Abstract
Deployments of tear gas and pepper spray have rapidly increased worldwide. Large amounts of tear gas have been used in densely populated cities, including Cairo, Istanbul, Rio de Janeiro, Manama (Bahrain), and Hong Kong. In the United States, tear gas was used extensively during recent riots in Ferguson, Missouri. Whereas tear gas deployment systems have rapidly improved—with aerial drone systems tested and requested by law enforcement—epidemiological and mechanistic research have lagged behind and have received little attention. Case studies and recent epidemiological studies revealed that tear gas agents can cause lung, cutaneous, and ocular injuries, with individuals affected by chronic morbidities at high risk for complications. Mechanistic studies identified the ion channels TRPV1 and TRPA1 as targets of capsaicin in pepper spray, and of the tear gas agents chloroacetophenone, CS, and CR. TRPV1 and TRPA1 localize to pain‐sensing peripheral sensory neurons and have been linked to acute and chronic pain, cough, asthma, lung injury, dermatitis, itch, and neurodegeneration. In animal models, transient receptor potential inhibitors show promising effects as potential countermeasures against tear gas injuries. On the basis of the available data, a reassessment of the health risks of tear gas exposures in the civilian population is advised, and development of new countermeasures is proposed.
Keywords: tear gas, pepper spray, capsaicin, chlorobenzalmalononitrile, CS, CN, CR, TRPV1, TRPA1
Tear gas agents for riot control
Over the past several decades, tear gas has been used as a common riot‐control agent (RCA) by law enforcement to quell protests, riots, and civil unrest. Tear gas use has dramatically increased in recent years, with very large amounts released in population centers in Turkey,1 the United States,2 Hong Kong,3 Greece,4 Brazil,5 Egypt, and Bahrain.6, 7
Tear gas is generally perceived to be a sublethal incapacitant.8 A 2003 analysis of several tear gases and incapacitants concluded that, on the basis of available toxicological evidence, commonly used tear gases have a large safety margin for life‐threatening or irreversible toxic effects.9 Another medical review published in 2013 concluded that, in the majority of exposures, significant clinical effects are not anticipated.10 However, there are debates surrounding the acceptability of tear gas use for riot‐control purposes, especially in the background of the recent massive use. Many believe the risks of tear gas exposure are understated and that perceived risks are based on insufficient human epidemiological and mechanistic data.
The major RCAs used since World War II include o‐chlorobenzylidene malononitrile (CS), oleoresin capsicum (OC, pepper spray), dibenz [b,f]‐1,4‐oxazepine (CR), and 1‐chloroacetophenone (CN)11 (Fig. 1). The most commonly used RCA until the 1950s was CN, but a search for an alternative to CN was initiated in response to dissatisfaction with the potency and stability of the compound.12 CS was discovered by two American scientists in 192813 but was only developed for use as an RCA decades later.14 This compound—considered to be more potent but less toxic than CN at that time—was adopted as the standard RCA of the U.S. Army in 1959.12, 15 In the following decades, CS became the most common RCA, and it is now used widely.16 CS, CN, and CR tear gas agents are electrophilic agents, and their structures are presented in Figure 1.
Figure 1.
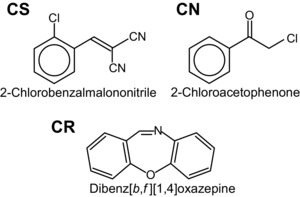
Chemical structures of commonly used tear gas agents o‐chlorobenzylidene malononitrile (CS), 1‐chloroacetophenone (CN), and dibenz[b,f]‐1,4‐oxazepine (CR).
OC is a mixture of several compounds extracted from chili peppers, with capsaicin as the major active ingredient.14 Pepper spray was developed as an animal repellent in the 1960s, but law enforcement agencies in the United States began using the compound for personal protection in the 1980s and 1990s.11 The rise in popularity of OC was similar to the rise in popularity of CS: OC was adopted as a less dangerous alternative to Mace, an aerosol self‐defense spray whose primary component was CN.11 CS and OC are now almost exclusively used as the RCAs of choice. The use of tear gas agents in warfare between military forces is banned under the 1993 International Chemical Weapons Convention, Geneva (Organisation for the Prohibition of Chemical Weapons), likely due to fear of escalation of chemical warfare. Some countries, including the United States, issued executive orders permitting the use of tear gas by military forces against rioting civilians and nonmilitary combatants and for troop extraction.17 The domestic use of tear gas agents is not covered by the Geneva Convention.17 Lack of epidemiological and mechanistic data on the spectrum of health effects of tear gas hinders the development of treatment plans and countermeasures and the medical understanding of long‐term effects. In this review, we summarize the existing epidemiological data on the health effects and biological mechanistic effects of tear gas agents and make recommendations to bridge the paucity of knowledge in this area.
Deployment technologies
Though commonly referred to as “tear gas,” the active compounds are not actually gases but solids. RCAs are deployed in many different ways, as personal defense sprays or from grenades or canisters.12 Sprays use a liquid formulation that is released from a pressurized dispenser, while grenades and canisters use a powdered form blended with a pyrotechnic mixture that can be aerosolized for dispersion as a smoke or fog.12 A common solvent for sprays is methyl isobutyl ketone (hexone), which is also considered hazardous.18, 19 CS tear gas agent is typically aerosolized as 3‐ to 10‐μm microencapsulated microparticles in aerosols. The typical pyrotechnic composition for dissemination of CS riot control agent consists of 45% CS agent, 30% potassium chlorate, 14% epoxy resin, 7% maleic anhydride, 3% methyl nadic anhydride, and 0.03% mixed residual balance.20 Although the intrinsic toxicities of these ingredients in the pyrotechnic composition were not studied in detail, their safety data sheets show significant toxicities. These pyrotechnic devices can be thrown by hand or fired from launchers, engaging targets up to 400 m away and penetrating window glass,12 with dispersion area ranges from 60 to 300 m2.21 Aircraft‐ and vehicle‐mounted dispersers can be used for RCA deployment, and aerial drone–based RCA deployment systems have been tested and requested by several law enforcement agencies, with widespread adoption likely once the technology has sufficiently advanced.12
Pepper spray (OC) is typically dispersed from a handheld canister.21 However, it is also available in a number of different types of grenades and projectiles.22 CN can be dissolved in a solvent, used in irritant sprays like Mace, or deployed from thermal grenades.11
Health effects of tear gas exposures
Exposure to tear gas agents produces a wide spectrum of health effects, including acute and chronic effects. Studies have demonstrated specific receptor‐mediated mechanisms of action of tear gas agents.23, 24 Whereas these specific receptors contribute to their acute painful and irritant effects, the electrophilic reactivity of the agents, together with the toxicities of solvents and pyrotechnic reaction products, engages multiple toxicological mechanisms that remain to be studied.
Immediate exposure effects
CS and OC produce similar symptoms. Acute CS exposure at concentrations generally used by law enforcement for riot‐control purposes results in instantaneous irritation to the eyes, nose, mouth, skin, and respiratory tract.9 Dermal effects include itching, stinging, and redness, with potential blistering and allergic contact dermatitis.25 Ocular exposure can result in lacrimation, blepharospasm, itching, and burning sensation.26 When inhaled, CS often leads to coughing, choking, salivation, and chest tightness.11 OC exposure causes pain and tingling in the respiratory tract, accompanied by coughing.11 Signs and symptoms of OC contact with eyes include lacrimation, inflammation of the conjunctiva, blepharospasm, redness, pain, burning, and edema.14 Some evidence indicates that OC may also temporarily inhibit the blink reflex and limit responsiveness to mechanical and chemical stimuli to the eye.14 Like CS, dermal effects of OC can include pain, tingling, redness, swelling, and blistering.14 The effects of CN are similar to those of CS and OC,27 but are significantly more severe and potentially life threatening. CN is a more toxic lacrimator than CS and is more likely to cause serious injury to the skin.11
Respiratory effects
Much of the research surrounding the effects of tear gas exposure was derived from laboratory animal research or from small studies of previously healthy individuals in controlled conditions, and several believe this level of research is inadequate for safety assessment.17, 28 For example, one study concluding that tear gas exposure was not associated with increased airway resistance was conducted on a sample of only seven healthy military volunteers, and those with a history of chronic respiratory illness were excluded.16 Tear gas use in riots or instances of large‐scale civil disorder could result in extended, repeated, or highly concentrated exposures, which pose a greater threat to respiratory health.14, 29 High concentrations of CS or OC can cause severe respiratory symptoms, such as reactive airways dysfunction syndrome, in an individual exposed to both CS and OC and hemoptysis.30, 31, 32 Capsaicin infiltration of the lower respiratory tract can induce pulmonary edema, apnea, and respiratory arrest.11 Surveys performed after recent massive‐scale tear gas deployments in Turkey reported persistent cough, chest pain, sputum production, hemoptysis, breathing difficulties, and nasal discharge, sometimes lasting for weeks after exposure.28 Lung function tests observed restriction and medium and small airway obstruction that was more severe in women.22 Respiratory effects were also observed in residents of the areas where tear gas was deployed, suggesting that tear gas agents represent a persistent environmental health hazard.29
Another Turkish study with 93 males frequently exposed to tear gas and 55 unexposed subjects found that tear gas–exposed subjects were at greater risk for chronic bronchitis.33 A study of 34 young adults exposed to CS in a confined space during a confrontation with the police reported no long‐term sequelae.34
CS‐induced respiratory illness during military training
Unexpected respiratory risks linked to tear gas exposures were discovered in epidemiological studies by the U.S. Army, analyzing health effects in more than 6000 army recruits exposed to CS in chambers during gas mask–confidence training. This relatively young and healthy population developed a high risk of presenting with acute respiratory illness in the time after CS exposure, with increasing risk at higher exposure concentrations.35, 36 Exposure levels considered for many years safe and necessary for training were determined to far exceed the National Institute for Occupational and Safety and Health and Occupational Safety and Health Administration safety levels.37 These findings led to immediate measures limiting exposure concentrations and times, improving decontamination procedures, and imposing frequent hygiene and health monitoring. Measures of respiratory illness included throat pain, cough, bronchitis, nasopharyngitis, sinusitis, and other indications. CS exposures were also associated with an increase in respiratory infections, including influenza. Follow‐up studies demonstrated that lowering CS exposures during training effectively reduced the risk for respiratory illness.38 Whether these lower concentrations are also safe for diverse civilian populations remains unclear. Follow‐up epidemiological studies in military populations would represent a unique opportunity to identify potential long‐term health effects of tear gas exposures.
Ocular effects
Tear gas deployed at close range can cause severe ocular injuries, including corneal stromal edema, conjunctival tearing, and deep vascularization of the eye.39 Other ocular complications include vitreous hemorrhage, traumatic optic neuropathy, symblepharon, pseudopterygium, infective keratitis, trophic keratopathy, glaucoma, and cataracts.39 One report described four subjects who developed corneal erosion following exposure to pepper spray, indicating that OC or a solvent in the spray may cause nerve damage.40
Skin burns and dermatitis
Physicians examining CS‐exposed patients often report skin burns, especially when large quantities are used, as in a case involving a riot at a Vietnamese refugee detention center in Hong Kong.30, 32 Multiple cases of unusually severe skin reactions in response to CS exposure have been reported, including severe facial erythema and swelling that obscured vision.41 Physicians from the Department of Dermatology at San Francisco General Hospital observed severe CS‐induced erythematous dermatitis of the face, neck, and hands.42 Cases of allergic contact sensitization were reported with erythematous patches and multiple vesicular eruptions on the skin following heavy exposure to CS.43 Ninety percent of workers in a plant manufacturing a CS agent reported a history of dermatitis on the arms and neck, with 7% showing positive patch‐test reactions to CS, suggesting that CS may act as a contact sensitizer.44
Cardiovascular and gastrointestinal effects
Irritation of the gastrointestinal tract due to ingestion of compounds like CS may cause nausea, vomiting, diarrhea, and hematemesis.14, 32 Various cardiovascular effects, including tachycardia and transient hypertension, have been observed in some individuals, likely initiated by sensory–autonomic reflexes or anxiety, pain, or psychological distress.45
Severe injuries and deaths
There have been numerous case reports of injuries and fatalities associated with exposure to high concentrations of tear gas or exposure in enclosed spaces or for extended periods of time. Deaths and respiratory tract injuries were reported after release of tear gas in prisons.46, 47, 48 CS and OC are increasingly used in prison systems, often in enclosed and poorly ventilated spaces. Deaths of inmates with preexisting respiratory conditions have been linked to multiple CS and OC exposures and lack of decontamination.49 Other studies documented cases of death within 1 h of exposure to OC, though a direct causal link has not yet been established.14 Severe injuries and deaths have been reported during the massive‐scale deployments of tear gas munitions in Egypt, Turkey, Bahrain, and Brazil. These were often caused by direct or close impact of tear gas munitions causing severe head and eye injuries and burns.50, 51 A well‐documented case is the death of 37 Egyptian inmates in a prisoner van into which tear gas munitions were fired.52 Circumstantial reports suggest a correlation between CS exposure and miscarriage.16, 51, 53
Lack of epidemiological research in tear gas–exposed civilian populations and high‐risk groups
There is a significant amount of research examining the acute effects of RCA exposure among small samples of healthy individuals in controlled conditions, but little information has been gathered on the consequences of exposure in the field. A review on exposure to the CS tear gas agent attempted to compile case reports on the basis of PubMed and Scopus literature searches.54 The real‐world conditions in which tear gas is used make it difficult to discriminate between the effects of different RCAs and to conduct effective epidemiological investigations.16 It is often not possible to ascertain the exposure concentration and duration among exposed individuals, and weather or terrain factors can further complicate analysis. Because of the difficulties associated with conducting epidemiological investigations of RCA effects and the lack of public support for these studies, few epidemiological studies have been published, and the reliability of the results is often deficient. The situation is further complicated by the fact that research conducted by various military organizations is often classified,55 and organizations may be denied access to health information during instances of civil unrest.56
While prolonged exposure can lead to increased severity of symptoms, conclusions from past research indicated that most effects should resolve within minutes of removal from exposure. However, evidence supporting this conclusion came from significantly limited studies. For example, one oft‐cited study deemed CS tear gas safe on the basis of outcomes of controlled exposures of 35 healthy male volunteers, without considering the effects on children, women, the elderly, or subjects affected by preexisting conditions.26 Deficiencies in the currently available research have impeded understanding of all of the risks potentially associated with chemical RCAs. The effects of RCA exposure among sensitive populations and among those with underlying health conditions are one such area where the level of risk is unclear. Individuals suffering from asthma or reactive airways disease could be at greater risk for more serious adverse effects from tear gas exposure, as chemical RCAs cause significant respiratory symptoms, which could plausibly be exacerbated in the presence of underlying respiratory illness. Current research on the issue, however, remains equivocal on the topic. A study of CS exposure in one group found no increase in airway resistance after exposure, but the study subjects only included healthy volunteers, and those with a history of asthma were excluded from the study.16 A report of CS exposure in a nightclub indicated that patients with asthma experienced no greater sensitivity to the RCA,57 and similar results were published in the report of an inquiry into a CS exposure in Londonderry in 1969.58 However, according to a study of RCA exposure in South Korea, physicians reported that patients with asthma and chronic obstructive pulmonary disease experienced deterioration of lung function following tear gas exposure—some to a serious degree necessitating a longer stay at the hospital.16
Other populations besides those with underlying respiratory conditions may also be at a greater susceptibility to harm from RCAs. The British Department of Health and other sources reported that individuals with hypertension or cardiovascular disease, as well as those taking neuroleptic medications, may be more susceptible to CS, calling for more research to be done in these populations.11, 59 In one case study, a 40‐year‐old male was diagnosed with acute myocardial infarction (AMI) following exposure to pepper spray, indicating that OC could potentially be a triggering factor.60 There is a strong relationship between inhalation of particulate matter and AMI.61 While the acute pain and cardiorespiratory distress following exposure can contribute to triggering AMI, the consequences of inhalation of microencapsulated and precipitated particles, oil droplets, and particles generated during combustion from tear gas sprays or munitions need to be further investigated.
Biological targets and mechanisms
TRPV1: the target of capsaicin in pepper spray
The active noxious agent in pepper spray is capsaicin, purified and enriched from pungent chili peppers. The molecular target of capsaicin, TRPV1, was discovered in 1997.62 TRPV1 is a transient receptor potential (TRP) ion channel expressed in nociceptors, the pain‐sensing peripheral sensory nerves of the trigeminal, vagal, and dorsal root ganglia (DRG). Nociceptor nerve endings are present in all organs and the body surface, including the skin, cornea, conjunctiva, and the mucous membranes of the upper and lower airways and lung. TRPV1 is a nonselective cation channel that, when activated by capsaicin, promotes neuronal depolarization. TRPV1 is also activated when nerve endings are exposed to noxious heat, acting as a thermal warning sensor for imminent tissue damage. Tissue acidification or acid exposures lead to sensitization or activation of TRPV1. TRPV1 is sensitized through signaling from a range of G protein–coupled receptors and receptor tyrosine kinases activated during injury and inflammation. These include the bradykinin receptor, prostaglandin receptors, nerve growth factor receptors, and cytokine and chemokine receptors.
TRPA1: the reactive irritant receptor mediating the acute effects of tear gas agents
The tear gas agents CS, CN, and CR are structurally dissimilar, suggesting they might bind to different targets (Fig. 1). However, a single target, TRPA1, was identified as mediating the acute noxious effects of these agents and of many similarly acting chemical exposures.24, 63 TRPA1 is also a TRP ion channel and, similar to TRPV1, is expressed in nociceptors. Pain neurobiological studies initially revealed that TRPA1 is the target of mustard oil (allyl isothiocyanate), the pain‐inducing and lachrymatory product in mustard, wasabi, and horseradish.64 Together with capsaicin, mustard oil was used as an important chemical tool to characterize the function of nociceptor subtypes in pain transduction. Mustard oil is an electrophile, and TRPA1 was also found to be responsive to similar naturally occurring isothiocyanates and related compounds in onions and garlic.64, 65 Isocyanates, such as mustard oil, are electrophiles thought to act as defensive agents of the plants against herbivores. Mustard oil is not to be mistaken with sulfur mustard and nitrogen mustard, the blistering agents belonging to a different class of chemical warfare agents in mustard gas. While these agents share a similar odor with mustard oil, mustard gas exposure is not immediately painful and has delayed effects.
Intriguingly, pretreatment of animals with capsaicin desensitized them to perceiving pain from mustard oil, suggesting that receptors for these agents may be expressed in the same nerve fibers, where they desensitize each other. Indeed, TRPV1 and TRPA1 were found to be expressed in the same population of nociceptors. Toxicological studies have shown that capsaicin pretreatment desensitizes neuronal responses to a wide range of chemical sensory irritants targeting nociceptors. One example is the volatile electrophile acrolein, an unsaturated aldehyde and the major airborne irritant in smoke from fires and combusted tobacco and in diesel exhaust.66 Acrolein was used briefly as an irritant gas in warfare in World War I. Capsaicin pretreatment was shown to render mouse nasal trigeminal neurons unresponsive to airborne acrolein.66 Studies in heterologous expression systems revealed that both rodent and human TRPA1 channels were activated by acrolein.23 Similar effects were seen with croton aldehyde, another tobacco smoke aldehyde, and even saturated aldehydes, such as formaldehyde and acetaldehyde.
The discovery of TRPA1 as an electrophilic irritant receptor inspired additional studies that in 2008 identified TRPA1 as the principal target of the tear gas agents CN, CS, and CR, in vitro and in vivo.24, 63 These three agents are among the most potent TRPA1 agonists known, with CS and CR activating human TRPA1 channels in the low nanomolar or subnanomolar range, more than 10,000‐fold more potent than mustard oil and other natural TRPA1 agonists. Modified electrophilic CR‐based chemicals were even more potent than the parent compound.67 Mice with a targeted deletion in Trpa1 displayed no or only minimal acute pain behavior when exposed to CN or CS, confirming the essential role of TRPA1 in their sensory detection.24 In human studies, the potency of tear gas agent derivatives toward TRPA1 showed clear correlation with their perceived irritancy, suggesting that TRPA1 also contributes to tear gas sensing in humans.68
TRPA1 is activated by a large variety of structurally unrelated irritant chemicals.69 This sensitivity to multiple chemicals cannot be explained by a traditional pharmacological ligand–receptor model. Biochemical studies revealed a reactivity‐based activation mechanism of TRPA1, in which electrophilic and oxidizing activators modify cysteine residues in the N‐terminal domain of TRPA1, resulting in covalent modification leading to channel activation.70 Thus, TRPA1 can be considered a peripheral neuronal reactivity detector, signaling the danger of imminent injury by electrophilic or oxidant exposures.
Health effects related to TRP channel activation
The discovery of TRP ion channels as the primary sensory detectors for environmental, chemical, and physical stimuli in peripheral sensory neurons was a watershed in sensory neurobiology and pharmacology. While initial studies focused on the roles of TRPV1 and TRPA1 in pain, more recent discoveries revealed that these ion channels play fundamental roles in reflex responses in the respiratory, cardiovascular, digestive, and other organ systems and in acute and chronic inflammatory and degenerative conditions. These pathological mechanisms need to be taken into consideration when reassessing the acute and chronic health effects of tear gas exposures, especially in exposed individuals affected by chronic health conditions within a diverse civilian population.
Pain
Tear gas and pepper spray exposures cause immediate and severe pain leading to incapacitation. Ocular and nasal pain are sensed almost immediately and are initiated by activation of trigeminal nerve endings in the cornea and nasal passages that are highly sensitive to chemical exposures. Since TRPV1 and TRPA1 were identified as major pain‐initiating receptors, the pharmaceutical industry has developed a wide range of inhibitors for development as analgesics. Analgesic action was demonstrated in animal models of acute and inflammatory pain. In clinical trials, TRPV1 inhibitors alleviated heat‐induced pain, with moderate effects toward other pain modalities. The development of TRPA1 inhibitors began later, with clinical trials ongoing at this time.
Cough and airway obstruction
Cough is elicited when a chemical irritant activates vagal sensory nerve endings in the larynx. Vagal sensory nerves express higher levels of TRPV1 and TRPA1 than the trigeminal ganglia and DRG. Capsaicin is often used as a cough stimulus in clinical settings, and many airborne TRPA1 agonists are cough triggers. Tear gas and pepper spray exposures trigger cough directly but also cause profuse secretions within the airways due to sensory–autonomic reflexes. Secretions further aggravate cough and contribute to incapacitation by obstructing normal breathing and eliciting the fear of suffocation. Recent studies have implicated TRPA1 sensitization and heightened activity in chronic cough and cough hypersensitivity.71
Asthma
Recent studies in animal models of allergen‐induced asthma have shown that TRPA1 plays a critical role in the initiation and maintenance of asthmatic inflammation, airway hyperreactivity, and smooth muscle contraction.72, 73 Human genetic studies have associated polymorphisms in TRPA1 with reduced control of asthma.74 Irritant‐induced asthma, manifesting as airway hyperreactivity following irritant inhalation, was also shown to depend on TRPA1.75 With an asthma prevalence of 8.4% in the U.S. population and similar levels around the world, there is a high chance of exposure to tear gas and the development of complications in asthmatics. Indeed, the most severe complications reported after tear gas deployment involve asthma attacks.34
Chronic obstructive pulmonary disorder and lung injury
TRPA1 is activated by many of the principal irritants in tobacco smoke, including acrolein, crotonaldehyde, and smoke particulates.23, 74 In chronic smokers, inhalation of these irritants contributes to the etiology of chronic obstructive pulmonary disorder. TRPA1 has also been implicated in ventilator‐induced lung injury, in which mechanical stress and oxygen activate sensory neurons that may contribute to the observed inflammatory response. Activation of TRPA1 in the lung was shown to trigger the release of proinflammatory neuropeptides, such as CGRP, substance P, and neurokinin A.24 While additional research is needed to delineate the role of TRPA1 in lung injury, these findings suggest that TRPA1 activation may aggravate preexisting pulmonary inflammation, injury, and remodeling processes in smokers and other affected individuals.
Cardiac arrhythmia
Irritant exposures have been linked to cardiovascular stress and sensory–autonomic dysregulation of cardiovascular function. Studies in rats prone to arrhythmia have shown that respiratory exposures to diesel exhaust or acrolein strongly increase the risk of arrhythmia through sympathetic activation.76, 77 Rats treated with a TRPA1 inhibitor were resistant to exposure‐induced arrhythmias, suggesting that TRPA1 is a key chemical detector triggering circuits that alter cardiovascular control.76, 77 Tear gas exposures may have similar effects in humans, suggesting that exposed individuals with preexisting cardiovascular conditions and arrhythmias may be at increased risk of developing cardiovascular complications.
Dermatitis and itch
TRPA1 agonists, such as mustard oil, are known to cause skin inflammation and edema that are diminished in TRPA1‐deficient mice. TRPA1 plays a key role in the neuronal control of skin inflammation and in the neuronal transduction of itch signals mediated by a specialized subpopulation of sensory neurons.78 TRPA1 is coupled to pruritogen receptors and is essential for full development of inflammation and itch in hapten‐induced contact dermatitis.78 Tear gas exposures of the skin cause pain, skin edema, and inflammation with itching. Tear gas agents can likely act as haptens themselves, and skin hypersensitivity reactions to tear gas agents, including allergic contact dermatitis, have been reported.34, 43
Thus, atopic and exposed individuals affected by contact dermatitis may be at risk of developing adverse skin reactions. The appearance of clinical cutaneous symptoms varies widely from a few minutes to several weeks. A summary of dermal clinical symptoms following CS tear gas exposure and latency periods has been published.54
Peripheral nerve damage
The analgesic properties of TRPA1 inhibitors are currently being tested in clinical trials in patients affected by diabetic neuropathy, a painful neurodegenerative condition. TRPA1 plays a key role in the peripheral neuronal hypersensitivity to metabolic stress in diabetic animal models.79 Chronic TRPA1 activity leads to nerve calcium overload and excitotoxicity, resulting in chronic pain signaling and peripheral neurodegeneration, leading to loss of sensation or altered sensations. Other chemically induced neurodegenerative conditions were also linked to TRPA1, including chemotherapy‐induced neuropathies.80 With more than 9% of the U.S. population affected by diabetes and diabetes rates approaching similar levels in other countries, this population needs to be considered at risk during tear gas exposures. Ligands of TRPV1 and TRPA1, when applied topically, are known to cause extensive desensitization and remodeling of cutaneous nerve endings. With tear gas agents being so highly potent, it is likely that similar effects occur. While desensitized nerve endings can recover and normal sensory capacity can be reestablished, high‐level exposures and local contamination may cause long‐term damage to the underlying sensory innervation.
TRP channel inhibitors as countermeasures against tear gas effects
At this time, no mechanism‐based countermeasures are available to alleviate the noxious effects of tear gas and pepper spray exposures. Countermeasures mostly involve decontamination strategies, including rinsing with water and buffered solutions, discarding contaminated clothing, and medical supportive treatment.
Highly potent and selective TRPV1 inhibitors have been developed and tested in animal studies and in clinical trials with proven efficacies for capsaicin‐induced and thermally induced pain.81 It remains unclear whether these inhibitors will be developed further toward U.S. Food and Drug Administration approval for pain indications and will be considered for testing as countermeasures against pepper spray exposures.
A TRPA1 inhibitor was efficacious for prevention of tear gas agent (CN and CS) exposure–induced ocular pain in mice.24 Since anti‐inflammatory and analgesic effects have been observed for TRPA1 inhibitors in multiple models of chemical injury and inflammation, it is highly likely that TRPA1 inhibitors will alleviate at least some of the tear gas–induced effects. There remains concern about the specificity and poor solubility of the tool compounds available for TRPA1 inhibition. The conclusion of clinical trials using TRPA1 inhibitors in diabetic neuropathic patients is eagerly awaited with the hope that more advanced inhibitors will be made available for testing in other conditions, including irritant and tear gas agent exposures.
Other targets of tear gas agents
Owing to their electrophilic properties, tear gas agents likely react with many other biomolecules in the eyes, respiratory tract, and skin. The nature of these targets is largely unknown. Similar to acrolein and related electrophiles, tear gas agents may damage and deplete biological redox systems in the lining fluids of epithelia and within cells and mitochondria, modify structural proteins and nucleic acids, and inactivate enzymes. There has been minimal research on endocrine effects, immunologic consequences, and histological changes from CS exposure, but some animal studies indicate that potential effects may occur. Studies in rats injected with CS found histological changes in the adrenal gland and the thyroid, though it is not clear whether dermal or inhalational exposure to CS in humans would result in a similar response.82 Another study of CS exposure in mice reported suppression of the humoral immune response and elevated corticosteroid levels.83 The mutagenicity and potential carcinogenic effects of RCAs are also not well understood, with research clearly lacking. Some laboratory studies indicate that CS is not mutagenic84 or is weakly mutagenic,85 but the results of carcinogenicity studies have not been confirmed,86 and much of the research has been limited to laboratory studies rather than human studies. The mutagenicity of capsaicinoids has been tested extensively, but the results have been conflicting.14 Dispersal of RCAs involving pyrotechnic mixtures can produce thermal degradation by‐products that could potentially be dangerous to human health.87
Discussion and recommendations
The decision by law enforcement to use tear gas during civil disorder is understandable, as CN, CS, and OC are effective RCAs and reduce the risk of injury to law enforcement personnel and demonstrators when used instead of physical force.10, 88 However, the massive increase in tear gas deployments worldwide, accompanied by advances in formulations and deployment technologies and the often‐observed absence or disregard of evidence‐based deployment rules and operating procedures, is of great concern. Epidemiological research on tear gas health effects is clearly deficient and has received little public support. Evidence from the limited epidemiological studies available and from case studies demonstrates that tear gas agents have the potential to cause serious harm and present specific threats to vulnerable populations, including children, women, and individuals affected by respiratory, cutaneous, and cardiovascular morbidities. While breakthroughs in mechanistic basic science have been made, discovering TRPV1 and TRPA1 as agent receptors, evidence that these targets are involved in multiple morbidities has not been taken into consideration for reassessment of tear gas use, and mechanism‐based countermeasures development has not progressed.
Based on these deficiencies, we make the following recommendations:
-
(1)
The toxicological effects of tear gas agents should be reassessed using state‐of‐the‐art toxicological techniques utilizing toxicology in the 21st century (Tox21) collaboration approaches (https://www.epa.gov/chemical-research/toxicology-testing-21st-century-tox21) and animal models of respiratory, cutaneous, and cardiovascular morbidities, taking into account age and sex differences.
-
(2)
Epidemiological research networks should be established to develop standardized questionnaires; collect medical data, tissue, and fluid samples from exposed patients; curate biorepositories; perform environmental analysis; and conduct follow‐up investigations. Mandatory cooperation of law enforcement with this network before, during, and after deployment needs to be specified in standard operating procedures.
-
(3)
Epidemiological investigation of tear gas–exposed military populations should be extended to identify potential long‐term effects.
-
(4)
Efficient countermeasures need to be developed for treatment of individuals exposed to high levels of tear gas agents. Countermeasures should include new decontamination strategies based on the chemical properties of tear gas agents, their solvents, and pyrotechnic products and novel pharmacological inhibitors of the TRP ion channels TRPV1 and TRPA1, currently in clinical trials for pain indications.
-
(5)
Efforts must be made to make tear gas munitions traceable to document use volume, deployment locations, and numbers.
-
(6)
Efficient export and world trade controls must be enacted to prevent procurement and manufacture of RCAs by state actors and organizations that repeatedly deploy tear gas agents resulting in deaths, mass injuries, and widespread contamination and prohibit medical care and act against medical personnel treating the exposed.
Conflicts of interest
Sven‐Eric Jordt is serving on the Scientific Advisory Board of Hydra Biosciences Inc., a biopharmaceutical company developing TRP ion channel inhibitors for the treatment of pain and inflammation.
Acknowledgments
This work was supported by the NIH CounterACT Program; the Office of the Director, National Institutes of Health; and the National Institute of Environmental Health Sciences (NIEHS), Grant numbers U01ES015674 and R21ES022875 (to S‐E.J.) and R01ES15532 (to E.R.S.). [Correction added on October 7, 2016, after first online publication: The preceding Acknowledgments section was added.]
References
- 1. AFP . 2013. Turkey violence flares after police storm protest park. Accessed May 11, 2016. https://fr.news.yahoo.com/video/turkey-violence-flares-police-storm-191500809.html.
- 2. Lowery, W. 2014. Police use tear gas on crowd in Ferguson, Mo., protesting teen's death. Accessed May 20, 2016. http://www.washingtonpost.com/news/post-nation/wp/2014/08/12/police-use-tear-gas-on-crowd/.
- 3. Pomfret, J. & Lee Y.. 2014. Hong Kong democracy protesters defy tear gas, baton charge in historic standoff. Accessed May 12, 2016. http://www.reuters.com/article/us-hongkong-china-idUSKCN0HN03Q20140928.
- 4. Edmonds, L. 2013. Greece rocked by night of riots after anti‐fascist rapper was stabbed to death by member of far‐right group Golden Dawn. Accessed May 12, 2016. http://www.dailymail.co.uk/news/article-2424967/Pavlos-Fyssas-murder-Golden-Dawn-sparks-Greece-riots.html.
- 5. Romero, S. 2013. Thousands gather for protests in Brazil's largest cities. Accessed May 20, 2016. http://www.nytimes.com/2013/06/18/world/americas/thousands-gather-for-protests-in-brazils-largest-cities.html.
- 6. CBS News . 2011. Egypt police tear gas Tahrir Square protesters. Accessed May 12, 2016. http://www.cbsnews.com/news/egypt-police-tear-gas-tahrir-square-protesters/.
- 7. BBC . 2012. Bahrain authorities ‘weaponising’ tear gas. Accessed May 20, 2016. http://www.bbc.com/news/world-middle-east-19078659.
- 8. Feigenbaum, A. 2014. 100 years of tear gas. Accessed May 20, 2016. http://www.theatlantic.com/international/archive/2014/08/100-years-of-tear-gas/378632/?single_page=true.
- 9. Blain, P.G. 2003. Tear gases and irritant incapacitants. 1‐Chloroacetophenone, 2‐chlorobenzylidene malononitrile and dibenz[b,f]‐1,4‐oxazepine. Toxicol. Rev. 22: 103–110. [DOI] [PubMed] [Google Scholar]
- 10. Schep, L.J. , Slaughter R.J. & McBride D.I.. 2013. Riot control agents: the tear gases CN, CS and OC—a medical review. J. R. Army Med. Corps 161: 94–99. [DOI] [PubMed] [Google Scholar]
- 11. Tuorinsky, S.D. & Sciuto A.M.. 2008. “Medical aspects of chemical warfare” In Textbooks of Military Medicine. Tuorinsky S.D., Ed.: 339–370. Washington, DC: Office of the Surgeon General. [Google Scholar]
- 12. Olajos, E.J. & Stopford W.. 2004. Riot Control Agents: Issues in Toxicology, Safety, and Health. Boca Raton, FL: CRC Press. [Google Scholar]
- 13. Corson, B.B. & Stoughton R.W.. 1928. Reactions of alpha, beta‐unsaturated dinitriles. J. Am. Chem. Soc. 50: 2825–2837. [Google Scholar]
- 14. Olajos, E.J. & Salem H.. 2001. Riot control agents: pharmacology, toxicology, biochemistry and chemistry. J. Appl. Toxicol. 21: 355–391. [DOI] [PubMed] [Google Scholar]
- 15. Ballantyne, B. & Swanston D.W.. 1978. The comparative acute mammalian toxicity of 1‐chloroacetophenone (CN) and 2‐chlorobenzylidene malononitrile (CS). Arch. Toxicol. 40: 75–95. [DOI] [PubMed] [Google Scholar]
- 16. Hu, H. , Fine J., Epstein P., et al 1989. Tear gas—harassing agent or toxic chemical weapon? JAMA 262: 660–663. [DOI] [PubMed] [Google Scholar]
- 17. Kastan, B. 2012. The chemical weapons convention and riot control agents: advantages of a “methods” approach to arms control. Duke J. Comp. & Int'l L. 22: 267–290. [Google Scholar]
- 18. Smith, J. & Greaves I.. 2002. The use of chemical incapacitant sprays: a review. J. Trauma 52: 595–600. [DOI] [PubMed] [Google Scholar]
- 19. CDC . 2016. Isobutyl methyl ketone, methyl isobutyl ketone, 4‐methyl 2‐pentanone, MIBK. Accessed May 03, 2016. http://www.cdc.gov/niosh/npg/npgd0326.html.
- 20. Ledgard, J. 2007. The Preparatory Manual of Black Powder and Pyrotechnics. Raleigh, NC: Lulu.com. [Google Scholar]
- 21. US Marine Corps . 1996. Flame, Riot Control, and Herbicide Operations. Washington, DC: U.S. Marine Corps. [Google Scholar]
- 22. Dagli, E. , Uslu E., Ozkan G., et al 2014. Immediate effects of tear gas on lung functions. Am. J. Respir. Crit. Care Med. 189: A5775. [Google Scholar]
- 23. Bautista, D.M. , Jordt S.E., Nikai T., et al 2006. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282. [DOI] [PubMed] [Google Scholar]
- 24. Bessac, B.F. , Sivula M., von Hehn C.A., et al 2008. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 23: 1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holland, P. & White R.G.. 1972. The cutaneous reactions produced by o‐chlorobenzylidenemalononitrile and ω‐chloroacetopheneone when applied directly to the skin of human subjects. Br. J. Dermatol. 86: 150–154. [DOI] [PubMed] [Google Scholar]
- 26. Beswick, F.W. , Holland P. & Kemp K.H.. 1972. Acute effects of exposure to orthochlorobenzylidene malononitrile (CS) and the development of tolerance. Br. J. Ind. Med. 29: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee, R.J. , Yolton R.L., Yolton D.P., et al 1996. Personal defense sprays: effects and management of exposure. J. Am. Optom. Assoc. 67: 548–560. [PubMed] [Google Scholar]
- 28. Dagli, E. , Uslu E., Ozkan G., et al 2014. Respiratory effects of tear gas exposure. Accessed May 20, 2016. http://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A3142.
- 29. Dagli, E. , Uslu E., Ozkan G., et al 2014. Respiratory effects of tear gas exposure on innocent by‐standers. Accessed May 20, 2016. http://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A3143.
- 30. Zekri, A.M. , King W.W., Yeung R., et al 1995. Acute mass burns caused by o‐chlorobenzylidene malononitrile (CS) tear gas. Burns 21: 586–589. [DOI] [PubMed] [Google Scholar]
- 31. Roth, V.S. & Franzblau A.. 1996. RADS after exposure to a riot‐control agent: a case report. J. Occup. Environ. Med. 38: 863–865. [DOI] [PubMed] [Google Scholar]
- 32. Anderson, P.J. , Lau G.S., Taylor W.R., et al 1996. Acute effects of the potent lacrimator o‐chlorobenzylidene malononitrile (CS) tear gas. Hum. Exp. Toxicol. 15: 461–465. [DOI] [PubMed] [Google Scholar]
- 33. Arbak, P. , Baser I., Kumbasar O.O., et al 2014. Long term effects of tear gases on respiratory system: analysis of 93 cases. Sci. World J. 2014: 963638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karagama, Y.G. , Newton J.R. & Newbegin C.J.. 2003. Short‐term and long‐term physical effects of exposure to CS spray. J. R. Soc. Med. 96: 172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hout, J.J. , White D.W., Artino A.R., et al 2014. O‐chlorobenzylidene malononitrile (CS riot control agent) associated acute respiratory illnesses in a U.S. Army basic combat training cohort. Mil. Med. 179: 793–798. [DOI] [PubMed] [Google Scholar]
- 36. Hout, J.J. , White D.W., Stubner A., et al 2014. O‐chlorobenzylidene malononitrile (CS riot control agent) exposure in a U.S. Army basic combat training cohort. J. Environ. Health 77: 14–21. [PubMed] [Google Scholar]
- 37. Hout, J.J. , Kluchinsky T., LaPuma P.T., et al 2011. Evaluation of CS (o‐chlorobenzylidene malononitrile) concentrations during U.S. Army mask confidence training. J. Environ. Health 74: 18–21. [PubMed] [Google Scholar]
- 38. Hout, J.J. , White D.W., Stevens M., et al 2014. Evaluation of an intervention to reduce tear gas exposures and associated acute respiratory illnesses in a US Army basic combat training cohort. Open Epidemiol. J. 7: 34–45. [Google Scholar]
- 39. Gray, P.J. & Murray V.. 1995. Treating CS gas injuries to the eye. Exposure at close range is particularly dangerous. BMJ 311: 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holopainen, J.M. , Moilanen J.A., Hack T., et al 2003. Toxic carriers in pepper sprays may cause corneal erosion. Toxicol. Appl. Pharmacol. 186: 155–162. [DOI] [PubMed] [Google Scholar]
- 41. Varma, S. & Holt P.J.. 2001. Severe cutaneous reaction to CS gas. Clin. Exp. Dermatol. 26: 248–250. [DOI] [PubMed] [Google Scholar]
- 42. Parneix‐Spake, A. , Theisen A., Roujeau J.C., et al 1993. Severe cutaneous reactions to self‐defense sprays. Arch. Dermatol. 129: 913. [DOI] [PubMed] [Google Scholar]
- 43. Ro, Y.S. & Lee C.W.. 1991. Tear gas dermatitis. Allergic contact sensitization due to CS. Int. J. Dermatol. 30: 576–577. [PubMed] [Google Scholar]
- 44. Shmunes, E. & Taylor J.S.. 1973. Industrial contact dermatitis. Effect of the riot control agent ortho‐chlorobenzylidene malononitrile. Arch. Dermatol. 107: 212–216. [DOI] [PubMed] [Google Scholar]
- 45. Beswick, F.W. 1983. Chemical agents used in riot control and warfare. Hum. Toxicol. 2: 247–256. [DOI] [PubMed] [Google Scholar]
- 46. Thorburn, K.M. 1982. Injuries after use of the lacrimatory agent chloroacetophenone in a confined space. Arch. Environ. Health 37: 182–186. [DOI] [PubMed] [Google Scholar]
- 47. Stein, A.A. & Kirwan W.E.. 1964. Chloracetophenone (tear gas) poisoning: a clinico‐pathologic report. J. Forensic Sci. 9: 374–382. [PubMed] [Google Scholar]
- 48. Chapman, A.J. & White C.. 1978. Death resulting from lacrimatory agents. J. Forensic Sci. 23: 527–530. [PubMed] [Google Scholar]
- 49. Brown, J.K. 2014. After Florida inmate's lethal gassing, claims of cover‐up. Accessed March 30, 2016. http://www.miamiherald.com/news/politics-government/article1985286.html.
- 50. Clarot, F. , Vaz E., Papin F., et al 2003. Lethal head injury due to tear‐gas cartridge gunshots. Forensic Sci. Int. 137: 45–51. [DOI] [PubMed] [Google Scholar]
- 51. Atkinson, H.G. & Sollom R.. 2012. Bahrain's unprecedented use of toxic chemical agents against civilians. Accessed March 30, 2016. http://physiciansforhumanrights.org/library/reports/weaponizing-tear-gas.html.
- 52. BBC . 2014. Egypt police convicted over detainee tear‐gas deaths. Accessed March 30, 2016. http://www.bbc.com/news/world-middle-east-26626367.
- 53. Hayman, M. 2011. Chile suspends use of tear gas amid concerns over miscarriages. Accessed May 30, 2016. http://latindispatch.com/2011/05/19/chile-suspends-use-of-tear-gas-amid-concerns-over-miscarriages/.
- 54. Dimitroglou, Y. , Rachiotis G. & Hadjichristodoulou C.. 2015. Exposure to the riot control agent CS and potential health effects: a systematic review of the evidence. Int. J. Environ. Res. Public Health 12: 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schindel, H.J. 1993. [Assessment of health effects of CS gas]. Gesundheitswesen 55: 372–375. [PubMed] [Google Scholar]
- 56. Human Rights Watch . 2011. Egypt: documented death toll from protests tops 300. Accessed June 11, 2016. https://www.hrw.org/news/2011/02/08/egypt-documented-death-toll-protests-tops-300.
- 57. Breakell, A. & Bodiwala G.G.. 1998. CS gas exposure in a crowded night club: the consequences for an accident and emergency department. J. Accid. Emerg. Med. 15: 56–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Himsworth, H. , Dornhorst H.C. & Thompson R.H.S. 1969. Report of an enquiry into the medical and toxicological aspects of CS (orthochlorohenzylidene malononitrile). Part I. Enquiry into the medical situation following the use of CS in Londonderry on the 13 and 14 August 1969. HMSO, London.
- 59. Fraunfelder, F.T. 2000. Is CS gas dangerous? Current evidence suggests not but unanswered questions remain. BMJ 320: 458–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cil, H. , Atilgan Z.A., Islamoglu Y., et al 2012. Is the pepper spray a triggering factor in myocardial infarction? A case report. Eur. Rev. Med. Pharmacol. Sci. 16(Suppl. 1): 73–74. [PubMed] [Google Scholar]
- 61. Claeys, M.J. , Rajagopalan S., Nawrot T.S., et al 2016. Climate and environmental triggers of acute myocardial infarction. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehw151. [DOI] [PubMed] [Google Scholar]
- 62. Caterina, M.J. , Schumacher M.A., Tominaga M., et al 1997. The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 389: 816–824. [DOI] [PubMed] [Google Scholar]
- 63. Brone, B. , Peeters P.J., Marrannes R., et al 2008. Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol. Appl. Pharmacol. 231: 150–156. [DOI] [PubMed] [Google Scholar]
- 64. Jordt, S.E. , Bautista D.M., Chuang H.H., et al 2004. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265. [DOI] [PubMed] [Google Scholar]
- 65. Bautista, D.M. , Movahed P., Hinman A., et al 2005. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. U.S.A. 102: 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morris, J.B. , Stanek J. & Gianutsos G.. 1999. Sensory nerve‐mediated immediate nasal responses to inspired acrolein. J. Appl. Physiol. (1985) 87: 1877–1886. [DOI] [PubMed] [Google Scholar]
- 67. Gijsen, H.J. , Berthelot D., Zaja M., et al 2010. Analogues of morphanthridine and the tear gas dibenz[b,f][1,4]oxazepine (CR) as extremely potent activators of the human transient receptor potential ankyrin 1 (TRPA1) channel. J. Med. Chem. 53: 7011–7020. [DOI] [PubMed] [Google Scholar]
- 68. Lindsay, C.D. , Green C., Bird M., et al 2015. Potency of irritation by benzylidenemalononitriles in humans correlates with TRPA1 ion channel activation. R. Soc. Open Sci. 2: 140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu, B. , Fan L., Nilius B., et al 2015. Transient receptor potential channels: TRPA1. Accessed June 11, 2016. http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=485.
- 70. Hinman, A. , Chuang H.H., Bautista D.M., et al 2006. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. U.S.A. 103: 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chung, K.F. , Canning B. & McGarvey L.. 2015. Eight International London Cough Symposium 2014: cough hypersensitivity syndrome as the basis for chronic cough. Pulm. Pharmacol. Ther. 35: 76–80. [DOI] [PubMed] [Google Scholar]
- 72. Caceres, A.I. , Brackmann M., Elia M.D., et al 2009. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. U.S.A. 106: 9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Raemdonck, K. , de Alba J., Birrell M.A., et al 2012. A role for sensory nerves in the late asthmatic response. Thorax 67: 19–25. [DOI] [PubMed] [Google Scholar]
- 74. Deering‐Rice, C.E. , Shapiro D., Romero E.G., et al 2015. Activation of transient receptor potential ankyrin‐1 by insoluble particulate material and association with asthma. Am. J. Respir. Cell Mol. Biol. 53: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hox, V. , Vanoirbeek J.A., Alpizar Y.A., et al 2013. Crucial role of transient receptor potential ankyrin 1 and mast cells in induction of nonallergic airway hyperreactivity in mice. Am. J. Respir. Crit. Care Med. 187: 486–493. [DOI] [PubMed] [Google Scholar]
- 76. Hazari, M.S. , Griggs J., Winsett D.W., et al 2014. A single exposure to acrolein desensitizes baroreflex responsiveness and increases cardiac arrhythmias in normotensive and hypertensive rats. Cardiovasc. Toxicol. 14: 52–63. [DOI] [PubMed] [Google Scholar]
- 77. Hazari, M.S. , Haykal‐Coates N., Winsett D.W., et al 2011. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ. Health Perspect. 119: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu, B. , Escalera J., Balakrishna S., et al 2013. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 27: 3549–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Koivisto, A. , Hukkanen M., Saarnilehto M., et al 2012. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol. Res. 65: 149–158. [DOI] [PubMed] [Google Scholar]
- 80. Trevisan, G. , Materazzi S., Fusi C., et al 2013. Novel therapeutic strategy to prevent chemotherapy‐induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 73: 3120–3131. [DOI] [PubMed] [Google Scholar]
- 81. Kaneko, Y. & Szallasi A.. 2014. Transient receptor potential (TRP) channels: a clinical perspective. Br. J. Pharmacol. 171: 2474–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chowdhury, A.R. , Deshmukh M.B., Raghuveeran C.D., et al 1978. Histological changes in thyroid of rat under the acute exposure of O‐chloro‐benzylidine malononitrile. Experientia 34: 1327. [DOI] [PubMed] [Google Scholar]
- 83. Nagarkatti, M. , Nagarkatti P.S. & Raghuveeran C.D.. 1981. Short‐term toxicity studies of O‐chlorobenzylidene malononitrile on humoral immunity in mice. Toxicol. Lett. 8: 73–76. [DOI] [PubMed] [Google Scholar]
- 84. Meshram, G.P. , Malini R.P. & Rao K.M.. 1992. Mutagenicity evaluation of riot control agent o‐chlorobenzylidene malononitrile (CS) in the Ames Salmonella/microsome test. J. Appl. Toxicol. 12: 377–384. [DOI] [PubMed] [Google Scholar]
- 85. von Daniken, A. , Friederich U., Lutz W.K., et al 1981. Tests for mutagenicity in Salmonella and covalent binding to DNA and protein in the rat of the riot control agent o‐chlorobenzylidene malononitrile (CS). Arch. Toxicol. 49: 15–27. [DOI] [PubMed] [Google Scholar]
- 86. Committee on Toxicology, Board on Toxicology and Environmental Health Hazards, Commission on Life Sciences & National Research Council . 1984. Possible Long‐Term Health Effects of Short‐Term Exposure to Chemical Agents. Cholinesterase Reactivators, Psychochemicals and Irritants and Vesicants. Vol. 2 Washington, DC: The National Academies Press. [Google Scholar]
- 87. Hout, J.J. , Hook G.L., LaPuma P.T., et al 2010. Identification of compounds formed during low temperature thermal dispersion of encapsulated o‐chlorobenzylidene malononitrile (CS riot control agent). J. Occup. Environ. Hyg. 7: 352–357. [DOI] [PubMed] [Google Scholar]
- 88. MacDonald, J.M. , Kaminski R.J. & Smith M.R.. 2009. The effect of less‐lethal weapons on injuries in police use‐of‐force events. Am. J. Public Health 99: 2268–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]


