Abstract
PURPOSE
The 21-gene recurrence score (RS) assay is prognostic in estrogen receptor-positive (HR+), HER2 negative, node negative breast cancer (BC). The interaction between RS and host factors including metabolic syndrome (MS) is unclear. MS conditions such as obesity have been associated with worse BC prognosis. The aim of this study was to identify associations between presence of MS conditions and RS group or breast cancer recurrence.
METHODS
Demographic, pathologic and treatment data, MS criteria and menopausal status were abstracted from medical records of women with stage I–II, HR+, HER2 negative BC evaluated with the RS assay at a single institution since 2005. MS was defined as presence of ≥3 of the following within 2 years of diagnosis: body mass index ≥27.7 kg/m2; hypertension; impaired fasting glucose; HDL <50mg/dL; hypertriglyceridemia.
RESULTS
Of 533 eligible women, 22% had MS. MS was more common in post- vs pre-menopausal women (30% vs 9%; p<0.0001). There was no significant association between RS group and overall MS status or any individual criterion, controlling for stage, and no association after stratification by menopausal status. Postmenopausal status was associated with higher RS group (p=0.039), independent of stage. With 4.2 year median follow-up, no association between disease recurrence and MS was identified.
CONCLUSIONS
Although MS has been associated with worse BC outcomes, we were unable to identify associations between RS group and MS criteria. Identification of prognostic factors other than RS that underlie this higher risk will be important for optimizing breast cancer treatment-decision making in patients with MS.
Keywords: recurrence score, metabolic syndrome, obesity, breast cancer
INTRODUCTION
In early stage hormone receptor (HR) positive breast cancer, the benefit from chemotherapy in addition to endocrine therapy is uncertain. In addition to standard pathologic assessment, tumor gene expression is being increasingly used to better understand tumor biology. Integration of both anatomic and biologic tumor factors has allowed for improvement in the ability to predict recurrence and guide treatment decisions in early stage breast cancer. The 21-gene recurrence score (RS) assay (OncotypeDX) has been validated to be prognostic for breast cancer recurrence in HR positive, HER2 negative, node negative breast cancer [1–3]. Patients with low risk RS (0–17) are generally recommended to receive endocrine therapy with tamoxifen or an aromatase inhibitor without chemotherapy. Those with high RS (31–100) benefit from both chemotherapy and endocrine therapy. Intermediate risk patients (RS 18–30) are also recommended to have endocrine therapy but the additional benefit from chemotherapy is less clear. More recently the RS assay was evaluated retrospectively for use in patients with node positive breast cancer [4]. As with node negative breast cancer, those with node positive disease and low RS were found to have minimal benefit from chemotherapy, whereas those with high RS demonstrated significant reduction in risk of recurrence with chemotherapy.
Metabolic syndrome is a constellation of pathologic disorders involving energy metabolism. Metabolic syndrome and its comprising conditions of central obesity, diabetes, hyperlipidemia and hypertension (HTN) are rising epidemics in our society. While studies have associated obesity with increased incidence and poorer prognosis of breast cancer it is unclear how other MS conditions, individually and in combination, affect risk of BC development or prognosis. It is also uncertain whether the 21-gene RS assay accurately predicts recurrence in patients with metabolic syndrome. One study showed a higher risk of recurrence in metabolic syndrome within the low RS group [5]. It has been proposed that metabolic syndrome creates a complex biochemical milieu with increased levels of estrogen, adipokines and inflammatory cytokines IL-6 and TNFα, which have been implicated in tumorigenesis and metastasis.
For this study, we hypothesized that the 21-gene RS assay may not fully capture the proposed higher risk tumor microenvironment in metabolic syndrome and thus may underestimate the actual risk of breast cancer recurrence within this population. In order to investigate this hypothesis we performed a retrospective analysis of patients who underwent testing with the RS assay at a single institution since 2005. Our goal was to investigate the association between metabolic syndrome conditions, both individually and in combination, and the 21-gene RS, as well as the risk of breast cancer recurrence, metastasis and death.
METHODS
Patients, Metabolic Syndrome Criteria Variables and Outcomes
We retrospectively reviewed data from 534 women with stage I-II, HR positive, HER2 negative breast cancer treated at the University of Michigan since 2005 and who had Oncotype DX testing performed. Data collected from the Michigan Breast Oncology Quality Initiative database and the University of Michigan Tumor Registry included demographic, pathologic, Recurrence Score (RS) and treatment data. Metabolic syndrome criteria and menopausal status at the time of diagnosis were abstracted from the University of Michigan electronic medical records (MiChart and CareWeb) with the assistance of the EMERSE search engine [6]. Institutional Review Board approval was obtained for this analysis, including a waiver of informed consent.
Metabolic syndrome was defined, based on a modification of the Adult Treatment Panel III (ATPIII) criteria [7], as having any 3 or more of the following: body mass index (BMI) ≥ 27.7 kg/m2; HTN ≥ 130/85 mmHg on at least 3 clinic visits or anti-hypertensive medication use; hemoglobin A1c>=5.7 or insulin, metformin or other hypoglycemic medication use; HDL < 50 mg/dL; triglycerides ≥ 150 mg/dL. Modifications from ATPIII include the use of BMI ≥ 27.7 as a surrogate for elevated waist circumference ≥ 88 cm and the use of hemoglobin A1c ≥ 5.7 as a surrogate for elevated fasting plasma glucose ≥ 100 mg/dl. The relevant laboratory and clinical data used to determine each MS criteria were limited to a window encompassing two years before and two years after breast cancer diagnosis date, and data available closest to diagnosis date was preferred and used for statistical analysis. BMI was obtained at the date of diagnosis. In cases without available data the corresponding metabolic syndrome criteria was counted as negative. Outcomes of interest were secondary breast cancer event (SBCE), including local recurrence, secondary breast cancer, or metastasis, or death from any cause. The follow-up time was time to SBCE, death, or last clinic visit at the University of Michigan.
Statistical Analysis
Associations between menopausal status and metabolic syndrome and the number of metabolic syndrome criteria and RS group were assessed using Chi-Square or Fisher’s exact tests. Associations between RS group and other categorical variables were assessed univariably using Chi-Square or Fisher’s exact tests and multivariably using multinomial logistic regression, controlling for stage of disease. Continuous RS were assessed by clinical and disease categorical characteristics using Wilcoxon rank sum tests. Time to a SBCE was compared by MS status using the Kaplan-Meier method and log-rank test and a Cox proportional hazards model controlling for age and stage.
RESULTS
Patients and Disease Characteristics
There were 534 women with stage I-II, HR+, HER2- breast cancer. One patient was excluded from analysis since she had bariatric surgery with substantial change in weight and resolution of diabetes, hypertension and hypertriglyceridemia just prior to diagnosis (Online Resource 1). At the time of diagnosis 74% of women had Stage I disease, 61% were postmenopausal, and the mean age was 56 years old (standard deviation (SD) 9.7 years) (Table 1).
Table 1.
Summary of baseline patient demographic, pathologic and metabolic syndrome criteria.
| Characteristic | N | % |
|---|---|---|
| Age, mean (SD) | 55.9 (9.7) | |
| Height (centimeters), mean (SD) | 163.0 (7.0) | |
| Weight at diagnosis (kilograms), mean (SD) | 75.3 (18.2) | |
| Body mass index, mean (SD) | 28.5 (7.0) | |
| 21- gene Recurrence Score, mean (SD) | 17.9 (9.1) | |
| Follow-up Time (years), mean (SD) | 4.4 (2.3) | |
| Stage | ||
| I | 392 | 73.5 |
| II | 141 | 26.5 |
| Estrogen Receptor Status | ||
| Positive | 531 | 99.6 |
| Negative | 2 | 0.4 |
| Progesterone Receptor Status | ||
| Positive | 481 | 90.2 |
| Negative | 50 | 9.4 |
| Unknown | 2 | 0.4 |
| Menopausal Status | ||
| Pre | 204 | 38.3 |
| Post | 324 | 60.8 |
| Unknown | 5 | 0.9 |
| Charlson Comorbidity Index | ||
| 1 | 499 | 93.6 |
| 2 | 21 | 3.9 |
| 3 | 12 | 2.3 |
| Unknown | 1 | 0.2 |
| Path Stage T | ||
| T1a | 10 | 1.9 |
| T1b | 127 | 23.8 |
| T1c | 292 | 54.8 |
| T2 | 104 | 19.5 |
| Path Stage N | ||
| N0 | 477 | 89.5 |
| N1 | 55 | 10.3 |
| Unknown | 1 | 0.2 |
| Body Mass Index Criteria | ||
| No | 303 | 56.8 |
| Yes | 230 | 43.2 |
| Hypertension Criteria | ||
| No | 312 | 58.5 |
| Yes | 221 | 41.5 |
| Triglycerides Criteria | ||
| No | 350 | 65.7 |
| Yes | 183 | 34.3 |
| HDL Criteria | ||
| No | 463 | 86.9 |
| Yes | 70 | 13.1 |
| Diabetes or Impaired Glucose Tolerance Criteria | ||
| No | 458 | 85.9 |
| Yes | 75 | 14.1 |
| Metabolic Syndrome (≥3 of 5 criteria) | ||
| No | 416 | 78.0 |
| Yes | 117 | 22.0 |
| Number of Metabolic Syndrome Criteria | ||
| 0 | 164 | 30.8 |
| 1 | 146 | 27.4 |
| 2 | 106 | 19.9 |
| 3 | 67 | 12.6 |
| 4 | 30 | 5.6 |
| 5 | 20 | 3.8 |
| Chemotherapy | ||
| No | 391 | 73.4 |
| Yes | 142 | 26.6 |
| Endocrine Therapy | ||
| No | 27 | 5.1 |
| Yes | 506 | 94.9 |
Abbreviations: HDL, high density lipoprotein, SD, standard deviation
Prevalence of Metabolic Syndrome Conditions
Of the metabolic syndrome conditions, elevated BMI was most commonly identified (43%), followed by hypertension (41%), hypertriglyceridemia (34%), impaired fasting glucose (14%) and low HDL (13%). Metabolic syndrome, defined as three of more conditions, was seen in 22% of women. Postmenopausal women had a 4.1 greater odds of having MS than premenopausal women (95% confidence interval (CI) 2.4–7.0, P < 0.0001).
Association of Metabolic Syndrome with the 21- gene Recurrence Score
Most patients had low (55%) or intermediate (38%) recurrence scores, with a mean 21-gene recurrence score of 18 (SD 9.1). Controlling for stage, postmenopausal status was associated with a significantly higher RS group than premenopausal status (P = 0.039; odds ratio (OR) of high vs low RS 2.7, 95% CI 1.2–6.2 for postmenopausal vs premenopausal women; OR of intermediate vs low RS 1.3, 95% CI 0.9–1.8 for postmenopausal vs premenopausal women) (Table 2). There was no difference in the distribution of RS groups between women with versus without MS (low: 54% vs. 55%, intermediate: 41% vs. 37%, high 5% vs. 8%; P = 0.55) or based on any individual MS condition. When stratified by menopausal status there were also no significant associations between RS group and any MS criteria. An analysis of obesity without MS versus MS did not reveal any statistically significant associations (Online Resource 2).
Table 2.
Associations between Recurrence Score group and individual metabolic syndrome (MS) conditions.
| Recurrence Score Group | |||||||
|---|---|---|---|---|---|---|---|
| Variable |
Low (N=292) |
Intermediate (N=203) |
High (N=38) |
P value | |||
| Number of MS Criteria | N | % | N | % | N | % | |
| 0 | 99 | 33.9 | 52 | 25.6 | 13 | 34.2 | 0.66 |
| 1 | 74 | 25.3 | 60 | 29.6 | 12 | 31.6 | |
| 2 | 56 | 19.2 | 43 | 21.2 | 7 | 18.4 | |
| 3 | 33 | 11.3 | 29 | 14.3 | 5 | 13.2 | |
| 4 | 19 | 6.5 | 11 | 5.4 | 0 | 0 | |
| 5 | 11 | 3.8 | 8 | 3.9 | 1 | 2.6 | |
| BMI Criteria | |||||||
| No | 167 | 57.2 | 112 | 55.2 | 24 | 63.2 | 0.65 |
| Yes | 125 | 42.8 | 91 | 44.8 | 14 | 36.8 | |
| Hypertension Criteria | |||||||
| No | 179 | 61.3 | 109 | 53.7 | 24 | 63.2 | 0.20 |
| Yes | 113 | 38.7 | 94 | 46.3 | 14 | 36.8 | |
| Triglycerides Criteria | |||||||
| No | 189 | 64.7 | 133 | 65.5 | 28 | 73.7 | 0.55 |
| Yes | 103 | 35.3 | 70 | 34.5 | 10 | 26.3 | |
| HDL Criteria | |||||||
| No | 260 | 89.0 | 169 | 83.3 | 34 | 89.5 | 0.15 |
| Yes | 32 | 11.0 | 34 | 16.7 | 4 | 10.5 | |
| Diabetes or Impaired Glucose Criteria | |||||||
| No | 249 | 85.3 | 175 | 86.2 | 34 | 89.5 | 0.77 |
| Yes | 43 | 14.7 | 28 | 13.8 | 4 | 10.5 | |
| Metabolic Syndrome (≥3 criteria) | |||||||
| No | 229 | 78.4 | 155 | 76.4 | 32 | 84.2 | 0.55 |
| Yes | 63 | 21.6 | 48 | 23.6 | 6 | 15.8 | |
| Menopausal Status* | |||||||
| Pre | 122 | 41.8 | 74 | 36.5 | 8 | 21.1 | 0.033 |
| Post | 167 | 57.2 | 127 | 62.6 | 30 | 78.9 | |
Abbreviations: BMI, body mass index; HDL, high density lipoprotein.
5 missing menopausal status, n=528
Association of Metabolic Syndrome with Outcomes
Women were followed for a mean of 4.4 years (SD 2.3). During this time within our 533 patient cohort there were a total of 24 events. There were 20 secondary breast cancer events (SBCE), which included 5 ductal carcinoma in situ, 3 local recurrences, 1 second primary breast cancer and 11 distant recurrences. There were 7 deaths from any cause, 3 of which were preceded by a SBCE. There was no significant difference between the time to SBCE based on MS status univariably (hazard ratio 0.6, 95% CI 0.2–1.7; P = 0.30) or multivariably (hazard ratio 0.5, 95% CI 0.2–1.7; P = 0.30), controlling for age and stage (Figure 1).
Figure 1. Survival based on metabolic syndrome (MS) status.
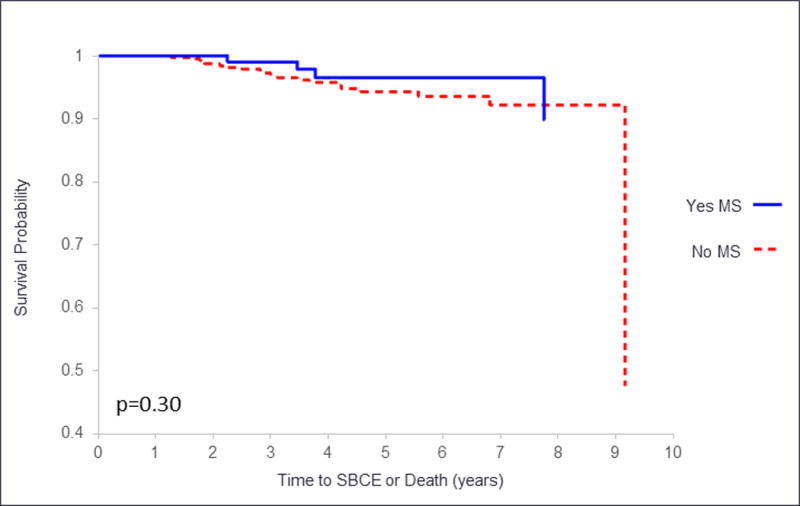
Time to second breast cancer event (SBCE) or death is given on the x axis. The solid line represents survival of those with MS, and the dashed line represents survival of those without MS.
DISCUSSION
While several studies have demonstrated that obesity and other metabolic syndrome conditions are associated with an increased incidence of breast cancer [8–19], few studies have explored the associations with risk of breast cancer recurrence. In our study, we demonstrated a fairly high prevalence of MS in our cohort of patients with breast cancer, as well as an association between MS status and menopausal status.
We were unable to identify an association between risk of recurrence as determined by RS and the presence of MS. Other reports have similarly demonstrated a lack of association between RS and both obesity [20] and overall MS status, however our study is the first to show this for all individual MS conditions.
Despite prior studies showing worse breast cancer prognosis with metabolic syndrome, our study was unable to demonstrate a significant difference in incidence of or time to SBCE or mortality based on MS status or individual criteria. This is in contrast to a previously reported study which demonstrated an association between increased incidence of SBCE and metabolic syndrome status specifically in the low RS risk group. Our inability to demonstrate a similar association between recurrence events and the presence of MS may be due to the overall low events in the relatively short follow-up time. Further validation studies to assess a difference in recurrence and survival among those with MS conditions based on RS group could have important prognostic and treatment implications.
Strengths of our study include analysis of a large cohort of patients with a high prevalence of MS followed for up to 10 years, including the collection of data on individual conditions comprising metabolic syndrome in addition to metabolic syndrome as a whole. Additionally, the classification of patients as having individual metabolic syndrome conditions was made thorough a detailed chart review including clinical notes, laboratory studies and medication use at the time of diagnosis rather than relying solely on medical record billing code diagnoses, which may lead to under-reporting of diagnoses.
Even though we examined a cohort of patients with a mean follow-up of 4.4 years and a prevalence of metabolic syndrome of 22%, our study was limited by the low overall event rate of 20 SBCE and 7 deaths (of which 3 were preceded by breast cancer recurrence). The excellent prognosis of this cohort of women with hormone sensitive, HER2 negative breast cancer and primarily node-negative disease is superior to the 89% 5-year recurrence rate reported by the Oxford Overview for patients with ER-positive, node negative disease [21].
Our study is also limited by the definition of metabolic syndrome, which is an issue that affects all retrospective studies examining metabolic syndrome factors [22, 23]. We used a modification of the established Adult Treatment Panel III criteria for metabolic syndrome [7] which may result in incorrect categorization of some patients. Laboratory and clinical data used to determine MS criteria were obtained closest to diagnosis date, however data did span a window within two years of diagnosis date based on limitations in availability in our retrospective review of the electronic medical record. Thus we are unable to determine whether all components of MS were present at the exact time of diagnosis. In addition, we were unable to take into account variation in the status of patients’ metabolic syndrome criteria over the study period. There may be important effects of adjuvant therapy or alternative medication or lifestyle changes that altered MS criteria following diagnosis that were not captured in our study. Changes in MS conditions may play an important role in their proposed mechanism of interaction with the tumor microenvironment. It is hypothesized that metabolic syndrome invokes a pro-inflammatory state that may alter tumor aggressiveness or metastatic potential. Thus it will be important to consider the effect of medications or lifestyle changes which may also alter cytokine stimulation, inflammation and insulin resistance. For example, we were unable to account for potential differences between patients with well-controlled versus poorly managed hypertension or diabetes mellitus during the follow-up period. In addition, there are emerging data on the anti-inflammatory effects of medications including metformin and aspirin that suggest they may result in reduction in breast cancer recurrence [24, 25]; the effect of metformin is currently being prospectively evaluated [25, 26]. Another area of investigation is the impact of dietary and weight loss interventions on reducing BC recurrence [27–30].
It is unclear whether the presence of MS impacts BC recurrence directly and/or via response to therapy. Some studies have suggested that obesity negatively impacts endocrine therapy or chemotherapy response due to underdosing [8]. Additionally patients with MS may have associated comorbidities such as renal dysfunction or cardiovascular complications; these comorbidities may impact the decision to pursue chemotherapy as opposed to endocrine therapy alone, the duration of treatment, tolerability and overall outcomes. However these factors this would only impact the minority of patients with intermediate or high risk RS who would typically be considered for chemotherapy.
Current evidence has associated metabolic syndrome and comprising conditions with increased risk of breast cancer recurrence. Our study adds to the suggestion that the increased risk of disease recurrence associated with metabolic syndrome may not be adequately captured by the 21-gene RS. This highlights the importance of considering host factors including metabolic syndrome along with tumor gene expression when determining BC prognosis and for making treatment decisions.
Studies are beginning to reveal the complex biochemical interplay involving insulin resistance and associated inflammatory cytokines, which has led to clinical trials examining pharmacologic or non-pharmacologic interventions. Understanding more about the modifiable risk factors for breast cancer recurrence may lead to improvements in patient management following diagnosis. Lifestyle interventions targeting weight loss and glycemic control as well as optimization of cholesterol profiles and blood pressure may prove to be important and previously underappreciated adjuncts to our endocrine and chemotherapies in breast cancer.
Supplementary Material
Acknowledgments
NLH was supported in part by a National Cancer Institute Clinical Cancer Investigator Team Leadership Award (supplement to 3-P30-CA046592, PI M Wicha).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
No authors had any conflicts of interest to disclose.
References
- 1.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE, Dees EC, Perez EA, Olson JA, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Jr, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. doi: 10.1016/S1470-2045(09)70314-6S1470-2045(09)70314-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakhani A, Guo A, Duan X, Ersahin C, Gaynor ER, Godellas C, Kay C, Lo SS, Mai H, Perez C, Albain K, Robinson P. Metabolic syndrome and recurrence within the 21-gene recurrence score assay risk categories in lymph node negative breast cancer. Cancer Res. 2012;72(24 Supplement) Abstract PD10-02. [Google Scholar]
- 6.Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: A report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE) Journal of Biomedical Informatics. 2015;55:290–300. doi: 10.1016/j.jbi.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 8.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 9.Chlebowski RT. Obesity and breast cancer outcome: adding to the evidence. J Clin Oncol. 2012;30:126–128. doi: 10.1200/JCO.2011.39.7877. [DOI] [PubMed] [Google Scholar]
- 10.Ligibel JA, Goodwin PJ. NEW and RENEW: building the case for weight loss in breast cancer. J Clin Oncol. 2012;30:2294–2296. doi: 10.1200/JCO.2012.42.5496. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Chen CM, Zhou Y, Zhou RJ, Yu KD, Shao ZM. Obesity or overweight is associated with worse pathological response to neoadjuvant chemotherapy among Chinese women with breast cancer. PLoS One. 2012;7:e41380. doi: 10.1371/journal.pone.0041380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewertz M, Gray KP, Regan MM, Ejlertsen B, Price KN, Thürlimann B, Bonnefoi H, Forbes JF, Paridaens RJ, Rabaglio M, Gelber RD, Colleoni M, Láng I, Smith IE, Coates AS, Goldhirsch A, Mouridsen HT. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the Breast Iinternational Group 1–98 trial. J Clin Oncol. 2012;30:3967–3975. doi: 10.1200/JCO.2011.40.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 14.Loi S, Milne RL, Friedlander ML, McCredie MR, Giles GG, Hopper JL, Phillips KA. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686–1691. doi: 10.1158/1055-9965.EPI-05-0042. [DOI] [PubMed] [Google Scholar]
- 15.Healy LA, Ryan AM, Carroll P, Ennis D, Crowley V, Boyle T, Kennedy MJ, Connolly E, Reynolds JV. Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol (R Coll Radiol) 2010;22:281–288. doi: 10.1016/j.clon.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Bhandari R, Kelley GA, Hartley TA, Rockett IR. Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. Int J Breast Cancer. 2014;2014:189384. doi: 10.1155/2014/189384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Rafaniello C, Giugliano D. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013;20:1301–1309. doi: 10.1097/GME.0b013e31828ce95d. [DOI] [PubMed] [Google Scholar]
- 18.Rosato V, Bosetti C, Talamini R, Levi F, Montella M, Giacosa A, Negri E, La Vecchia C. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann Oncol. 2011;22:2687–2692. doi: 10.1093/annonc/mdr025. [DOI] [PubMed] [Google Scholar]
- 19.Calip GS, Malone KE, Gralow JR, Stergachis A, Hubbard RA, Boudreau DM. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res Treat. 2014;148:363–377. doi: 10.1007/s10549-014-3157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chun J, Refinetti AP, Schnabel F, Price A, Billig J, Cimeno A, Schwartz S, Guth A, Axelrod D. Tumor characteristics in obese women with breast cancer. ASCO Breast Cancer Symposium 2012. 2012 abstract 179. [Google Scholar]
- 21.Early Breast Cancer Trialists’Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/s0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 22.Magliano DJ, Shaw JE, Zimmet PZ. How to best define the metabolic syndrome. Ann Med. 2006;38:34–41. doi: 10.1080/07853890500300311. [DOI] [PubMed] [Google Scholar]
- 23.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodwin PJ, Parulekar WR, Gelmon KA, Shepherd LE, Ligibel JA, Hershman DL, Rastogi P, Mayer IA, Hobday TJ, Lemieux J, Thompson AM, Pritchard KI, Whelan TJ, Mukherjee SD, Chalchal HI, Oja CD, Tonkin KS, Bernstein V, Chen BE, Stambolic V. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCIC Clinical Trials Group. A Phase III Randomized Trial of Metformin vs Placebo in Early Stage Breast Cancer. https://clinicaltrials.gov/ct2/show/NCT01101438. Accessed May 25, 2016.
- 27.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 28.Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, Kealey S, Jones VE, Caan BJ, Gold EB, Haan M, Hollenbach KA, Jones L, Marshall JR, Ritenbaugh C, Stefanick ML, Thomson C, Wasserman L, Natarajan L, Thomas RG, Gilpin EA. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23:728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 29.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 30.Ligibel JA, Alfano CM, Hershman D, Ballard RM, Bruinooge SS, Courneya KS, Daniels EC, Demark-Wahnefried W, Frank ES, Goodwin PJ, Irwin ML, Levit LA, McCaskill-Stevens W, Minasian LM, O’Rourke MA, Pierce JP, Stein KD, Thomson CA, Hudis CA. Recommendations for Obesity Clinical Trials in Cancer Survivors: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;33:3961–3967. doi: 10.1200/jco.2015.63.1440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


