Abstract
Basal insulin peglispro (BIL) is a novel basal insulin with hepato‐preferential action, resulting from reduced peripheral effects. This report summarizes hypoglycaemia data from five BIL phase III studies with insulin glargine as the comparator, including three double‐blind trials. Prespecified pooled analyses (n = 4927) included: patients with type 2 diabetes (T2D) receiving basal insulin only, those with T2D on basal‐bolus therapy, and those with type 1 diabetes (T1D). BIL treatment resulted in a 36–45% lower nocturnal hypoglycaemia rate compared with glargine, despite greater reduction in glycated haemoglobin (HbA1c) and higher basal insulin dosing. The total hypoglycaemia rate was similar in patients with T2D on basal treatment only, trended towards being higher (10%) in patients with T2D on basal‐bolus treatment (p = .053), and was 15% higher (p < .001) with BIL versus glargine in patients with T1D, with more daytime hypoglycaemia in the T1D and T2D groups who were receiving basal‐bolus therapy. In T1D, during the maintenance treatment period (26‐52 weeks), the total hypoglycaemia rate was not significantly different. There were no differences in severe hypoglycaemia in the T1D or T2D pooled analyses. BIL versus glargine treatment resulted in greater HbA1c reduction with less nocturnal hypoglycaemia in all patient populations, higher daytime hypoglycaemia with basal‐bolus therapy in the T1D and T2D groups, and an associated increase in total hypoglycaemia in the patients with T1D.
Keywords: basal insulin peglispro, hypoglycaemia, insulin glargine, nocturnal hypoglycaemia
1. INTRODUCTION
Despite advances in insulin therapy, hypoglycaemia remains a significant concern and limits optimization of insulin dosing to achieve glycaemic targets.1 Hypoglycaemia is associated with increased morbidity and cardiovascular risk.1 Patients/caregivers may be fearful of nocturnal hypoglycaemia, which may affect sleep and well‐being.2
Basal insulin peglispro (BIL) is PEGylated insulin lispro and has a flat pharmacokinetic profile with a 2‐3 day half‐life.3 BIL has an hepato‐preferential action versus glargine as a result of a reduced peripheral action rather than an enhanced effect on the liver 4 (Fig 2). The primary objective of the BIL phase III studies was to demonstrate the non‐inferiority of glycated haemoglobin (HbA1c) for BIL versus glargine. The three double‐blind studies were also powered for the key secondary objective of superiority of BIL versus glargine for reduction in nocturnal hypoglycaemia rate.
In five phase III IMAGINE trials, BIL treatment met the primary objective of non‐inferiority to glargine for HbA1c.5, 6, 7, 8, 9 The five IMAGINE trials also resulted in 0.2–0.5% greater HbA1c reduction versus glargine over 26, 52 and 78 weeks in patients with type 1 diabetes (T1D) and those with type 2 diabetes (T2D), meeting the key secondary objective of statistical superiority (with multiplicity adjustment), with higher basal insulin doses.5, 6, 7, 8, 9
The present report summarizes prespecified pooled hypoglycaemia analyses from the IMAGINE studies with a glargine comparator.
2. METHODS
Table 1 provides an overview of the five phase III IMAGINE studies of BIL versus glargine that were included in the prespecified pooled analyses of: patients with T2D receiving basal insulin only; patients with T2D receiving basal‐bolus insulin; and patients with T1D receiving basal‐bolus insulin. Entry criteria, study design, dosing algorithms and results, including individual study glycaemic/hypoglycaemia data, have been previously reported.5, 6, 7, 8, 9 All were treat‐to‐target trials with the same basal‐bolus insulin‐dosing algorithms applied to both treatments and with a fasting/pre‐meal self‐monitored blood glucose (SMBG) target of 5.6 mmol/L.
Table 1.
Overview of IMAGINE studies, patient clinical characteristics, glycated haemoglobin and insulin dose
| Overview of IMAGINE studies | |||||
|---|---|---|---|---|---|
| T2D basal only | T2D basal‐bolus | T1D | |||
| Study | IMAGINE 2 | IMAGINE 5 | IMAGINE 41 | IMAGINE 11 | IMAGINE 31 |
| Prior treatment | Insulin‐naïve | Basal insulin | ≥1 insulin injection | Basal‐bolus | Basal‐bolus |
| OAMs during study | ≤3 | ≤3 | Metformin only | N/A | N/A |
| Blinding | Double‐blind | Open‐label | Double‐blind | Open‐label | Double‐blind |
| HbA1c inclusion criteria, % | 7‐11 | ≤9 | ≥7, <12 | <12 | <12 |
| Study duration, weeks | 52‐78 | 52 | 26 | 78 | 52 |
| Summary of patient clinical characteristics and HbA1c | ||||||
|---|---|---|---|---|---|---|
| T2D basal only | T2D basal‐bolus | T1D | ||||
| GL (N = 694) | BIL (N = 1305) | GL (N = 676) | BIL (N = 689) | GL (N = 608) | BIL (N = 955) | |
| Age2, years | 59.6 ± 9.9 | 59.5 ± 9.6 | 57.8 ± 9.2 | 57.4 ± 9.2 | 41.4 ± 13.5 | 41.0 ± 13.4 |
| Men, n (%) | 401 (57.8) | 725 (55.3) | 404 (59.6) | 376 (54.4) | 368 (60.3) | 535 (55.8) |
| BMI2, kg/m2 (baseline) | 32.0 ± 5.1 | 32.2 ± 5.2 | 33.0 ± 5.6 | 33.3 ± 5.7 | 26.2 ± 4.0 | 26.2 ± 3.9 |
| Diabetes duration2, years | 11.3 ± 6.7 | 11.1 ± 6.4 | 14.2 ± 7.8 | 14.1 ± 7.0 | 19.0 ± 12.5 | 18.9 ± 12.1 |
| HbA1c5, % | ||||||
| Baseline | 8.2 ± 0.04 | 8.2 ± 0.03 | 8.5 ± 0.04 | 8.4 ± 0.04 | 7.8 ± 0.05 | 7.8 ± 0.04 |
| Endpoint3 | 7.2 ± 0.04 | 6.9 ± 0.03 | 7.0 ± 0.04 | 6.8 ± 0.04 | 7.6 ± 0.03 | 7.4 ± 0.03 |
| LS mean difference (95% CI) | −0.33 (−0.42, −0.23)4 | −0.21 (−0.31, −0.11)4 | −0.23 (−0.31, −0.14)4 | |||
| Insulin dose at study endpoint | ||||||||
|---|---|---|---|---|---|---|---|---|
| T2D basal only | T2D basal‐bolus | T1D | ||||||
| IMAGINE 2 | IMAGINE 5 | IMAGINE 4 | ||||||
| GL | BIL | GL | BIL | GL | BIL | GL | BIL | |
| Basal insulin dose3, 5, U | 39.0 ± 1.0 | 42.7 ± 0.84 | 47.0 ± 1.6 | 54.5 ± 1.24 | 60.3 ± 1.2 | 67.6 ± 1.24 | 28.3 ± 0.6 | 35.1 ± 0.54 |
| Bolus insulin dose3, 5, U | 62.8 ± 1.7 | 61.1 ± 1.7 | 35.8 ± 0.7 | 26.2 ± 0.64 | ||||
| Total insulin dose3, 5, U | 121.0 ± 2.6 | 125.9 ± 2.6 | 62.8 ± 1.0 | 59.5 ± 0.94 | ||||
GL, insulin glargine; LS, least squares; OAM, oral antihyperglycaemic medication; s.d., standard deviation; s.e., standard error.
Electronic diaries used.
Mean ± s.d.
52 weeks for T2D basal only; 26 weeks for T2D basal‐bolus; 52 weeks for T1D.
p < .05 for between treatment group comparison.
LS mean ± s.e.
Hypoglycaemia was defined as SMBG ≤3.9 mmol/L or hypoglycaemia signs/symptoms. Nocturnal hypoglycaemia was an event between bedtime and waking for T2D basal‐only studies (IMAGINE 2 and 5). For studies using electronic diaries (IMAGINE 1, 3 and 4) with direct transfer of time/date stamped SMBG values,10 nocturnal hypoglycaemia was defined both as between bedtime and waking and between 22:00 and 10:00 hours. Hypoglycaemia at other times was daytime hypoglycaemia. Severe hypoglycaemia was investigator‐determined and defined as episodes accompanied by neurological impairment requiring medical assistance to administer carbohydrates, glucagon or other resuscitative actions.
Study durations ranged from 26 to 78 weeks (Table 1). For the pooled analyses of patients with T2D on basal insulin only (IMAGINE 2 and 5) and patients with T1D (IMAGINE 1 and 3), 52‐week data were included, as this was a consistent time point. IMAGINE 4, the only T2D basal‐bolus trial, was a 26‐week study.
Blinded continuous glucose monitoring (CGM) was performed in patient subsets in the three double‐blind studies (IMAGINE 2, 3 and 4) to assess duration (min) with glucose values ≤3.9 mmol/L from 00:00 to 06:00 hours and over 24 hours and duration of individual hypoglycaemic episodes. The Medtronic CGMS iPro Continuous Glucose Recorder (IMAGINE 3) or the iPro2 Professional CGM (IMAGINE 2 and 4) were used over 3 or 6 days, respectively. CGM data were centrally collected (Phase V Technologies, Wellesley, Massachusetts) at baseline/prespecified time points during treatment. The 52‐week data are presented for IMAGINE 2 and 3 and 26‐week data for IMAGINE 4 (Table S1, Appendix S1).
Analyses (SAS 9.1, Cary, North Carolina) were conducted on the modified intention‐to‐treat population of all randomized patients who received ≥1 study insulin dose. All tests were conducted at a two‐sided α value = 0.05 and 95% confidence intervals (CIs) were calculated. Pooling of studies for HbA1c was prespecified. All continuous variables were analysed using mixed linear models with repeated measurements. Total/nocturnal hypoglycaemia frequencies were analysed using negative binomial regression and a model‐based rate for each treatment was estimated.11 The rate of severe hypoglycaemia was analysed using an empirical method; hypoglycaemia incidence was assessed using a logistic regression model or Cochran–Mantel–Haenszel test (if <10 patients with events). Analysis of variance was used for continuous variables collected at baseline.
3. RESULTS
A total of 4927 patients randomized to bedtime BIL (n = 2949) or glargine (n = 1978) were included. Baseline characteristics were similar between groups (Table 1). Across the studies, 49–72% of patients with T2D and 64–71% of patients with T1D were previously treated with glargine.
The pooled analyses were consistent with the individual study results, with a 0.21–0.33% greater reduction in HbA1c with BIL versus glargine (Table 1).
Basal insulin doses were 10–24% higher with BIL versus glargine across phase III studies (Table 1).5, 6, 7, 8, 9 Bolus insulin doses were 27% lower with BIL versus glargine in T1D (Table 1), but not statistically significantly different in patients with T2D receiving basal‐bolus insulin (Table 1).6
Nocturnal hypoglycaemia rates were reduced by 36% in patients with T2D on basal insulin only, 45% in patients with T2D on basal‐bolus insulin, and 43% in patients with T1D with BIL versus glargine (Figure 1A). Nocturnal hypoglycaemia incidence was statistically significantly lower with BIL versus glargine in all patient populations (Figure 1B). The nocturnal hypoglycaemia rate and incidence sensitivity analyses were consistent with the primary results including: prespecified analyses of the alternative nocturnal hypoglycaemia definition for basal only and basal‐bolus e‐diary studies and post hoc analyses including events between 00:01 and 05:59 hours (Figure S1, Appendix S1).
Figure 1.
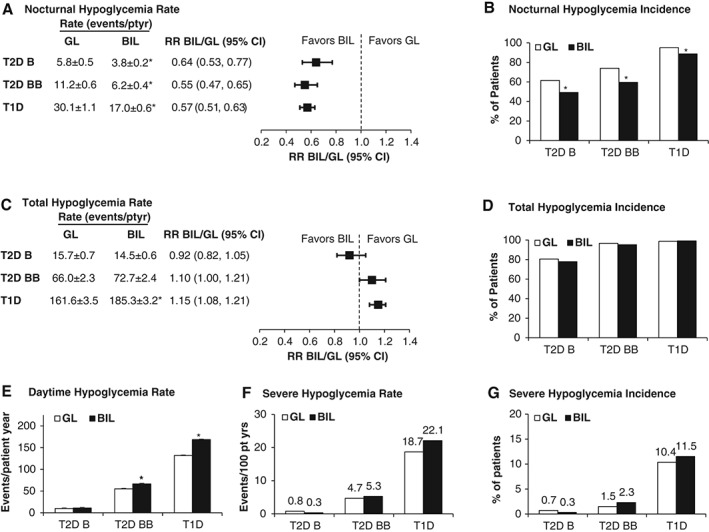
Nocturnal, total, daytime and severe hypoglycaemia. A, Nocturnal hypoglycaemia rate per patient‐year ± standard error (s.e.). B, Nocturnal hypoglycaemia incidence. C, Total hypoglycaemia rate per patient‐year ± s.e. D, Total hypoglycaemia incidence. E, Daytime hypoglycaemia rate per patient‐year ± s.e. F, Severe hypoglycaemia rate per 100 patient‐years. Values are aggregated rate, calculated using the total number of severe hypoglycaemia episodes divided by the cumulative days on treatment from all patients within that treatment, then times 36 525 days (for rate per 100 years). G, Severe hypoglycaemia incidence. Weeks of study data included: T2D basal only (0‐52 weeks), T2D basal‐bolus (0‐26 weeks), T1D (0‐52 weeks). B, basal only; BB, basal‐bolus; GL, insulin glargine; RR, relative rate. *p < .05 for between‐treatment‐group comparisons.
The total hypoglycaemia rate was not significantly different in patients with T2D on basal insulin only, there was a 10% higher trend in patients with T2D on basal‐bolus insulin (p = .053), and the rate was 15% higher in patients with T1D (p < .001) with BIL versus glargine (Figure 1C). The total hypoglycaemia incidence was not different between treatments in any population (Figure 1D). In patients with T1D or T2D on basal‐bolus insulin, daytime hypoglycaemia was significantly higher with BIL versus glargine (Figure 1E) from 0 to 52 and 0 to 26 weeks, respectively. In T1D patients, during the maintenance period of 26‐52 weeks, the total hypoglycaemia relative rate (BIL/glargine) was 1.07 (95% CI 1.00, 1.14; p = .062; Figure S2, Appendix S1).
There were no significant differences in the rate or incidence of severe hypoglycaemia between treatments in all three patient populations (Figure 1F and G).
In the three double‐blind studies, CGM was performed in patient subsets (BIL, n = 313, glargine, n = 215). In patients with T1D or T2D, there were no significant treatment differences in duration with glucose level ≤3.9 mmol/L (00:00–06:00 hours or over 24 hours) or in the average duration of individual hypoglycaemic episodes with BIL versus glargine (Table S1, Appendix S1).
4. DISCUSSION
The present analyses provide a summary of prespecified and pooled hypoglycaemia analyses from the five IMAGINE studies with glargine comparator. BIL treatment resulted in a 36–45% reduction in nocturnal hypoglycaemia rate versus glargine, despite greater reduction in HbA1c and higher basal insulin dosing. There was a 15% increase in total hypoglycaemia in T1D with an associated increase in daytime hypoglycaemia with basal‐bolus therapy in T1D and T2D. CGM in patient subsets showed no evidence of prolongation or shortening of hypoglycaemic episodes with BIL versus glargine. There were no significant differences in severe hypoglycaemia among the three populations. Nocturnal hypoglycaemia was also reduced with morning dosing of BIL versus glargine in T1D and T2D phase II studies12, 13 and nocturnal/total hypoglycaemia were similar with bedtime versus 8‐40‐hours variable‐time dosing of BIL in a T1D phase III study.14
The significant reduction in nocturnal hypoglycaemia was achieved simultaneously with greater improvements in HbA1c with BIL versus glargine. In contrast, significant reductions in nocturnal hypoglycaemia rates were reported in clinical studies of insulin degludec15 and glargine U‐30016 versus glargine U‐100, but with non‐inferiority of HbA1c. This may reflect the hepato‐preferential action of BIL as well as longer duration of action, lower peak‐to‐trough ratio, and reduced glucose variability versus glargine U‐100 seen across the BIL phase III trials.5, 6, 7, 8, 9 A study in the conscious dog has shown that peripheral insulin delivery increased hypoglycaemia risk versus portal vein insulin delivery, suggesting that hepato‐preferential insulin analogues may be associated with a lower risk of hypoglycaemia.17 Glucose variability has also been independently associated with the risk of hypoglycaemia.18, 19
Increased daytime hypoglycaemia with BIL versus glargine in basal‐bolus therapy may also reflect the hepato‐preferential action and longer duration of action of BIL. In T1D, the adjustment of basal‐bolus dosing occurred gradually during the titration period and resulted in significantly reduced bolus insulin requirements with BIL compared with glargine. During the maintenance period of 26‐52 weeks and after basal‐bolus dose adjustment and stabilization, total hypoglycaemia decreased in T1D and was not significantly different between groups (Figure S2, Appendix S1).
The strengths of the present pooled analyses of five BIL phase III studies include the double‐blind design of three trials, which were also powered to detect differences in nocturnal hypoglycaemia, the large sample size of >4900 patients, as well as the global nature of the studies. The use of an e‐diary in three basal‐bolus studies10 allowed wireless, direct capture of SMBG values and hypoglycaemic events. Treat‐to‐target fasting SMBG levels were not significantly different in the BIL and glargine groups. Limitations include the ability to translate the findings outside of a clinical trial setting and exclusion of patients with T1D with >1 and patients with T2D with ≥1 severe hypoglycaemic episode within 6 months of screening. In addition, it is unknown from these studies if other basal‐bolus insulin‐dosing algorithms, including algorithms different from those used with conventional basal insulins, could more rapidly optimize bolus insulin dosing with BIL.
In conclusion, across five phase III trials, BIL treatment resulted in greater HbA1c reduction with less nocturnal hypoglycaemia in all patient populations compared with glargine despite higher basal insulin doses. Basal‐bolus treatment with BIL versus glargine resulted in higher daytime hypoglycaemia in T1D and T2D, with an associated increase in total hypoglycaemia in patients with T1D.
Supporting information
Table S1. Duration of hypoglycemia as assessed by continuous glucose monitoring (CGM) in three double‐blind IMAGINE studies.
Figure S1. Sensitivity analyses for nocturnal hypoglycemia between 00:00 and 05:59 hours. A, Nocturnal hypoglycemia rate per patient year ± SE. B, Nocturnal hypoglycemia incidence. Weeks of study data included: T2D basal only (0‐52 weeks), T2D basal‐bolus (0‐26 weeks), T1D (0‐52 weeks). Abbreviations: B, basal only; BB, basal‐bolus; BIL, basal insulin peglispro; CI, confidence interval; GL, insulin glargine; pt, patient; RR, relative rate; SE, standard error; T1D, type 1 diabetes; T2D,type 2 diabetes; yr, year. *p < .05 for between treatment group comparisons.
Figure S2. Total hypoglycemia rate in T1D. Values are rate per patient year ± SE. Abbreviations: BIL, basal insulin peglispro; GL, insulin glargine; SE, standard error; T1D, type 1 diabetes. *p < .05 for between treatment group comparisons.
ACKNOWLEDGMENTS
The authors would like to thank the study participants, and the investigators, nurses and study coordinators who cared for them. The authors thank Michelle Carey, PhD, (inVentiv Health Clinical) for writing and editorial assistance.
Conflict of interest
J. R. has served on scientific advisory boards and received honoraria or consulting fees from Eli Lilly, Novo Nordisk, Sanofi, Merck, Daiichi Sankyo, Janssen, Boehringer Ingelheim, AstraZeneca and Intarcia. He has received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Takeda, AstraZeneca, Hanmi, Janssen, Daiichi Sankyo, Asahi, MannKind, Boehringer Ingelheim, Intarcia and Lexicon. M. M. has served on advisory boards for Abbott, Intarcia, Merck Sharpe & Dohme, Novo Nordisk, Sanofi and Servier, has received honoraria or speaking fees from Abbott, Eli Lilly and Company, Merck Sharpe & Dohme, Novo Nordisk, Sanofi and Servier and has received research grants from Merck Sharpe & Dohme, Novartis, Novo Nordisk, Sanofi and Servier. M.J.P. was an employee of Eli Lilly and Company during these clinical trials. E. J. B. was an employee of Eli Lilly and Company during these clinical trials and is currently a consultant to Viacyte, Inc., San Diego, California. Y. Q., S. Z. and A. M. C. are employees and shareholders of Eli Lilly and Company.
Author contributions
J. R. and M. M. participated as trial investigators and in the discussion/interpretation of the research, and reviewed/edited the manuscript. E. J. B. and M. J. P. participated in study design, the conduct of the studies, the data analysis/interpretation of the research, and reviewed/edited the manuscript. Y. Q. and S. Z. contributed to the study design, designed and conducted the statistical analyses, and participated in the interpretation of the research and reviewed/edited the manuscript. A. M. C. contributed to the study design, the conduct of the studies, the data analysis/interpretation of the research and in writing the manuscript. All authors approved the final manuscript to be published.
Funding informationThis study was funded by Eli Lilly and Company.
References
- 1. International Hypoglycaemia Study Group . Minimizing hypoglycemia in diabetes. Diabetes Care. 2015;38:1583–1591. [DOI] [PubMed] [Google Scholar]
- 2. King P, Kong MF, Parkin H, Macdonald IA, Tattersall RB. Well‐being, cerebral function, and physical fatigue after nocturnal hypoglycemia in IDDM. Diabetes Care. 1998;21:341–345. [DOI] [PubMed] [Google Scholar]
- 3. Sinha VP, Howey DC, Choi SL, Mace KF, Heise T. Steady‐state pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 dosed once‐daily in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:344–350. [DOI] [PubMed] [Google Scholar]
- 4. Henry RR, Mudaliar S, Ciaraldu TP, et al. Basal insulin peglispro demonstrates preferential hepatic versus peripheral action relative to insulin glargine in healthy subjects. Diabetes Care. 2014;37:2609–2615. [DOI] [PubMed] [Google Scholar]
- 5. Bergenstal RM, Lunt H, Franek E, et al. A randomized, double‐blind clinical trial comparing basal insulin peglispro and insulin glargine, in combination with prandial insulin lispro, in patients with type 1 diabetes: IMAGINE 3. Diabetes Obes Metab. 2016, DOI: 10.1111/dom.12698. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blevins T, Pieber TR, Colon VG, Zhang S, Bastyr EJ III, Chang AM. Randomized double‐blind clinical trial comparing basal insulin peglispro and insulin glargine, in combination with prandial insulin lispro, in patients with type 2 diabetes: IMAGINE 4. Diabetes Obes Metab. 2016, DOI: 10.1111/dom.12696. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buse JB, Rodbard HW, Trescoli SC, et al. Randomized clinical trial comparing basal insulin peglispro and insulin glargine in patients with type 2 diabetes previously treated with basal insulin: IMAGINE 5. Diabetes Care. 2016;39:92–100. [DOI] [PubMed] [Google Scholar]
- 8. Davies MJ, Russell‐Jones D, Selam JL, et al. Basal insulin peglispro vs insulin glargine in insulin‐naive type 2 diabetes: IMAGINE 2 randomized trial. Diabetes Obes Metab. 2016, DOI: 10.1111/dom.12712. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garg S, Dreyer M, Jinnouchi H, et al. A randomized clinical trial comparing basal insulin peglispro and insulin glargine, in combination with prandial insulin lispro, in patients with type 1 diabetes: IMAGINE 1. Diabetes Obes Metab. 2016, DOI: 10.1111/dom.12738. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Bastyr EJ III, Zhang S, Mou J, Hackett AP, Raymond SA, Chang AM. Performance of an electronic diary system for intensive insulin management in global diabetes clinical trials. Diabetes Technol Ther. 2015;17:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qu Y, Luo J. Estimation of group means when adjusting for covariates in generalized linear models. Pharm Stat. 2015;14:56–62. [DOI] [PubMed] [Google Scholar]
- 12. Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once‐daily LY2605541, a novel long‐acting basal insulin, versus insulin glargine in basal insulin‐treated patients with type 2 diabetes. Diabetes Care. 2012;35:2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenstock J, Bergenstal RM, Blevins TC, et al. Better glycemic control and weight loss with the novel long‐acting basal insulin LY2605541 compared with insulin glargine in type 1 diabetes: a randomized, crossover study. Diabetes Care. 2013;36:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garg S, Selam JL, Bhargava A, et al. Similar HbA1c reduction and hypoglycaemia with variable‐ vs fixed‐time dosing of basal insulin peglispro (BIL) in type 1 diabetes: IMAGINE 7 Study. Diabetes Obes Metab. 2016, DOI: 10.1111/dom.12740. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritzel R, Roussel R, Bolli GB, et al. Patient‐level meta‐analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gregory JM, Kraft G, Scott MF, et al. Insulin delivery into the peripheral circulation: a key contributor to hypoglycemia in type 1 diabetes. Diabetes. 2015;64:3439–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50:2553–2561. [DOI] [PubMed] [Google Scholar]
- 19. Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulin‐treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther. 2012;14:1008–1012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Duration of hypoglycemia as assessed by continuous glucose monitoring (CGM) in three double‐blind IMAGINE studies.
Figure S1. Sensitivity analyses for nocturnal hypoglycemia between 00:00 and 05:59 hours. A, Nocturnal hypoglycemia rate per patient year ± SE. B, Nocturnal hypoglycemia incidence. Weeks of study data included: T2D basal only (0‐52 weeks), T2D basal‐bolus (0‐26 weeks), T1D (0‐52 weeks). Abbreviations: B, basal only; BB, basal‐bolus; BIL, basal insulin peglispro; CI, confidence interval; GL, insulin glargine; pt, patient; RR, relative rate; SE, standard error; T1D, type 1 diabetes; T2D,type 2 diabetes; yr, year. *p < .05 for between treatment group comparisons.
Figure S2. Total hypoglycemia rate in T1D. Values are rate per patient year ± SE. Abbreviations: BIL, basal insulin peglispro; GL, insulin glargine; SE, standard error; T1D, type 1 diabetes. *p < .05 for between treatment group comparisons.


