Abstract
Purpose
To report the outcome using radiation therapy (RT) for pediatric patients with high grade spinal cord tumors.
Methods and Materials
A retrospective chart review was conducted that included 17 children with high-grade spinal cord tumors treated with RT at St. Jude Children’s Research Hospital between 1981 and 2007. Three patients had gross total resection, 11 had subtotal resection, and 3 underwent biopsy. The tumor diagnosis was glioblastoma multiforme (n = 7), anaplastic astrocytoma (n = 8), or anaplastic oligodendroglioma, (n = 2). Seven patients received craniospinal irradiation (34.2–48.6Gy). The median dose to the primary site was 52.2 Gy (range 38–66 Gy).
Results
The median progression-free and overall survivals were 10.8 and 13.8 months, respectively. Local tumor progression at 12 months (79% vs. 30%, p = 0.02) and median survival (13.1 vs. 27.2 months, p = 0.09) were worse for patients with glioblastoma multiforme compared to anaplastic astrocytoma or oligodendroglioma. The median overall survival was shorter for patients when failure included neuraxis dissemination (n=8) compared to local failure alone (n=5), 9.6 vs. 13.8 months, p = 0.08. Three long-term survivors with WHO grade III tumors were alive with follow-up ranging from 88–239 months.
Conclusions
High-grade spinal cord primary tumors in children have a poor prognosis. The propensity for neuraxis metastases as a component of progression after RT suggests the need for more aggressive therapy.
Keywords: Radiotherapy, Pediatrics, CNS Neoplasms, Spinal Cord Tumors
Introduction
Tumors arising in the spinal cord are uncommon, comprising less than 5% of all central nervous system (CNS) tumors. In adults, spinal tumors are often extramedullary and benign, whereas the majority of cases in children are intramedullary tumors and typically low-grade astrocytomas and ependymomas.1
High-grade spinal cord tumors (HGSCT) are even rarer, comprising less than 1% of all central nervous system tumors seen in children and adolescents. There are relatively few reported experiences describing the treatment of HGSCT in the pediatric population.2–6 Though there are no established guidelines for the treatment of these rare tumors, patients at St. Jude Children’s Research Hospital (SJCRH) have been consistently treated with maximal surgical resection followed by postoperative radiation therapy. In most cases, chemotherapy is also given, but the optimal type and method of delivering chemotherapy is not clear.
In this report we update the previously described institutional experience treating pediatric patients with HGSCT.7 The objective of this study is to provide a guideline for clinicians regarding treatment outcomes in these rare cases.
Materials and Methods
Patient Characteristics
Between March 1981 and October 2007, 17 patients with high-grade spinal cord tumors, according to the World Health Organization classification (WHO grade III-IV), were treated at St. Jude Children’s Research Hospital and LeBonheur Children’s Medical Center in Memphis, Tennessee. The median age of patients in this series was 11 years (range 3 – 23 years). There were 10 female and 7 male patients. The records of these patients were retrospectively reviewed, with approval from the institutional review board. Information obtained from these records included: diagnosis, date of birth, sex, race, date of histologic diagnosis, therapeutic intervention, relapse, death, and extent of disease at presentation and relapse, extent of surgery, use of chemotherapy, and doses and volumes of radiation therapy. The relevant clinical and treatment-related parameters are detailed in Tables 1 and 2.
Table 1.
Patient characteristics and outcomes.
| N | % | |
|---|---|---|
| Gender | ||
| Female | 10 | 59% |
| Male | 7 | 41% |
| Histology | ||
| AA | 8 | 47% |
| AO | 2 | 12% |
| GBM | 7 | 41% |
| Surgery | ||
| Biopsy | 3 | 18% |
| STR | 11 | 64% |
| GTR | 3 | 18% |
| Primary EBRT | ||
| CSI | 7 | 41% |
| Focal | 10 | 59% |
| Failure Type | ||
| None | 4 | 24% |
| Local only | 5 | 29% |
| Diffuse only | 2 | 12% |
| Local + diffuse | 6 | 35% |
| Leptomeningeal disease | ||
| None | 9 | 53% |
| At presentation | 4 | 24% |
| At failure | 4 | 24% |
| Intracranial disease | ||
| None | 10 | 59% |
| At presentation | 3 | 18% |
| At failure | 4 | 24% |
AA = anaplastic astrocytoma. AO = anaplastic oligodendroglioma. GBM = glioblastoma multiforme. STR = subtotal resection. GTR = gross total resection. CSI = craniospinal irradiation.
Table 2.
Individual clinical and treatment characteristics of pediatric patients with high-grade spinal cord tumors.
| Age, sex | Site | Histology | Surgery | Radiation (Gy) | Failure | Time to Failure |
|---|---|---|---|---|---|---|
| 12, M | T11-L1 (M3) | AO | STR | CSI 48.6 | Diffuse | 7 months |
| 9, F | T11-L2 | AA | STR | Focal 38 | Local + diffuse | 1 month |
| 23, F | C3-C5 | AO | GTR | Focal 50.4 | Local | 24 months |
| 11, F | C1-T1 | AA | GTR | Focal 50 | None | Stable 150 months |
| 5, F | C3-T2 (M2) | AA | BX | CSI 38, focal 48 | None | Stable 239 months |
| 10, M | C1-C2, T6-T8 (M2) | AA | STR | CSI 48.4, focal 66 | Local + diffuse | 2 months |
| 10, M | C2-T6 | AA | STR | Focal 54 | Local | 6 years |
| 11, F | C2-C7 | AA | STR | Focal 54 | Diffuse | 1 month |
| 18, F | C7-T7 | GBM | GTR | Focal 55 | Local | 10 months |
| 14, M | C7-T2 | GBM | STR | CSI 38.5 | Local | 4 months |
| 10, F | C3-T7 | GBM | BX | Focal 48 | Local + diffuse | 14 months |
| 17, M | C1-C5 | GBM | STR | Focal 54 | Local | 4 months |
| 13, F | C1-T1 | GBM | BX | Focal 54 | Local + diffuse | 1 month |
| 3, M | C3-T8 (M3) | AA | STR | CSI 34.2, focal 52.2 | None | Stable 88 months |
| 15, F | T10-T11 | GBM | STR | CSI 39.6, focal 51.3 | Local + diffuse | 1 month |
| 12, M | T12 | AA | STR | Focal 54 | Local + diffuse | 4 months |
| 9, F | T2-T7 | GBM | STR | CSI 36, focal 54 | None | Stable 10 months |
Surgery
All patients in this series initially underwent a biopsy or an attempt at resection. Three patients were considered to have had a gross total resection (GTR) of their primary tumor based on the impression of the operating surgeon and postoperative imaging. The resection was considered to be subtotal (STR) in 11 patients, and was characterized as a biopsy only in the remaining 3 patients.
Pathology and Extent of Disease at Diagnosis
Included in this study are patients with histologically confirmed primary high-grade tumors of the spinal cord: glioblastoma multiforme (GBM, WHO grade IV, n = 7), anaplastic astrocytoma (AA, WHO grade III, n = 8), and anaplastic oligodendroglioma (AO, WHO grade III, n = 2). Neuro-imaging was performed in all patients (computed tomography (CT) myelogram or magnetic resonance (MR) imaging). All primary lesions originated in the cervical or thoracic spine. Four patients had imaging evidence of metastatic or multi-focal disease at presentation. Four of 17 patients underwent cerebrospinal fluid evaluation, however, no patient had cytologic evidence of tumor dissemination at presentation.
Radiation Therapy and Chemotherapy
All patients received radiation therapy following surgery. Seven patients were initially treated with craniospinal irradiation (CSI) over a range of 34.2–48.6 Gy, followed by a boost to gross residual tumor, while ten patients received only local radiotherapy. One patient received CSI at the time of recurrence. The median dose to the primary site for all patients was 52.2 Gy (range 38–66 Gy). One patient received 66 Gy in 1.1 Gy twice daily fractions, while five patients received less than 50 Gy for a variety of clinical indications. Three patients received planned chemotherapy prior to irradiation, and 4 received concurrent chemotherapy with irradiation; otherwise chemotherapy was given only at the time of recurrence (n = 9).
Definitions of Endpoints
Progression-free survival (PFS) was measured from day 1 of radiotherapy to the time of imaging evidence of disease progression. Overall survival (OS) was measured from the date of histologic diagnosis to the date of death from any cause. Failures were classified as local, diffuse or combined, and calculated from the date of starting radiotherapy to the date of failure. Patients categorized as diffuse failures had imaging evidence of neuraxis metastases. In defining the extent of disease and location of failure, the following system was adopted: M1 - positive cerebrospinal fluid cytology, M2 - intracranial leptomeningeal disease, M3 - spinal leptomeningeal disease, and M4 - extra-neuraxis dissemination.
Statistical Analysis
Survival curves were computed to estimate PFS and OS using Kaplan-Meier methodology.8 The cumulative incidences of local and diffuse failures were calculated by Gray’s method.9
Results
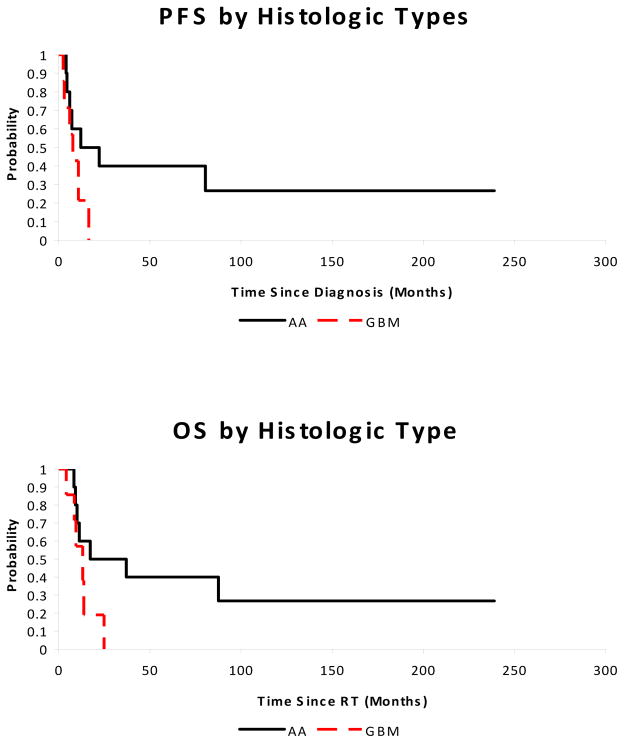
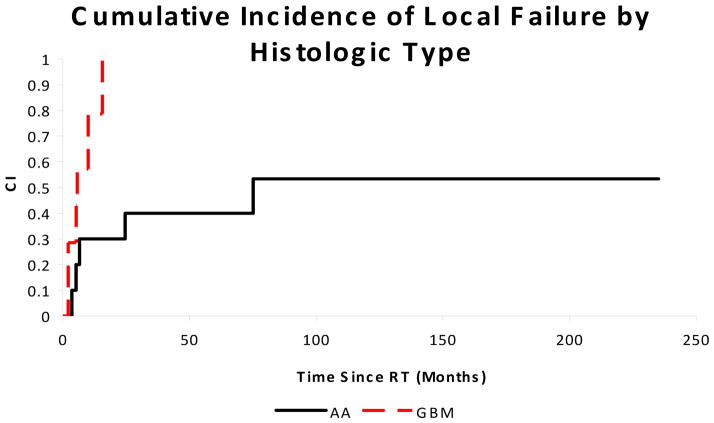
The median PFS and OS were 10.8 and 13.8 months, respectively. Patients with WHO grade IV tumors had shorter PFS (7.8 months vs. 17.2 months, p = 0.11) and OS (13.1 months vs. 27.2 months, p = 0.09) than those with WHO grade III tumors. The risk of local progression at 12 months was 79% for WHO grade IV tumors compared to 30% for WHO grade III tumors (p = 0.02). These results are shown in Figures 1 and 2. Factors such as gender, race, radiotherapy volume (local vs. CSI), extent of surgical resection, and presence of neuraxis dissemination at presentation did not significantly impact OS or PFS in this small cohort.
Figure 1.
Progression-free (p = 0.11) and overall survival (p = 0.09) for pediatric patients with high-grade spinal cord tumors by histologic subtype. AA = anaplastic astrocytoma or oligodendroglioma. GBM = glioblastoma multiforme.
Figure 2.
Cumulative incidence of local failure (p = 0.02) for pediatric patients with high-grade spinal cord tumors by histologic subtype. AA = anaplastic astrocytoma or oligodendroglioma. GBM = glioblastoma multiforme.
Four patients had neuraxis metastases at the time of presentation and four patients with initially localized tumor developed neuraxis dissemination at the time of failure. For the patients who had diffuse failure (n = 8), the median OS was 9.6 months compared to 13.8 months for those with local failure (p = 0.08). Seven patients had evidence of intracranial dissemination, either at diagnosis or at failure, which represented 41% of the cohort. Early treatment failures predicted for a shorter OS. Patients with neuroimaging evidence of progression less than 3 months from completion of radiotherapy had a median OS of 8.6 months, compared to 17.3 months for those who did not progress by 3 months (p = 0.0002).
Only three long-term survivors were noted, all with AA; a fourth survivor with GBM and without evidence of progression was only eight months post-therapy at the time of data analysis. These patients were alive 88, 150, and 239 months post-treatment. Their medical records were assessed for function and performance. The 150-month survivor was diagnosed with an AA at the age of 11 years at C1-C5 and received focal irradiation to 50 Gy following gross total resection. Despite being quadraparetic postoperatively, this patient was fully employed at last follow-up, and suffered only from loss of left hand fine motor function. The 239-month survivor was diagnosed with an AA at 5 years of age, located at C3-T2 with synchronous intracranial leptomeningeal dissemination. The patient received 4 courses of pre-irradiation cisplatin and etoposide, after which 38 Gy CSI was administered followed by a boost to the brain and primary site to 48 Gy. This patient continued to suffer from a seizure disorder that was present at initial diagnosis, and subsequently developed multiple endocrine deficiencies. The 88-month survivor was diagnosed at age 3 with an AA at C3-T8. He underwent subtotal resection, followed by CSI of 34.2 Gy, with a boost to the primary tumor to 52.2 Gy. He did not receive chemotherapy, and had no evidence of disease progression at last follow-up. However, post-therapeutic sequelae include cataracts, kyphoscoliosis, spastic paraparesis, and endocrine dysfunction.
Discussion
Spinal cord neoplasms in children are uncommon entities, and high-grade tumors in this population are found even more rarely. They typically arise in the cervical or thoracic cord and account for less than 1% of all cases of central nervous system tumors. It remains uncertain whether HGSCT in children are clinically or biologically similar to their adult counterparts. Most series describing the treatment of spinal cord tumors in children also include adult patients with low-grade astrocytoma and ependymoma.10–13 To our knowledge, our cohort is among the largest series specifically documenting the clinical outcomes of pediatric patients with HGSCT.
The rarity of pediatric HGSCT and short survival of these patients limits our ability to reliably identify prognostic factors that impact survival. Among primary spinal cord tumors, histological subtype and grade appear to be the most consistent predictive factors for outcome across many series.3, 10, 14–16 Grade IV spinal cord tumors have a dismal prognosis, and only a handful of reports in the literature have documented long-term survivors with spinal GBM.17 Our data is consistent with this observation, as there were no long-term survivors among our cohort of children with grade IV spinal tumors. Although the overall and progression-free survival outcomes did not reach statistical significance when stratified by histology, local failure was statistically worse for grade IV tumors than for grade III tumors. Of note, there were three long-term survivors with grade III tumors, however all experienced significant co-morbidities as a consequence of their disease.
Prognostic factors other than histological grade are not as well described in patients with primary spinal cord tumors. In our cohort, a short time to clinical failure did predict for poor OS. Patients who progressed within 3 months after radiotherapy had a significantly worse survival than those whose disease remained controlled at that time point. In other series, a shorter duration of symptoms before diagnosis was also predictive of a poor outcome.3
Although there is no well-defined standard therapy for HGSCT, maximal surgical resection and post-operative radiotherapy remains the most common treatment strategy. In a child presenting with an intramedullary spinal cord tumor, the very rapid progression of signs and symptoms alone may indicate malignant histology. After high resolution imaging of the entire neuraxis, the child should be taken to surgery with the limits of the tumor extension noted on MR confirmed with ultrasound prior to opening of the dura. Dorsal myelotomy should be performed over the length of the tumor, even if limited resection or biopsy are planned, to minimize post-surgical morbidity resulting from edema. At our institution, we advocate immediate biopsy and frozen section and in the case of malignant tumors, very judicious resection removing only frankly obvious abnormal tissue. Because malignant tumors are infiltrative, radical resection leads to permanent post-operative deficits.12 Because the extent of surgical resection appears to be an unreliable prognostic factor across many series, there is no proven advantage to radical resection. In thoracic tumors, there may be a temptation to perform a cordectomy, especially at the time of tumor progression, in those with no evidence of metastatic disease. Fred Epstein, an experienced spinal cord tumor surgeon, reported a series of malignant spinal cord tumors.18 Cordectomy was performed three times and was unsuccessful in all three (personal communication). We have not performed cordectomy at our institution.
Despite aggressive local treatment, both local and distant leptomeningeal failures are common. It has been noted that high-grade tumors of the spinal cord, especially GBM, are associated with a high rate of ultimate leptomeningeal dissemination, up to 58% in some series and 47% in our cohort.4, 15, 18–21 This rate of neuraxis dissemination with HGSCT appears to be higher than that observed with high-grade astrocytomas arising in the brain, which occurs in approximately 5–10% at diagnosis.22–24 It is felt that this discordant rate of leptomeningeal dissemination may be related to the closer anatomic proximity of spinal tumors to the subarachnoid space compared to their intracranial counterparts.
Patients with documented leptomeningeal disease at diagnosis are generally candidates for craniospinal irradiation, but it is unclear whether prophylactically treating such an extensive volume should be considered in HGSCT patients without radiographic evidence of distant spread. Current imaging techniques to evaluate dissemination of disease prior to radiation therapy may in fact underestimate the actual extent of disease. However, in our series, 6 of the 8 diffuse failures progressed locally in addition to regions beyond the primary site, therefore, improved definition of the extent of disease may not be sufficient to improve outcome. The ability to identify occult disease impacts radiotherapeutic management and the potential to achieve disease control. Unfortunately, the discrepancy between the dose that can be delivered to the neuraxis with acceptable toxicity and the dose required to sterilize even microscopic amounts of a high-grade glial tumor ultimately results in disseminated failures in a large proportion of patients. It seems appropriate that the radiotherapy dose and volume should be determined on an individual basis.
It also remains unclear whether GBM arising in the spinal cord are biologically analogous to those arising in the brain. Although spinal GBM and intracranial GBM have different clinical courses, it is felt that they are histologically identical entities. Despite aggressive surgery and post-operative radiotherapy, the poor outcomes of HGSCT patients suggest that alternative modalities should be investigated. Given the lack of prospective evidence in this rare disease, it is reasonable to take treatment strategies that were successful in patients with intracranial GBM and apply them to children with spinal GBM. Unfortunately, attempts at improving local control in intracranial GBM by radiation dose escalation, brachytherapy, radiosensitizers, and altered fractionation have yielded no improvements over standard therapy.25–29
However, chemotherapy has recently been established as a standard of care in adult patients with intracranial GBM. A randomized trial demonstrated that the addition of concurrent and adjuvant temozolomide to radiotherapy improves both OS and PFS in adults with GBM.30 Temozolomide, an oral alkylating agent with penetration of the blood-brain-barrier, is being investigated in the pediatric population.31–33 There is strong evidence that adults who exhibit methylation of the methylguanine-DNA methyltransferase (MGMT) promoter have a better outcome with alkylating agents such as temozolomide, and the same appears to be true for children.34–36 Analyzing molecular factors such as MGMT status may help guide therapy and predict those who are predisposed to a more favorable outcome with combined radiotherapy and chemotherapy. The role of intra-thecal chemotherapy in the treatment of HGSCT is undefined. Likewise, targeted therapies such as inhibitors of the epidermal growth factor receptor or the vascular endothelial growth factor receptor remain investigational.
In conclusion, primary high-grade spinal tumors in children are very rare and carry a poor prognosis. The role of prophylactic craniospinal irradiation remains unclear, as a high proportion of patients die with both local failure and distant leptomeningeal dissemination despite aggressive therapy. The dose of radiotherapy that can be successfully delivered to the neuraxis is limited by the tolerance of surrounding normal spinal cord parenchyma and by the intrinsic resistance of malignant glioma cells. Further investigation into the optimal combination of surgery, radiation, chemotherapy, and targeted therapy is warranted. However, the rarity of this disease makes prospective evaluation difficult to perform in this population.
Acknowledgments
Supported in part by Cancer Center Support CORE Grant, P30 CA 21765 and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflicts of Interest Notification:
No actual or potential conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minehan KJ, Brown PD, Scheithauer BW, et al. Prognosis and Treatment of Spinal Cord Astrocytoma. International Journal of Radiation Oncology*Biology*Physics. 2009;73:727–733. doi: 10.1016/j.ijrobp.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 2.Constantini S, Houten J, Miller DC, et al. Intramedullary spinal cord tumors in children under the age of 3 years. J Neurosurg. 1996;85:1036–1043. doi: 10.3171/jns.1996.85.6.1036. [DOI] [PubMed] [Google Scholar]
- 3.Bouffet E, Pierre-Kahn A, Marchal J, et al. Prognostic factors in pediatric spinal cord astrocytoma. Cancer. 1998;83:2391–2399. doi: 10.1002/(sici)1097-0142(19981201)83:11<2391::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Ciappetta P, Salvati M, Capoccia G, et al. Spinal glioblastomas: report of seven cases and review of the literature. Neurosurgery. 1991;28:302–306. [PubMed] [Google Scholar]
- 5.DeSousa AL, Kalsbeck JE, Mealey J, Jr, et al. Intraspinal tumors in children. A review of 81 cases. J Neurosurg. 1979;51:437–445. doi: 10.3171/jns.1979.51.4.0437. [DOI] [PubMed] [Google Scholar]
- 6.Allen JC, Aviner S, Yates AJ, et al. Treatment of high-grade spinal cord astrocytoma of childhood with “8-in-1” chemotherapy and radiotherapy: a pilot study of CCG-945. Children’s Cancer Group. J Neurosurg. 1998;88:215–220. doi: 10.3171/jns.1998.88.2.0215. [DOI] [PubMed] [Google Scholar]
- 7.Merchant TE, Nguyen D, Thompson SJ, et al. High-grade pediatric spinal cord tumors. Pediatr Neurosurg. 1999;30:1–5. doi: 10.1159/000028751. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan ES, Meier P. Non-parametric estimations from incomplete observations. Am Stat Assoc J. 1958;53:457–482. [Google Scholar]
- 9.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1140–1154. [Google Scholar]
- 10.Jyothirmayi R, Madhavan J, Nair MK, et al. Conservative surgery and radiotherapy in the treatment of spinal cord astrocytoma. J Neurooncol. 1997;33:205–211. doi: 10.1023/a:1005758313700. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin MP, Marcus RB, Jr, Buatti JM, et al. Ependymoma: results, prognostic factors and treatment recommendations. Int J Radiat Oncol Biol Phys. 1998;40:845–850. doi: 10.1016/s0360-3016(97)00893-6. [DOI] [PubMed] [Google Scholar]
- 12.Minehan KJ, Shaw EG, Scheithauer BW, et al. Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg. 1995;83:590–595. doi: 10.3171/jns.1995.83.4.0590. [DOI] [PubMed] [Google Scholar]
- 13.Shirato H, Kamada T, Hida K, et al. The role of radiotherapy in the management of spinal cord glioma. Int J Radiat Oncol Biol Phys. 1995;33:323–328. doi: 10.1016/0360-3016(95)00179-3. [DOI] [PubMed] [Google Scholar]
- 14.Linstadt DE, Wara WM, Leibel SA, et al. Postoperative radiotherapy of primary spinal cord tumors. Int J Radiat Oncol Biol Phys. 1989;16:1397–1403. doi: 10.1016/0360-3016(89)90940-1. [DOI] [PubMed] [Google Scholar]
- 15.Kopelson G, Lingood RM. Intramedullary spinal cord astrocytoma versus glioblastoma: the prognostic importance of histologic grade. Cancer. 1982;50:732–735. doi: 10.1002/1097-0142(19820815)50:4<732::aid-cncr2820500418>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Wahab M, Etuk B, Palermo J, et al. Spinal cord gliomas: A multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys. 2006;64:1060–1071. doi: 10.1016/j.ijrobp.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 17.Caroli E, Salvati M, Ferrante L. Spinal glioblastoma with brain relapse in a child: clinical considerations. Spinal Cord. 2005;43:565–567. doi: 10.1038/sj.sc.3101749. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AR, Wisoff JH, Allen JC, et al. Malignant astrocytomas of the spinal cord. J Neurosurg. 1989;70:50–54. doi: 10.3171/jns.1989.70.1.0050. [DOI] [PubMed] [Google Scholar]
- 19.Johnson DL, Schwarz S. Intracranial metastases from malignant spinal-cord astrocytoma. Case report. J Neurosurg. 1987;66:621–625. doi: 10.3171/jns.1987.66.4.0621. [DOI] [PubMed] [Google Scholar]
- 20.Andrews AA, Enriques L, Renaudin J, et al. Spinal intramedullary glioblastoma with intracranial seeding. Report of a case. Arch Neurol. 1978;35:244–245. doi: 10.1001/archneur.1978.00500280062013. [DOI] [PubMed] [Google Scholar]
- 21.Asano N, Kitamura K, Seo Y, et al. Spinal cord glioblastoma multiforme with intracranial dissemination--case report. Neurol Med Chir (Tokyo) 1990;30:489–494. doi: 10.2176/nmc.30.489. [DOI] [PubMed] [Google Scholar]
- 22.Heideman RL, Kuttesch J, Jr, Gajjar AJ, et al. Supratentorial malignant gliomas in childhood: a single institution perspective. Cancer. 1997;80:497–504. doi: 10.1002/(sici)1097-0142(19970801)80:3<497::aid-cncr18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.Benesch M, Wagner S, Berthold F, et al. Primary dissemination of high-grade gliomas in children: experiences from four studies of the Pediatric Oncology and Hematology Society of the German Language Group (GPOH) J Neurooncol. 2005;72:179–183. doi: 10.1007/s11060-004-3546-5. [DOI] [PubMed] [Google Scholar]
- 24.Finlay JL, Boyett JM, Yates AJ, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group. J Clin Oncol. 1995;13:112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 25.Laperriere NJ, Leung PM, McKenzie S, et al. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1998;41:1005–1011. doi: 10.1016/s0360-3016(98)00159-x. [DOI] [PubMed] [Google Scholar]
- 26.Selker RG, Shapiro WR, Burger P, et al. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002;51:343–355. discussion 355–347. [PubMed] [Google Scholar]
- 27.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Werner-Wasik M, Scott CB, Nelson DF, et al. Final report of a phase I/II trial of hyperfractionated and accelerated hyperfractionated radiation therapy with carmustine for adults with supratentorial malignant gliomas. Radiation Therapy Oncology Group Study 83-02. Cancer. 1996;77:1535–1543. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1535::AID-CNCR17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Chang CH, Horton J, Schoenfeld D, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52:997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 31.Broniscer A, Chintagumpala M, Fouladi M, et al. Temozolomide after Radiotherapy for Newly Diagnosed High-grade Glioma and Unfavorable Low-grade Glioma in Children. Journal of Neuro-Oncology. 2006;76:313–319. doi: 10.1007/s11060-005-7409-5. [DOI] [PubMed] [Google Scholar]
- 32.Barone G, Maurizi P, Tamburrini G, et al. Role of temozolomide in pediatric brain tumors. Childs Nerv Syst. 2006;22:652–661. doi: 10.1007/s00381-006-0081-z. [DOI] [PubMed] [Google Scholar]
- 33.Loh KC, Willert J, Meltzer H, et al. Temozolomide and radiation for aggressive pediatric central nervous system malignancies. J Pediatr Hematol Oncol. 2005;27:254–258. doi: 10.1097/01.mph.0000162528.79186.fe. [DOI] [PubMed] [Google Scholar]
- 34.Donson AM, Addo-Yobo SO, Handler MH, et al. MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr Blood Cancer. 2007;48:403–407. doi: 10.1002/pbc.20803. [DOI] [PubMed] [Google Scholar]
- 35.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 36.Pollack IF, Hamilton RL, Sobol RW, et al. O6-Methylguanine-DNA Methyltransferase Expression Strongly Correlates With Outcome in Childhood Malignant Gliomas: Results From the CCG-945 Cohort. J Clin Oncol. 2006;24:3431–3437. doi: 10.1200/JCO.2006.05.7265. [DOI] [PubMed] [Google Scholar]