Abstract
Aims
To compare the efficacy and safety of basal insulin peglispro (BIL), which has a flat pharmacokinetic and pharmacodynamic profile and a long duration of action, with insulin glargine (GL) in patients with type 1 diabetes.
Materials and methods
In this phase III, 52‐week, blinded study, we randomized 1114 adults with type 1 diabetes in a 3 : 2 distribution to receive either BIL (n = 664) or GL (n = 450) at bedtime, with preprandial insulin lispro, using intensive insulin management. The primary objective was to compare glycated haemoglobin (HbA1c) in the groups at 52 weeks, with a non‐inferiority margin of 0.4%.
Results
At 52 weeks, mean (standard error) HbA1c was 7.38 (0.03)% with BIL and 7.61 (0.04)% with GL {difference −0.22% [95% confidence interval (CI) −0.32, −0.12]; p < 0.001}. At 52 weeks more BIL‐treated patients reached HbA1c <7% (35% vs 26%; p < 0.001), the nocturnal hypoglycaemia rate was 47% lower (p < 0.001) and the total hypoglycaemia rate was 11% higher (p = 0.002) than in GL‐treated patients, and there was no difference in severe hypoglycaemia rate. Patients receiving BIL lost weight, while those receiving GL gained weight [difference −1.8 kg (95% CI −2.3, −1.3); p < 0.001]. Treatment with BIL compared with GL at 52 weeks was associated with greater increases from baseline in levels of serum triglyceride [difference 0.19 mmol/l (95% CI 0.11, 0.26); p < 0.001] and alanine aminotransferase (ALT) levels [difference 6.5 IU/l (95% CI 4.1, 8.9), p < 0.001], and more frequent injection site reactions.
Conclusions
In patients with type 1 diabetes, treatment with BIL compared with GL for 52 weeks resulted in a lower HbA1c, more patients with HbA1c levels <7%, and reduced nocturnal hypoglycaemia, but more total hypoglycaemia and injection site reactions and higher triglyceride and ALT levels.
Keywords: basal insulin, glycaemic control, hypoglycaemia, randomized trial, type 1 diabetes
Introduction
Despite advances in insulin replacement therapy, and even with the advent of newer basal insulin analogues, an ideal basal insulin—characterized by a prolonged pharmacodynamic effect, low intra‐patient glucose variability, low risk of nocturnal hypoglycaemia and limited weight gain—has remained an unmet medical need for patients with type 1 diabetes1.
Basal insulin peglispro (BIL; LY2605541), a PEGylated insulin lispro, is slowly absorbed from the site of injection as an insulin monomer, principally through the lymphatic system2. BIL is cleared slowly from the circulation, with a half‐life of 2–3 days 3, 4. These properties confer a flat activity profile at steady state 3 and a prolonged duration of action of at least 36 h 4. BIL is hypothesized to have restricted passage through the vascular endothelium to peripheral tissues such as skeletal muscle and adipose tissue, but ready access to the liver through the fenestrations in the hepatic sinusoidal endothelium. In healthy subjects, BIL was shown to have a hepato‐preferential insulin action allowing a reduction in endogenous glucose production similar to GL (5 see Figure 2), but with less glucose disposal in the periphery (5 see Figure 1). Similar results were obtained in conscious dogs 6 and in patients with type 1 diabetes 7, 8.
Figure 2.
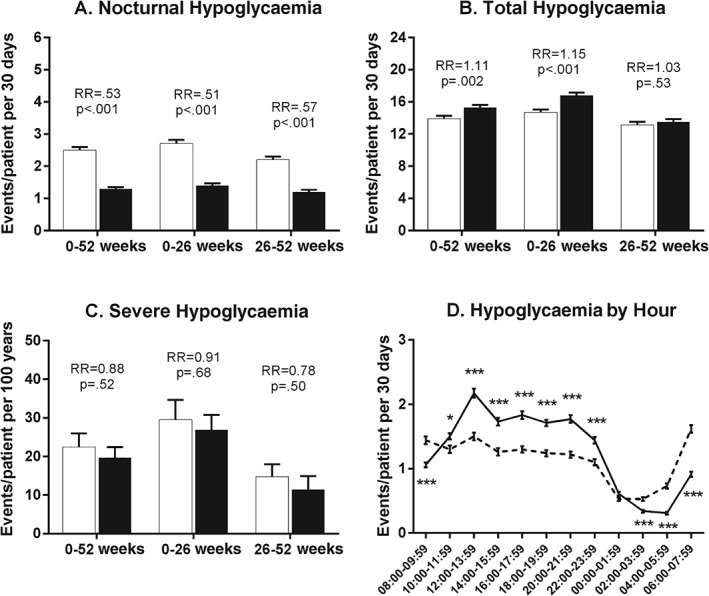
Hypoglycaemia rates during the study. (A) Nocturnal hypoglycaemia rates for overall, first half and second half of treatment period [events/patient/30 days; group mean ± standard error (s.e.)]. (B) Total hypoglycaemia rates for overall, first half and second half of treatment period (events/patient/30 days; group mean ± s.e.). (C) Severe hypoglycaemia rates for overall, first half and second half of treatment period (events/100 patient years; aggregated rate ± s.d.). White bars = glargine; black bars = BIL. (D) Hypoglycaemia rate in 2‐h intervals (group mean ± s.e.) at 52 weeks. Dashed line = glargine; solid line = BIL; RR = relative rate BIL/glargine. *p < 0.05, **p < 0.01, ***p < 0.001 for differences between treatments.
Figure 1.
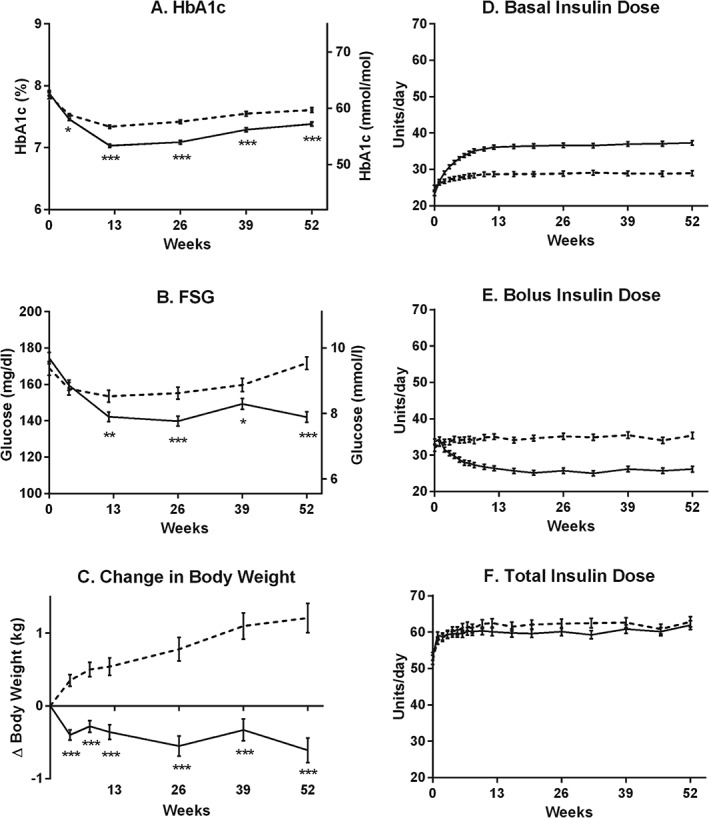
Outcome measures and insulin doses by weeks of treatment. (A) HbA1c (%, mmol/mol). (B) FSG (mg/dl, mmol/l). (C) Change from baseline in body weight (kg). (D) Basal insulin dose (U/day, p < 0.05 for treatment differences from weeks 2 to 52). (E) Bolus insulin dose (U/day, p < 0.05 for treatment differences from weeks 2 to 52). (F) Total insulin dose (U/day, p > 0.05 for treatment differences, except for week 32). Dashed lines = glargine; solid lines = BIL. All data are LS mean ± s.e. *p < 0.05, **p < 0.01, ***p < 0.001 for differences between treatments.
The aim of this double‐blind, phase III trial was to compare the safety and efficacy of BIL with that of GL, administered once daily in the evening, in combination with preprandial insulin lispro, in adults with type 1 diabetes.
Materials and Methods
This 52‐week, double‐blind, randomized, phase III trial was conducted in patients with type 1 diabetes at 132 sites in 20 countries (Appendix S1, Supporting Information) between 11 January 2012 and 3 February 2014. The study was divided into the following periods: 3‐week screening and baseline; 12‐week intensive insulin management; 40‐week maintenance insulin management; and a 4‐week post‐treatment follow‐up (Figure S1, Supporting Information).
Eligible participants were aged ≥18 years, had type 1 diabetes for at least 1 year, glycated haemoglobin (HbA1c) levels <12% and body mass index ≤35 kg/m2 at screening, and were currently taking basal‐bolus insulin therapy, administered either by insulin syringe or pen or continuous subcutaneous insulin infusion pump (Appendix S2, Supporting Information). The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. All participants signed an informed consent document, and the protocols and consent documents were approved by local ethical review boards before study initiation. An unblinded, independent data monitoring committee monitored patient safety.
Eligible participants were randomly assigned to BIL or GL basal insulin in a ratio of 3 : 2, stratified by baseline HbA1c (≤8.5%, >8.5%), baseline LDL cholesterol [<100 mg/dl (2.6 mmol/l) and ≥100 mg/dl], and prior basal insulin therapy (GL/insulin detemir/other basal insulin; Appendix S3, Supporting Information). Both basal insulins were injected at bedtime using a syringe and vials that allowed visual inspection of the insulin but hid all differentiating vial features. Insulin lispro was provided in a disposable pen for mealtime administration and correction of hyperglycaemia. Investigators calculated for each patient the initial basal insulin dosage (converted unit per unit to the blinded insulin) based on insulin doses during the 3 days before randomization. Basal and bolus doses were adjusted weekly during the initial 12 weeks and were evaluated at each visit during the remainder of the study using an intensive insulin regimen algorithm with a target pre‐meal self‐monitored blood glucose (SMBG) value of 5.6 mmol/l and pre‐bedtime value of 7.2 mmol/l (Appendix S4, Supporting Information). Investigators were permitted to make further insulin adjustments at any point, depending on clinical circumstances (e.g. hypoglycaemia). Intensive insulin adjustment as well as capture of SMBG and hypoglycaemia data was enhanced through the implementation of an electronic diary (e‐diary) system 9. The e‐diary supported investigator and patient preferences for bolus insulin dosing including a carbohydrate counting plan, a preprandial action plan, and a pattern adjustment plan. Individualized adjustments based upon carbohydrate intake, glucose correction factors, exercise and stress factors were supported by the e‐diary and could be modified by the investigator, and provided the opportunity for the patient to calculate an accurate bolus dose 9.
Hypoglycaemia was defined as an SMBG value <3.9 mmol/l or signs and symptoms consistent with hypoglycaemia without an SMBG measurement; nocturnal hypoglycaemia was defined as an event occurring between bedtime and waking and between 22:00 and 10:00 hours (Appendix S5, Supporting Information). Patients were asked to perform a nine‐point SMBG profile on 2 days in the week preceding selected clinic visits.
Statistical Analysis
To control the Type I error for multiple tests, a gate‐keeping strategy was used to test the primary objective of non‐inferiority of BIL to GL for HbA1c at 52 weeks (non‐inferiority margin 0.4%) 10, and five key secondary objectives for superiority of BIL to GL for (in order of hypothesis testing): nocturnal hypoglycaemia rate, HbA1c, proportion of patients with HbA1c <7%, fasting serum glucose (FSG) by laboratory measurement, and total hypoglycaemia rate. While most traditional methods control the overall type I error by splitting the α value, the gate‐keeping strategy controls the overall type I error by fixing the order of testing with the same α level. If a prior test fails (p > 0.05), the subsequent tests are deemed non‐significant regardless of the nominal p values 11, 12. This gate‐keeping strategy allows results from the gated secondary objectives to have the same statistical rigour as the primary objective.
A total of 1114 patients would provide >99% statistical power to demonstrate the non‐inferiority of BIL to GL for HbA1c, and >95% statistical power to demonstrate a difference in nocturnal hypoglycaemia events (assuming a 30% rate reduction) 13. Analyses were based on all randomized patients who received ≥1 dose of study medication. A mixed‐model repeated measures approach was used to analyse continuous variables; values are presented as least squares (LS) mean (standard error), unless otherwise indicated. The proportion of patients with the last non‐missing HbA1c value <7% was analysed using logistic regression. The hypoglycaemia rate was estimated and compared between treatments using negative binomial regression analysis, after adjustment for pre‐randomization (baseline) 14, 15, 16. Major adverse cardiovascular events (MACE; cardiovascular death, myocardial infarction or stroke) and MACE+ (MACE plus hospitalization for unstable angina) were analysed using a Cox proportional hazard model. The statistical software used was sas version 9.1 or higher (Cary, NC, USA).
Results
Trial Population
A total of 1114 patients were randomized and a similar percentage of patients in each treatment group completed the study (Figure S2, Supporting Information). The demographic and baseline characteristics were similar between BIL (n = 664) and GL (n = 450) groups (Table 1).
Table 1.
Patient demographic and baseline characteristics.
| Glargine (N = 450) | BIL (N = 664) | p value | |
|---|---|---|---|
| Age, years | 42.3 ± 13.2 | 41.6 ± 13.5 | 0.39 |
| Men, n (%) | 281 (62.4) | 397 (59.8) | 0.38 |
| Race, n (%) | — | — | 0.64 |
| Asian | 2 (0.4) | 6 (0.9) | — |
| Black or African‐American | 14 (3.1) | 15 (2.3) | — |
| Multiple/Other | 8 (1.7) | 18 (2.8) | — |
| White | 426 (94.7) | 625 (94.1) | — |
| Hispanic or Latino ethnicity, n (%) | 18 (4.0) | 23 (3.5) | 0.78 |
| Weight, kg, | 79.6 ± 15.2 | 79.0 ± 14.8 | 0.55 |
| Body mass index, kg/m2 | 26.5 ± 4.0 | 26.5 ± 3.9 | 0.77 |
| Duration of diabetes, years | 20.3 ± 12.9 | 19.4 ± 12.3 | 0.25 |
| Baseline insulin use, n (%) | — | — | 0.18 |
| Insulin glargine | 286 (63.6) | 428 (64.5) | — |
| Insulin detemir | 96 (21.3) | 124 (18.7) | — |
| NPH (isophane) | 35 (7.8) | 62 (9.3) | — |
| Pump | 31 (6.9) | 38 (5.7) | — |
| Other | 2 (0.4) | 12 (1.8) | — |
| Lipid‐lowering medications, n (%) | — | — | 0.57 |
| Statins | 149 (33.1) | 208 (31.3) | 0.56 |
| Non‐statin | 29 (6.4) | 47 (7.1) | 0.72 |
| Hypertension, n (%) | 171 (38.0) | 234 (35.2) | 0.37 |
Data are mean ± standard deviation, unless otherwise noted.
Glycaemic Control and Hypoglycaemia
Treatment with BIL was non‐inferior and also superior to GL treatment for HbA1c at 52 weeks {LS mean treatment difference −0.22% [95% confidence interval (CI) −0.32, −0.12]}, as the 95% CI upper limit was less than zero and the test for multiplicity was satisfied (Figure 1A and Table 2). At week 52, a higher proportion of patients treated with BIL achieved an HbA1c level of <7% (Table 2) and BIL‐treated patients had lower FSG levels compared with GL‐treated patients [LS mean difference −1.65 mmol/l (95% CI −2.14, −1.15); p < 0.001 (Figure 1B and Table 2)].
Table 2.
Baseline values and treatment outcomes at 52 weeks.
| Baseline | 52 weeks | ||||
|---|---|---|---|---|---|
| Outcome | Glargine | BIL | Glargine | BIL | p value * |
| N = 450 | N = 664 | N = 450 | N = 664 | ||
| HbA1c†, % | 7.84 ± 0.05 | 7.88 ± 0.04 | 7.61 ± 0.04 | 7.38 ± 0.03 | <0.001 |
| Change from baseline† | — | — | −0.24 ± 0.04 | −0.46 ± 0.03 | |
| HbA1c†, mmol/mol | 62.2 ± 0.6 | 62.6 ± 0.5 | 59.6 ± 0.4 | 57.2 ± 0.4 | <0.001 |
| Change from baseline† | — | — | −2.65 ± 0.41 | −5.07 ± 0.35 | |
| FSG†, mmol/l | 9.37 ± 0.21 | 9.70 ± 0.17 | 9.53 ± 0.19 | 7.88 ± 0.16 | <0.001 |
| Change from baseline† | — | — | −0.03 ± 0.19 | −1.68 ± 0.16 | |
| Patients with HbA1c <7%, n (%) | 92 (20.7) | 130 (20.1) | 116 (26.1) | 229 (35.3) | <0.001 |
| Total hypoglycaemia rate‡ | 14.5 ± 0.52 | 15.2 ± 0.46 | 13.9 ± 0.35 | 15.3 ± 0.32 | 0.002 |
| Total hypoglycaemia incidence, n (%) | 420 (93.5) | 616 (93.1) | 446 (99.3) | 657 (99.2) | 0.84 |
| Nocturnal hypoglycaemia rate‡ | 2.79 ± 0.16 | 2.62 ± 0.13 | 2.46 ± 0.10 | 1.31 ± 0.06 | <0.001 |
| Nocturnal hypoglycaemia incidence, n (%) | 266 (59.2) | 374 (56.5) | 432 (96.2) | 579 (87.5) | <0.001 |
| Daytime hypoglycaemia rate‡ | 11.7 ± 0.47 | 12.6 ± 0.42 | 11.4 ± 0.33 | 14.1 ± 0.31 | <0.001 |
| Daytime hypoglycaemia incidence, n (%) | 405 (90.2) | 603 (91.1) | 443 (98.7) | 657 (99.2) | 0.39 |
| Severe hypoglycaemia rate§ | 10.8 ± 7.6 | 11.0 ± 6.3 | 22.5 ± 3.5 | 19.7 ± 2.7 | 0.52 |
| Severe hypoglycaemia incidence, n (%) | 2 (0.4) | 3 (0.5) | 54 (12.0) | 70 (10.6) | 0.45 |
| Within‐day glucose variability, mmol/l† | 4.07 ± 0.07 | 3.98 ± 0.06 | 3.98 ± 0.07 | 3.69 ± 0.06 | 0.002 |
| Between‐day glucose variability, mmol/l† | 3.55 ± 0.08 | 3.56 ± 0.07 | 3.50 ± 0.08 | 3.00 ± 0.07 | <0.001 |
| Basal insulin dose, U/day, U/kg/day† | 25.0 ± 0.7 | 23.4 ± 0.5 | 29.0 ± 0.7 | 37.3 ± 0.6 | <0.001 |
| 0.31 ± 0.01 | 0.29 ± 0.01 | 0.35 ± 0.01 | 0.47 ± 0.01 | <0.001 | |
| Bolus insulin dose, U/day, U/kg/day† | 32.1 ± 1.0 | 33.7 ± 0.8 | 35.4 ± 0.9 | 26.0 ± 0.8 | <0.001 |
| 0.40 ± 0.01 | 0.42 ± 0.01 | 0.43 ± 0.01 | 0.33 ± 0.01 | <0.001 | |
| Total insulin dose, U/day, U/kg/day† | 52.4 ± 1.3 | 53.3 ± 1.1 | 62.9 ± 1.4 | 61.9 ± 1.2 | 0.57 |
| 0.65 ± 0.01 | 0.67 ± 0.01 | 0.77 ± 0.02 | 0.78 ± 0.01 | 0.63 | |
| Body weight, kg† | 79.8 ± 0.7 | 79.2 ± 0.6 | 80.9 ± 0.2 | 79.1 ± 0.2 | <0.001 |
| Change from baseline† | — | — | 1.2 ± 0.2 | −0.6 ± 0.2 | |
| Triglycerides, mmol/l† | 0.96 ± 0.03 | 1.00 ± 0.03 | 0.99 ± 0.03 | 1.18 ± 0.02 | <0.001 |
| Total cholesterol, mmol/l† | 4.63 ± 0.04 | 4.67 ± 0.03 | 4.61 ± 0.03 | 4.78 ± 0.03 | <0.001 |
| HDL cholesterol, mmol/l† | 1.63 ± 0.02 | 1.64 ± 0.02 | 1.54 ± 0.01 | 1.50 ± 0.01 | 0.021 |
| LDL cholesterol, mmol/l† | 2.56 ± 0.04 | 2.58 ± 0.03 | 2.62 ± 0.03 | 2.74 ± 0.02 | 0.002 |
| Systolic blood pressure, mm Hg† | 124 ± 0.7 | 123 ± 0.6 | 124 ± 0.6 | 126 ± 0.5 | 0.022 |
| Diastolic blood pressure, mm Hg† | 75 ± 0.5 | 75 ± 0.4 | 76 ± 0.4 | 77 ± 0.3 | 0.012 |
| ALT, IU/l† | 21.9 ± 0.6 | 22.1 ± 0.5 | 23.4 ± 0.9 | 29.9 ± 0.8 | <0.001 |
| ALT ≥3 × ULN (postbaseline), n (%) | — | — | 9 (2.0) | 31 (4.8) | 0.021 |
| Injection site reactions¶ | — | — | 1 (0.2) | 88 (13.3) | <0.001 |
| Anti‐BIL treatment‐emergent antibody response** , n (%) | — | — | 103 (23.2) | 249 (38.4) | <0.001 |
For difference between treatments at week 52.
Least squares mean [standard error (s.e.)].
Group mean (s.e.); events/patient/30 days: baseline, baseline to week 52.
Aggregated rate (standard deviation); events/100 patient years: baseline, baseline to week 52.
Based on the following Medical Dictionary for Drug Regulatory Activities preferred terms that were considered adverse events of special interest related to injection sites: injection site swelling; injection site oedema; lipohypertrophy; lipoatrophy; lipodystrophy acquired; injection site abscess; injection site induration; injection site inflammation; injection site hypertrophy; injection site mass; partial lipodystrophy; injection site atrophy; injection site abscess sterile.
Defined as change from baseline to post‐baseline in the anti‐BIL antibody level, either from undetectable to detectable, or from detectable to the value with at least 130% relative increase from baseline.
The nocturnal hypoglycaemia rate was lower with BIL during weeks 0–52 [relative rate 0.53 (95% CI 0.47, 0.61); Figure 2A, Table 2 and Figure S3A, Supporting Information]. The rate of total hypoglycaemia was higher with BIL during weeks 0–52 [relative rate 1.11 (95% CI 1.04, 1.18); Figure 2B, Table 2 and Figure S3B, Supporting Information], contributed to by a higher relative rate of daytime hypoglycaemia [relative rate 1.23 (95% CI 1.15, 1.32); p < 0.001 (Figure 2D and Table 2)]. In additional analyses, the total hypoglycaemia rate with BIL was higher in the first 26 weeks [relative rate 1.15 (95% CI 1.08, 1.22); p < 0.001], but was not different compared with GL from 26 to 52 weeks [relative rate 1.03 (95% CI 0.95, 1.11); p = 0.53 (Figure 2B)]. Importantly, there were no treatment differences during weeks 0–52 in severe hypoglycaemia rate or incidence (Figure 2C and Table 2).
With the gate‐keeping multiplicity adjustment applied to the results above, BIL treatment was found to be statistically superior to GL treatment for: HbA1c reduction; proportion of patients reaching HbA1c < 7%; nocturnal hypoglycaemia rate; and FSG.
In the nine‐point SMBG profiles at week 52, lower values were observed at all time points from the morning postprandial period up to bedtime in the BIL group (Figure S4, Supporting Information). The within‐day glucose variability [the standard deviation (s.d.) of the SMBG profiles in the week before a visit; LS mean difference −0.29 mmol/l (95% CI −0.48, −0.10); p = 0.002] and the between‐day glucose variability [the s.d. of fasting blood glucose (SMBG) for the 7 days before a visit; LS mean difference −0.51 mmol/l (95% CI −0.72, −0.30); p < 0.001], were lower in the BIL group at week 52 (Table 2).
The daily basal insulin dose increased progressively from weeks 2 to 12 and was higher with BIL than with GL at week 52 [LS mean difference 8.3 U/day (95% CI 6.7, 9.9); p < 0.001; 0.11 U/kg/day (95% CI 0.09, 0.13); p < 0.001], with a corresponding decrease in the bolus insulin dose in the BIL group [LS mean difference: −9.4 U/day (95% CI −11.5, −7.3); p < 0.001; −0.11 U/kg/day (95% CI −0.13, −0.08); p < 0.001], leading to similar total daily insulin dose between groups [LS mean difference −0.92 U/day (95% CI −4.1, 2.3); p = 0.57; 0.01 U/kg/day (95% CI −0.03, 0.05); p = 0.63 (Figure 1D–F and Table 2)]. Compared with baseline, patients treated with BIL lost weight during the study, while those treated with GL gained weight [LS mean difference: −1.8 kg (95% CI −2.3, −1.3); p < 0.001 (Figure 1C and Table 2)].
Side Effects and Adverse Events
Triglyceride levels increased after initiation of BIL from baseline to first measurement at 4 weeks, and then plateaued. At week 52, BIL‐treated patients had higher triglyceride [treatment difference 0.19 mmol/l (95% CI 0.11, 0.26); p < 0.001], lower HDL cholesterol [−0.04 mmol/l (95% CI: −0.07, −0.01); p = 0.021], higher LDL cholesterol [0.12 mmol/l (95% CI 0.04, 0.19); p = 0.002] and higher total cholesterol levels [0.17 mmol/l (95% CI 0.08, 0.26); p < 0.001 (Figure S5, Supporting Information and Table 2)]. At study endpoint after study drug withdrawal, all mean lipid values returned to baseline (Figure S5, Supporting Information).
Blood pressure increased from baseline with BIL, but not with GL [LS mean difference at 52 weeks: systolic blood pressure 1.8 mm Hg (95% CI 0.3, 3.3), p = 0.022; diastolic blood pressure 1.3 mm Hg (95% CI 0.3, 2.3), p = 0.012 (Table 2)]. There were no significant differences in the reporting of treatment‐emergent adverse events (TEAEs) of hypertension or clinical outcomes of cardiac congestive heart failure, peripheral oedema or cerebrovascular events with BIL compared with GL treatment. Although event rates were low, fewer BIL‐treated patients had an adjudicated MACE [BIL, n = 3 (0.5/100 patient‐years); GL, n = 8 (1.8/100 patient‐years); p = 0.056] or MACE+ [BIL, n = 3 (0.5/100 patient‐years); GL, n = 9 (2.0/100 patient‐years); p = 0.035].
Overall, TEAEs were similar between groups with the exception of events related to skin and injection sites, and elevations in liver enzymes, which were more common with BIL (Table S1, Supporting Information). More patients in the BIL group experienced TEAEs related to injection sites (Table 2); the most common of these were lipohypertrophy (7.7%), injection site hypertrophy (2.0%), and injection site swelling (1.7%). Of the 88 BIL patients with TEAEs related to injection sites, events resolved during the study in 65% of patients (41% on study drug and 24% after withdrawal from study drug).
Increases in mean alanine aminotransferase (ALT) levels were observed with BIL treatment [LS mean difference in change from baseline to 52 weeks: 6.5 (95% CI 4.1, 8.9); p < 0.001] (Table 2). Mean ALT levels increased from baseline to 4 weeks, remained steady during BIL treatment, and returned to baseline 30 days after BIL withdrawal (Figure S6, Supporting Information). There was a greater number of participants in the BIL group with an increase in ALT to ≥3 × upper limit of normal (ULN; Table 2); of these, 100% experienced a return to baseline of ALT levels or a decrease by at least 20% from the peak value, either while continuing BIL or after discontinuing. The proportion of patients with total bilirubin ≥2 × ULN at any time after baseline was higher with GL treatment (GL 0.9% vs BIL 0%; p = 0.027). No patients in the study met the criteria for Hy's Law for acute, serious drug‐induced liver injury 17.
A higher proportion of patients in the BIL group had anti‐BIL treatment‐emergent antibody response compared with the GL group (Table 2), but antibody levels were low (median percent binding in patients with detectable anti‐BIL antibodies was 1.80% in the BIL group and 1.08% in the GL group). Neither anti‐BIL nor anti‐PEG antibody levels were associated with changes in HbA1c, total hypoglycaemia rates or insulin dose during treatment.
Discussion
In this double‐blind study in patients with type 1 diabetes, basal insulin treatment with BIL compared with GL for 52 weeks resulted in a 0.22% lower HbA1c, a 1.7‐mmol/l lower FSG, and lower SMBG values throughout the day. The lower HbA1c levels obtained in the BIL treatment group were achieved with a similar total daily insulin dose, but with a higher proportion of basal to total insulin. Patients in the BIL group lost weight in spite of improved glycaemic control and more basal insulin, and experienced lower between‐day and within‐day glucose variability. All of these findings are consistent with the flat time–action profile and possibly the reduced peripheral effect of BIL 3, 7.
Interestingly, compared with glargine, nocturnal hypoglycaemia was 47% lower with BIL, in spite of a 29% or ∼8‐unit higher BIL basal insulin dose. By contrast, BIL‐treated patients had more total hypoglycaemia during the study, driven by increased daytime hypoglycaemia. Analyses of hypoglycaemia by time of day and SMBG profiles (Figure S4, Supporting Information) suggest the increased daytime hypoglycaemia was associated with use of bolus insulin. Bolus insulin requirements were 30% lower when BIL was used as the basal insulin, possibly because of its longer half‐life and flat pharmacokinetic/pharmacodynamic profile 3, 4. Investigators and patients gradually adjusted the bolus insulin dose in combination with BIL during the titration period, when the total hypoglycaemia rate was increased with BIL; however, when the bolus insulin dosages had been optimized during the maintenance period (26–52 weeks), the total hypoglycaemia rate did not differ between treatments, suggesting an empirical learning process for the BIL regimen. Additionally, in type 2 diabetes studies without bolus insulin use, BIL‐treated patients had similar or lower total hypoglycaemia rates compared with GL 18, 19.
The increase in serum triglycerides when BIL replaced a conventional basal insulin may be consistent with its lower peripheral insulin activity, which may enable lipolysis of adipocyte triglycerides, thus increasing free fatty acid flux to the liver 6, 7. Further support for this hypothesis derives from BIL studies of insulin‐naïve patients with type 2 diabetes, where no increases in triglyceride levels were observed through 26 weeks of treatment with BIL 19. Although there were small increases in blood pressure and LDL cholesterol in this study, this was not observed in phase III trials in patients with type 2 diabetes 18, 19. While cardiovascular safety for BIL will be assessed in a meta‐analysis of adjudicated MACE+ across all of the phase II and phase III trials with active comparators, no differences in treatment‐emergent cardiovascular events were detected in this study, and there was a small but statistically significant reduction in adjudicated MACE+ events in the BIL group.
The cause of the increase in mean ALT level is unknown and may be related to changes in liver fat metabolism associated with the increase in serum triglycerides. Several studies have demonstrated a reduction in liver fat content (LFC) associated with initiation of GL 20 or other conventional basal insulins 21, 22. In a study of insulin‐naïve patients with type 2 diabetes comparing GL with BIL, LFC decreased from baseline with GL, but was unchanged in BIL‐treated patients 19. By contrast, LFC increased from baseline with BIL treatment in patients with type 2 diabetes previously treated with insulin 18. There was an elevation in mean ALT level and more patients with ALT ≥3 × ULN in the absence of a mean increase in LFC during BIL treatment for insulin‐naïve patients 23. Thus, changes in liver fat may not be the only explanation for increases in ALT. Some PEGylated proteins, such as pegvisomant 24, and PEG used to treat constipation 25, are associated with increased aminotransferases, but this is not true of all PEGylated proteins. The increased incidence of ALT ≥3 × ULN noted with BIL was observed without evidence of severe, acute hepatocellular injury (no Hy's Law cases) 17. While the clinical importance of these changes has not been determined, the resolution of ALT elevations on and off drug may be suggestive of hepatic adaptation 26, 27.
Injection site lipohypertrophy and swelling were reported more frequently in patients receiving BIL than in those receiving GL; however, injection site events were not associated with systemic hypersensitivity, increased anti‐BIL antibody titres, or worsening clinical outcome measures, such as higher HbA1c or FSG. Increased rates of lipohypertrophy and injection site swelling may reflect slow absorption of BIL via the lymphatic system, and a trophic effect on adipocytes resulting from a longer exposure of subcutaneous tissue to BIL 2; however, the resolution rate of these events in this study (41% in the BIL group and 24% after withdrawal of BIL), was higher than that reported in a previous prospective study of insulin injection‐induced lipohypertrophy (18%) 28. Injection site rotation has been associated with reduced rates of lipohypertrophy 28, 29.
The strengths of the present study include its double‐blind design, the use of the e‐diary system, the large sample size powered for hypoglycaemia, and the adjustment for multiple comparisons. Limitations include: (i) adjustment of bolus insulin doses may not have been optimized for the randomized basal insulin; (ii) most patients who entered the trial were already on GL, which may have biased TEAE reporting; and (iii) as with any clinical trial, the intensity of interaction between patients and investigators was not typical of clinical practice.
In conclusion, BIL, when used with preprandial insulin lispro, provides statistically superior glycaemic control (lower HbA1c, lower FSG, more patients achieving HbA1c <7% and less nocturnal hypoglycaemia) with more total hypoglycaemia and injection site reactions, as well as higher triglycerides and ALT, when compared with GL.
Conflict of Interest
R. M. B. has served on a scientific advisory board, consulted or performed clinical research with Abbott Diabetes Care, Bayer, Becton Dickinson, Boehringer Ingelheim, Astra Zeneca, DexCom, Eli Lilly, Halozyme, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo‐Nordisk, Roche, Sanofi and Takeda. R. M. B.'s employer, non‐profit Park Nicollet Institute, contracts for his services and no personal income goes to him. He has inherited Merck stock. He is a volunteer for ADA and JDRF. H. L. has served on a speaker's bureau for Sanofi‐Aventis New Zealand Ltd. E. F. has served as a speaker for Astra Zeneca/BMS, Bioton, Boehringer Ingelheim, Eli Lilly, Janssen‐Cilag, Novo‐Nordisk, Merck/MSD, Sanofi, Servier, and TEVA, and was member of Advisory Boards for Janssen Cilag, Novartis, Novo‐Nordisk, and Merck/MSD. F. T. has served as a global expert panel member for Novo‐Nordisk, as a TECOS advisory board member, MSD board member, research board member for Lilly France, and conferences for Novartis. J. M., Y. Q., C. J. A, M. L. H., M. R. and S. J. J. are employees and stockholders of Eli Lilly and company. E. J. B. was an employee and shareholder of Eli Lilly and Company at the time the work was carried out.
R. M. B., H. L., E. F. and F. T. participated as trial investigators, in the discussion of the research, and in reviewing and editing the manuscript. J. M. and Y. Q. contributed to the study design, designed and conducted the statistical analyses, and participated in the interpretation of the research and in writing the manuscript. C. J. A. contributed to the discussion of the research and in writing the manuscript. S. J. J. participated in the study design, the data analysis and interpretation of the research, and in writing the manuscript. M. R. and M. L. H. participated in the data analysis and interpretation of the research, and in writing the manuscript. E. J. B. was responsible for medical oversight during the trial and contributed to the study design, the data analysis and interpretation of the research, and to writing the manuscript. E. J. B. is the guarantor of this work and, as such takes full responsibility for the work as a whole, including the study design, access to data reported in the manuscript, and the decision to submit and publish the manuscript. All authors approved the final manuscript to be published.
Supporting information
Appendix S1. List of investigators by country.
Appendix S2. Study inclusion and exclusion criteria.
Appendix S3. Randomization and blinding.
Appendix S4. Insulin dose algorithms.
Appendix S5. Definitions of hypoglycaemia.
Table S1. Percent of patients experiencing TEAEs and serious adverse events from randomization to the end of the study.
Figure S1. Study design.
Figure S2. Patient disposition.
Figure S3. Cumulative hypoglycaemia.
Figure S4. SMBG profiles at baseline and 52 weeks.
Figure S5. Blood lipid values.
Figure S6. Alanine aminotransferase values.
Acknowledgements
The authors would like to thank the study participants, and the investigators, nurses and study coordinators who cared for them. This study was funded by Eli Lilly and Company. Portions of this study were presented as abstracts at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, USA, 5–9 June , 2015, and at the 51st Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, 14–18 September 2015.
Funding information: Funded by Eli Lilly and Company, ClinicalTrials.gov number NCT01454284.
References
- 1. Owens DR, Rosenstock J. Current and new long‐acting insulin analogs in development: the quest for a better basal insulin In: Umpierrez GE, ed. Therapy for Diabetes Mellitus and Related Disorders. 6th edn. American Diabetes Association, 2014; 480–507. [Google Scholar]
- 2. Knadler MP, Nguyen T‐H, Campanale KM et al. Lymphatic absorption of basal insulin peglispro (BIL) in sheep. Diabetes 2015; 64(suppl. 1): A262. [Google Scholar]
- 3. Sinha VP, Howey DC, Choi SL, Mace KF, Heise T. Steady‐state pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 dosed once‐daily in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014; 16: 344–350. [DOI] [PubMed] [Google Scholar]
- 4. Sinha VP, Choi SL, Soon DK et al. Single‐dose pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 in healthy subjects. J Clin Pharmacol 2014; 54: 792–799. [DOI] [PubMed] [Google Scholar]
- 5. Henry RR, Mudaliar S, Ciaraldi TP et al. Basal insulin peglispro demonstrates preferential hepatic versus peripheral action relative to insulin glargine in healthy subjects. Diabetes Care 2014; 37: 2609–2615. [DOI] [PubMed] [Google Scholar]
- 6. Moore MC, Smith MS, Sinha VP et al. Novel PEGylated basal insulin LY2605541 has a preferential hepatic effect on glucose metabolism. Diabetes 2014; 63: 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mudaliar S, Henry RR, Ciaraldi TP et al. Basal insulin peglispro (BIL) demonstrates hepato‐preferential action vs. insulin glargine (GL) in patients with type 1 diabetes mellitus (T1DM). Diabetes 2015; 64(suppl 1A): LB22–LB23. [Google Scholar]
- 8. Morrow LA, Hompesch M, Jacober SJ et al. Glucodynamics of long‐acting basal insulin peglispro (BIL) compared with insulin glargine at steady state in subjects with type 1 diabetes: substudy of a randomized crossover trial. Diabetes Obes Metab 2016; DOI: 10.1111/dom.12691. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Bastyr EJ III, Zhang S, Mou J et al. Performance of an electronic diary system for intensive insulin management in global diabetes clinical trials. Diabetes Technol Ther 2015; 17: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Center for Drug Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services . Guidance for industry: diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention: 2008 draft. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071624.pdf Accessed June 2016.
- 11. Dmitrienko A, Tamhane AC, Wiens BL. General multistage gatekeeping procedures. Biom J 2008; 50: 667–677. [DOI] [PubMed] [Google Scholar]
- 12. U.S. Department of Health and Human Services, Food and Drug Administration . Guidance for industry: patient‐reported outcome measures: use in medical product development to support labeling claims: statistical considerations for using multiple endpoints, 2009. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm193282.pdf Accessed June 2016.
- 13. Workgroup on Hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005; 28: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 14. Luo J, Jacober SJ, Prince MJ, Carey MA, Qu Y. The effect of adjusting for baseline hypoglycemia when analyzing hypoglycemia data: a systematic analysis of 15 diabetes clinical trials. Diabetes Technol Ther 2013; 15: 654–661. [DOI] [PubMed] [Google Scholar]
- 15. Luo J, Qu Y. Analysis of hypoglycemic events using negative binomial models. Pharm Stat 2013; 12: 233–242. [DOI] [PubMed] [Google Scholar]
- 16. Qu Y, Luo J. Estimation of group means when adjusting for covariates in generalized linear models. Pharm Stat 2015; 14: 56–62. [DOI] [PubMed] [Google Scholar]
- 17. U.S. Department of Health and Human Services, Food and Drug Administration . Guidance for industry: drug‐induced liver injury: premarketing clinical evaluation, 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090.pdf Accessed June 2016.
- 18. Buse JB, Rodbard HW, Trescoli Serrano C et al. Randomized clinical trial comparing basal insulin peglispro and insulin glargine in patients with type 2 diabetes previously treated with basal insulin: imagine 5. Diabetes Care 2016; 39: 92–100. [DOI] [PubMed] [Google Scholar]
- 19. Davies MJ, Russel‐Jones D, Selam JL et al. Basal insulin peglispro vs insulin glargine in insulin‐naïve type 2 diabetes: IMAGINE 2 randomized trial. Diabetes Obes Metab 2016. [Accepted Manuscript]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang A, Rabasa‐Lhoret R, Castel H et al. Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes: a randomized trial. Diabetes Care 2015; 38: 1339–1346. [DOI] [PubMed] [Google Scholar]
- 21. Lingvay I, Raskin P, Szczepaniak LS. Effect of insulin‐metformin combination on hepatic steatosis in patients with type 2 diabetes. J Diabetes Complications 2007; 21: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juurinen L, Tiikkainen M, Hakkinen AM, Hakkarainen A, Yki‐Jarvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 292: E829–835. [DOI] [PubMed] [Google Scholar]
- 23. Ginsberg H, Cariou B, Orchard T et al. Lipid changes during basal insulin peglispro, insulin glargine, or NPH treatment in 6 IMAGINE trials. Diabetes Obes Metab 2016. [Accepted Manuscript]. [DOI] [PubMed] [Google Scholar]
- 24. Schreiber I, Buchfelder M, Droste M et al. Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol 2007; 156: 75–82. [DOI] [PubMed] [Google Scholar]
- 25. Pashankar DS, Loening‐Baucke V, Bishop WP. Safety of polyethylene glycol 3350 for the treatment of chronic constipation in children. Arch Pediatr Adolesc Med 2003; 157: 661–664. [DOI] [PubMed] [Google Scholar]
- 26. Au JS, Navarro VJ, Rossi S. Review article: drug‐induced liver injury–its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther 2011; 34: 11–20. [DOI] [PubMed] [Google Scholar]
- 27. Navarro VJ, Senior JR. Drug‐related hepatotoxicity. N Engl J Med 2006; 354: 731–739. [DOI] [PubMed] [Google Scholar]
- 28. Hauner H, Stockamp B, Haastert B. Prevalence of lipohypertrophy in insulin‐treated diabetic patients and predisposing factors. Exp Clin Endocrinol Diabetes 1996; 104: 106–110. [DOI] [PubMed] [Google Scholar]
- 29. Blanco M, Hernandez MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin‐injecting patients with diabetes. Diabetes Metab 2013; 39: 445–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of investigators by country.
Appendix S2. Study inclusion and exclusion criteria.
Appendix S3. Randomization and blinding.
Appendix S4. Insulin dose algorithms.
Appendix S5. Definitions of hypoglycaemia.
Table S1. Percent of patients experiencing TEAEs and serious adverse events from randomization to the end of the study.
Figure S1. Study design.
Figure S2. Patient disposition.
Figure S3. Cumulative hypoglycaemia.
Figure S4. SMBG profiles at baseline and 52 weeks.
Figure S5. Blood lipid values.
Figure S6. Alanine aminotransferase values.


