L. et al. (2004) Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253–268 doi: 10.1016/S1534-5807(04)00025-5 [DOI] [PubMed] [Google Scholar]
- 178.Watanabe Y. (2010) Temporal and spatial regulation of targeting aurora B to the inner centromere. Cold Spring Harb. Symp. Quant. Biol. 75, 419–423 doi: 10.1101/sqb.2010.75.035 [DOI] [PubMed] [Google Scholar]
- 179.Lampson M.A. and Cheeseman I.M. (2011) Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 21, 133–140 doi: 10.1016/j.tcb.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zaytsev A.V., Segura-Peña D., Godzi M., Calderon A., Ballister E.R., Stamatov R. et al. (2016) Bistability of a coupled Aurora B kinase-phosphatase system in cell division. eLife 5, e10644 doi: 10.7554/eLife.10644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bloom K.S. (2014) Centromeric heterochromatin: the primordial segregation machine. Annu. Rev. Genet. 48, 457–484 doi: 10.1146/annurev-genet-120213-092033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Goshima G. and Yanagida M. (2000) Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell 100, 619–633 doi: 10.1016/S0092-8674(00)80699-6 [DOI] [PubMed] [Google Scholar]
- 183.Cooke C.A., Heck M.M. and Earnshaw W.C. (1987) The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J. Cell Biol. 105, 2053–2067 doi: 10.1083/jcb.105.5.2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dawe R.K., Reed L.M., Yu H.G., Muszynski M.G. and Hiatt E.N. (1999) A maize homolog of mammalian CENPC is a constitutive component of the inner kinetochore. Plant Cell Online 11, 1227–1238 doi: 10.1105/tpc.11.7.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fujiwara T., Tanaka K., Kuroiwa T. and Hirano T. (2013) Spatiotemporal dynamics of condensins I and II: evolutionary insights from the primitive red alga Cyanidioschyzon merolae. Mol. Biol. Cell 24, 2515–2527 doi: 10.1091/mbc.E13-04-0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Prensier G. and Slomianny C. (1986) The karyotype of Plasmodium falciparum determined by ultrastructural serial sectioning and 3D reconstruction. J. Parasitol. 72, 731–736 doi: 10.2307/3281465 [DOI] [PubMed] [Google Scholar]
- 187.Ureña F. (1986) Three-dimensional reconstructions of the mitotic spindle and dense plaques in three species of Leishmania. Z. Parasitenkd. 72, 299–306 doi: 10.1007/BF00928739 [DOI] [PubMed] [Google Scholar]
- 188.Campbell C.S. and Desai A. (2013) Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature 497, 118–121 doi: 10.1038/nature12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.London N. and Biggins S. (2014) Signalling dynamics in the spindle checkpoint response. Nat. Rev. Mol. Cell Biol. 15, 736–747 doi: 10.1038/nrm3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vleugel M., Hoogendoorn E., Snel B. and Kops G.J.P.L. (2012) Evolution and function of the mitotic checkpoint. Dev. Cell 23, 239–250 doi: 10.1016/j.devcel.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 191.Vicente J.-J. and Cande W.Z. (2014) Mad2, Bub3, and Mps1 regulate chromosome segregation and mitotic synchrony in Giardia intestinalis, a binucleate protist lacking an anaphase-promoting complex. Mol. Biol. Cell 25, 2774–2787 doi: 10.1091/mbc.E14-05-0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li R. and Murray A.W. (1991) Feedback control of mitosis in budding yeast. Cell 66, 519–531 doi: 10.1016/0092-8674(81)90015-5 [DOI] [PubMed] [Google Scholar]
- 193.Hoyt M.A., Totis L. and Roberts B.T. (1991) S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507–517 doi: 10.1016/0092-8674(81)90014-3 [DOI] [PubMed] [Google Scholar]
- 194.He X., Patterson T.E. and Sazer S. (1997) The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl Acad. Sci. USA 94, 7965–7970 doi: 10.1073/pnas.94.15.7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Buffin E., Emre D. and Karess R.E. (2007) Flies without a spindle checkpoint. Nat. Cell Biol. 9, 565–572 doi: 10.1038/ncb1570 [DOI] [PubMed] [Google Scholar]
- 196.Wild T., Larsen M.S.Y., Narita T., Schou J., Nilsson J. and Choudhary C. (2016) The spindle assembly checkpoint is not essential for viability of human cells with genetically lowered APC/C activity. Cell Rep. 14, 1829–1840 doi: 10.1016/j.celrep.2016.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Craney A., Kelly A., Jia L., Fedrigo I., Yu H. and Rape M. (2016) Control of APC/C-dependent ubiquitin chain elongation by reversible phosphorylation. Proc. Natl Acad. Sci. USA 113, 1540–1545 doi: 10.1073/pnas.1522423113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fujimitsu K., Grimaldi M. and Yamano H. (2016) Cyclin dependent kinase 1-dependent activation of APC/C ubiquitin ligase. Science doi: 10.1126/science.aad3925 [DOI] [PubMed] [Google Scholar]
- 199.Jia L., Li B. and Yu H. (2016) The Bub1-Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation. Nat. Commun. 7, 10818 doi: 10.1038/ncomms10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Qiao R., Weissmann F., Yamaguchi M., Brown N.G., VanderLinden R., Imre R. et al. (2016) Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc. Natl Acad. Sci. USA 113, E2570–E2578 doi: 10.1073/pnas.1604929113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang S., Chang L., Alfieri C., Zhang Z., Yang J., Maslen S. et al. (2016) Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature 533, 260–264 doi: 10.1038/nature17973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ploubidou A., Robinson D.R., Docherty R.C., Ogbadoyi E.O. and Gull K. (1999) Evidence for novel cell cycle checkpoints in trypanosomes: kinetoplast segregation and cytokinesis in the absence of mitosis. J. Cell. Sci. 112(Pt 24), 4641–4650 PMID: [DOI] [PubMed] [Google Scholar]
- 203.Gluenz E., Sharma R., Carrington M. and Gull K. (2008) Functional characterization of cohesin subunit SCC1 in Trypanosoma brucei and dissection of mutant phenotypes in two life cycle stages. Mol. Microbiol. 69, 666–680 doi: 10.1111/j.1365-2958.2008.06320.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Singh N., Wiltshire T.D., Thompson J.R., Mer G. and Couch F.J. (2012) Molecular basis for the association of microcephalin (MCPH1) protein with the cell division cycle protein 27 (Cdc27) subunit of the anaphase-promoting complex. J. Biol. Chem. 287, 2854–2862 doi: 10.1074/jbc.M111.307868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Atkins M.S., Teske A.P. and Anderson O.R. (2000) A survey of flagellate diversity at four deep-sea hydrothermal vents in the Eastern Pacific Ocean using structural and molecular approaches. J. Eukaryot. Microbiol. 47, 400–411 doi: 10.1111/j.1550-7408.2000.tb00067.x [DOI] [PubMed] [Google Scholar]
- 206.López-García P., Philippe H., Gail F. and Moreira D. (2003) Autochthonous eukaryotic diversity in hydrothermal sediment and experimental microcolonizers at the Mid-Atlantic Ridge. Proc. Natl Acad. Sci. USA 100, 697–702 doi: 10.1073/pnas.0235779100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Flegontov P., Votýpka J., Skalický T., Logacheva M.D., Penin A.A., Tanifuji G. et al. (2013) Paratrypanosoma is a novel early-branching trypanosomatid. Curr. Biol. 23, 1787–1793 doi: 10.1016/j.cub.2013.07.045 [DOI] [PubMed] [Google Scholar]
- 208.d'Avila-Levy C.M., Boucinha C., Kostygov A., Santos H.L.C., Morelli K.A., Grybchuk-Ieremenko A. et al. (2015) Exploring the environmental diversity of kinetoplastid flagellates in the high-throughput DNA sequencing era. Mem. Inst. Oswaldo Cruz 110, 956–965 doi: 10.1590/0074-02760150253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moreira D., López-García P. and Vickerman K. (2004) An updated view of kinetoplastid phylogeny using environmental sequences and a closer outgroup: proposal for a new classification of the class Kinetoplastea. Int. J. Syst. Evol. Microbiol. 54, 1861–1875 doi: 10.1099/ijs.0.63081-0 [DOI] [PubMed] [Google Scholar]
- 210.Simpson A.G.B., Stevens J.R. and Lukeš J. (2006) The evolution and diversity of kinetoplastid flagellates. Trends Parasitol. 22, 168–174 doi: 10.1016/j.pt.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 211.Jackson A.P., Otto T.D., Aslett M., Armstrong S.D., Bringaud F., Schlacht A. et al. (2016) Kinetoplastid phylogenomics reveals the evolutionary innovations associated with the origins of parasitism. Curr. Biol. 26, 161–172 doi: 10.1016/j.cub.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Porcel B.M., Denoeud F., Opperdoes F., Noel B., Madoui M.-A., Hammarton T.C. et al. (2014) The streamlined genome of Phytomonas spp. relative to human pathogenic kinetoplastids reveals a parasite tailored for plants. PLoS Genet. 10, e1004007 doi: 10.1371/journal.pgen.1004007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tanifuji G., Kim E., Onodera N.T., Gibeault R., Dlutek M., Cawthorn R.J. et al. (2011) Genomic characterization of Neoparamoeba pemaquidensis (Amoebozoa) and its kinetoplastid endosymbiont. Eukaryotic Cell 10, 1143–1146 doi: 10.1128/EC.05027-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Walker G., Dorrell R.G., Schlacht A. and Dacks J.B. (2011) Eukaryotic systematics: a user's guide for cell biologists and parasitologists. Parasitology 138, 1638–1663 doi: 10.1017/S0031182010001708 [DOI] [PubMed] [Google Scholar]
- 215.Adl S.M., Simpson A.G.B., Lane C.E., Lukeš J., Bass D., Bowser S.S. et al. (2012) The revised classification of eukaryotes. J. Eukaryot. Microbiol. 59, 429–514 doi: 10.1111/j.1550-7408.2012.00644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.O'Neill E.C., Trick M., Hill L., Rejzek M., Dusi R.G., Hamilton C.J. et al. (2015) The transcriptome of Euglena gracilis reveals unexpected metabolic capabilities for carbohydrate and natural product biochemistry. Mol. Biosyst. 11, 2808–2820 doi: 10.1039/C5MB00319A [DOI] [PubMed] [Google Scholar]
- 217.Malik H.S. and Henikoff S. (2003) Phylogenomics of the nucleosome. Nat. Struct. Biol. 10, 882–891 doi: 10.1038/nsb996 [DOI] [PubMed] [Google Scholar]
- 218.Marande W., Lukes J. and Burger G. (2005) Unique mitochondrial genome structure in diplonemids, the sister group of kinetoplastids. Eukaryotic Cell 4, 1137–1146 doi: 10.1128/EC.4.6.1137-1146.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lukeš J., Flegontova O. and Horák A. (2015) Diplonemids. Curr. Biol. 25, R702–R704 doi: 10.1016/j.cub.2015.04.052 [DOI] [PubMed] [Google Scholar]
- 220.de Vargas C., Audic S., Henry N., Decelle J., Mahé F., Logares R. et al. (2015) Eukaryotic plankton diversity in the sunlit ocean. Science 348, 1261605 doi: 10.1126/science.1261605 [DOI] [PubMed] [Google Scholar]
- 221.Yubuki N., Edgcomb V.P., Bernhard J.M. and Leander B.S. (2009) Ultrastructure and molecular phylogeny of Calkinsia aureus: cellular identity of a novel clade of deep-sea euglenozoans with epibiotic bacteria. BMC Microbiol. 9, 16 doi: 10.1186/1471-2180-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yutin N. and Koonin E.V. (2012) Archaeal origin of tubulin. Biol. Direct 7, 10 doi: 10.1186/1745-6150-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Williams T.A., Foster P.G., Cox C.J. and Embley T.M. (2013) An archaeal origin of eukaryotes supports only two primary domains of life. Nature 504, 231–236 doi: 10.1038/nature12779 [DOI] [PubMed] [Google Scholar]
- 224.Spang A., Saw J.H., Jørgensen S.L., Zaremba-Niedzwiedzka K., Martijn J., Lind A.E. et al. (2015) Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 521, 173–179 doi: 10.1038/nature14447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Embley T.M. and Martin W. (2006) Eukaryotic evolution, changes and challenges. Nature 440, 623–630 doi: 10.1038/nature04546 [DOI] [PubMed] [Google Scholar]
- 226.Stechmann A. and Cavalier-Smith T. (2002) Rooting the eukaryote tree by using a derived gene fusion. Science 297, 89–91 doi: 10.1126/science.1071196 [DOI] [PubMed] [Google Scholar]
- 227.Rogozin I.B., Basu M.K., Csürös M. and Koonin E.V. (2009) Analysis of rare genomic changes does not support the unikont-bikont phylogeny and suggests cyanobacterial symbiosis as the point of primary radiation of eukaryotes. Genome Biol. Evol. 1, 99–113 doi: 10.1093/gbe/evp011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Katz L.A., Grant J.R., Parfrey L.W. and Burleigh J.G. (2012) Turning the crown upside down: gene tree parsimony roots the eukaryotic tree of life. Syst. Biol. 61, 653–660 doi: 10.1093/sysbio/sys026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cavalier-Smith T. (2010) Kingdoms Protozoa and Chromista and the eozoan root of the eukaryotic tree. Biol. Lett. 6, 342–345 doi: 10.1098/rsbl.2009.0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Allen J.W.A., Jackson A.P., Rigden D.J., Willis A.C., Ferguson S.J. and Ginger M.L. (2008) Order within a mosaic distribution of mitochondrial c-type cytochrome biogenesis systems? FEBS J. 275, 2385–2402 doi: 10.1111/j.1742-4658.2008.06380.x [DOI] [PubMed] [Google Scholar]
- 231.Nishino M., Choy J.W., Gushwa N.N., Oses-Prieto J.A., Koupparis K., Burlingame A.L. et al. (2013) Hypothemycin, a fungal natural product, identifies therapeutic targets in Trypanosoma brucei. elife 2, e00712 doi: 10.7554/eLife.00712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Merritt C., Silva L.E., Tanner A.L., Stuart K. and Pollastri M.P. (2014) Kinases as druggable targets in trypanosomatid protozoan parasites. Chem. Rev. 114, 11280–11304 doi: 10.1021/cr500197d [DOI] [PMC free article] [PubMed] [Google Scholar]