Abstract
Aims
To evaluate the efficacy and safety of basal insulin peglispro (BIL) with those of insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (T2D).
Methods
In this phase III, multicentre, double‐blind, 26‐week study, we randomized patients with T2D [glycated haemoglobin (HbA1c) ≥7 and <12%, on ≥1 insulin injections daily) to BIL (n = 691) or glargine (n = 678), in combination with lispro.
Results
At week 26, the primary objective of non‐inferiority of BIL versus glargine for HbA1c reduction was achieved (least squares mean difference −0.21%; 95% confidence interval −0.31 to −0.11%), with statistical superiority of BIL with multiplicity adjustment (p < 0.001). HbA1c at baseline was 8.4% versus 8.5% for BIL versus glargine and at 26 weeks it was 6.8% versus 7.0%. At 26 weeks, more patients reached HbA1c <7% with BIL than with glargine (63.3% vs 53.3%; p < 0.001), the nocturnal hypoglycaemia rate (≤3.9 mmol/l) was lower with BIL (0.51 vs 0.92 events/30 days; p < 0.001), but the daytime hypoglycaemia rate was higher with BIL (5.47 vs 4.53 events/30 days; p < 0.001). The total hypoglycaemia relative rate was 1.10 (p = 0.053). At 26 weeks, patients in the BIL group had lower fasting serum glucose levels, higher basal insulin dosing, with no statistically significant difference in prandial or total insulin dosing, reduced glucose variability and less weight gain (1.3 kg vs 2.2 kg) compared with the glargine group. The BIL group had higher mean triglyceride and aminotransferase levels.
Conclusions
In patients with T2D, BIL with insulin lispro provided greater improvement in glycaemic control with less nocturnal hypoglycaemia, lower glucose variability and less weight gain compared with glargine. The daytime hypoglycaemia rate and mean triglyceride and aminotransferase levels were higher with BIL.
Keywords: basal insulin, glycaemic control, hypoglycaemia, randomized trial, type 2 diabetes
Introduction
The development and progression of type 2 diabetes (T2D) is characterized by insulin resistance and defects in pancreatic β‐cell function 1, 2. Although T2D treatment is individualized, treatment intensification to basal insulin and eventually to basal‐bolus therapy is required for a significant number of patients 2, 3, 4. Intensive insulin therapy and glycaemic control increase the risk of hypoglycaemia 2, 4, 5. The development of an ideal basal insulin with 24‐h basal coverage, reduced hypoglycaemia risk, decreased glucose variability and effective suppression of hepatic glucose production, with minimal weight gain and/or weight loss, has not yet been fully achieved 6, although newer basal insulins such as degludec and glargine U‐300 have some of the aforementioned attributes 7, 8.
Basal insulin peglispro (BIL) is a novel basal insulin analogue comprised of insulin lispro covalently bound to a 20 kDa polyethylene glycol moiety, and has a larger hydrodynamic size than lispro 9. BIL has delayed absorption and reduced clearance, with a half‐life of 2–3 days in patients with T2D 10, 11. In healthy subjects and in patients with type 1 diabetes (T1D), BIL resulted in similar inhibition of hepatic glucose output, but reduced stimulation of glucose disposal compared with glargine 12, 13, 14. Patients with T1D treated with equivalent therapeutic concentrations of BIL versus glargine had significantly higher free fatty acid and glycerol levels during euglycaemic clamp 12. These human data are consistent with a conscious dog study showing that BIL had a peripheral‐to‐hepatic activity distribution more like endogenous insulin 15.
The present 26‐week, double‐blind, phase III study is the first trial to evaluate the efficacy and safety of BIL compared with insulin glargine U‐100, both in combination with prandial insulin lispro, in patients with T2D previously treated with insulin.
Materials and Methods
This phase III, double‐blind, randomized, controlled, multinational, parallel‐arm trial was approved by local ethics committees and conducted according to International Conference on Harmonisation Good Clinical Practice guidelines. Written informed consent was obtained from all patients. An unblinded independent data monitoring committee monitored patient safety. The study was conducted from January 2012 to August 2013 at 156 sites in 24 countries by endocrinologists or physicians with insulin clinical trial expertise (Appendix S1, Supporting Information). The clinical trial registry number was: NCT01468987.
Participants
Adults (aged ≥ 18 years) with T2D 16 [glycated haemoglobin (HbA1c) ≥7.0 and <12.0%] treated with one or more insulin injections daily were eligible. Participants were allowed to continue metformin; other oral antihyperglycaemic medications were discontinued at randomization. Appendix S2, Supporting Information shows the additional inclusion/exclusion criteria.
Study Design and Treatment
The study included a 2‐week pre‐randomization period, including electronic diary (e‐diary) collection of baseline hypoglycaemia data, 26 weeks of double‐blind treatment, and a 4‐week safety follow‐up period after discontinuation of study basal insulin (Figure S1, Supporting Information). Randomization (1 : 1 BIL:glargine) was stratified by HbA1c (≤8.5, >8.5%), LDL cholesterol (<2.6, ≥2.6 mmol/l), country and baseline number (1, 2, ≥3) of daily insulin injections (Appendix S3, Supporting Information). Lipid and hepatic criteria for study insulin discontinuation are listed in Appendix S4, Supporting Information.
Study basal insulins were injected once daily at approximately the same time at bedtime using a syringe and covered vial for blinding purposes. Based on phase II studies, BIL was formulated at 900 nmol/ml to achieve a U‐100 concentration, as 9 nmol BIL has approximately the same effect on glycaemic measures as 1 unit of glargine 17. Insulin lispro was injected by disposable pen with meals, starting at randomization. A customized wireless e‐diary system was used to collect self‐monitored blood glucose (SMBG) values, hypoglycaemic events/outcomes, and insulin dosing data 18. A treat‐to‐target algorithm was used with a fasting/pre‐meal SMBG goal of 5.6 mmol/l and a bedtime goal of 7.2 mmol/l (Appendix S5, Supporting Information). The e‐diary included the three protocol bolus dosing plans (Appendix S5, Supporting Information) and provided basal and bolus protocol algorithm dosing recommendations, which could be adjusted by investigators for individual patient considerations. Insulin dosing was assessed weekly for 12 weeks and thereafter at each visit, or more often as needed.
Hypoglycaemia was defined as SMBG ≤3.9 mmol/l or hypoglycaemia signs/symptoms. Nocturnal hypoglycaemia was defined both as an event between bedtime and waking, as recorded by the patient in the e‐diary, and between 22:00 and 10:00 hours, as e‐diaries collected SMBG values with the time/date of measurement. Hypoglycaemia at other times was defined as daytime hypoglycaemia. Documented symptomatic hypoglycaemia was defined as both signs/symptoms and SMBG ≤3.9 mmol/l. Severe hypoglycaemia was investigator‐determined as accompanied by neurological impairment requiring medical assistance to administer carbohydrates, glucagon, other resuscitative actions. Patients performed SMBG with daily 4‐point SMBG profiles (fasting, pre‐meal, bedtime), two 9‐point SMBG profiles (fasting, post‐morning meal, pre‐/post‐midday/evening meal, bedtime, 03:00 hours, next day fasting) the week before pre‐specified visits, and whenever hypoglycaemia was suspected.
Deaths and non‐fatal cardiovascular events including myocardial infarction, stroke and hospitalization for unstable angina were adjudicated by an independent Clinical Endpoint Committee. Lipid‐lowering therapy adjustments were prohibited from week 0 to week 12.
Sample Size and Statistical Analysis
A total of 1369 randomized patients provided ≥99% statistical power to demonstrate non‐inferiority of BIL to glargine [margin = 0.4%] for HbA1c change from 0 to 26 weeks, with assumptions of no treatment difference, standard deviation 1.1%, a two‐sided α value of 0.05, and a 20% dropout rate. This sample size also provided ≥80% power to demonstrate the superiority of BIL over glargine for HbA1c change, nocturnal hypoglycaemia rate, and fasting serum glucose (FSG) level.
Data were analysed according to the pre‐defined statistical analysis plan. The primary objective was non‐inferiority of BIL to glargine for HbA1c change from week 0 (baseline) to week 26. To control for overall type 1 error at α = 0.05, a sequential gatekeeping strategy was used to adjust for multiplicity for the primary and six key secondary objectives 19. The gated objective was achieved if all preceding objectives were met, and the objective reached statistical significance at α = 0.05. The gated secondary objectives (in order) were: superiority of BIL to glargine at 26 weeks for: nocturnal hypoglycaemia rate, proportion of patients with HbA1c <7% and no nocturnal hypoglycaemia, HbA1c change from baseline, proportion of patients with HbA1c <7%, FSG level and total hypoglycaemia rate. Other analyses were not adjusted for multiplicity.
Analyses (sas 9.1 or higher, Cary, NC, USA) were conducted in all randomized patients who received at least one study insulin dose. Mixed‐model repeated measures analysis was used for continuous outcomes with repeated post‐baseline measurements. Between‐treatment differences are presented as least squares (LS) mean (BIL‐glargine). Analysis of variance or analysis of covariance was used for continuous variables collected at baseline and/or endpoint. Logistic regression was used for categorical efficacy outcomes and Fisher's exact test for other categorical outcomes. Negative binomial regression was used to analyse the hypoglycaemia rate 20. The baseline value of the analysis variable was included as a covariate in the analysis model when applicable. All tests were conducted at two‐sided α = 0.05, and 95% confidence intervals (CIs) calculated.
Results
A total of 1369 patients were randomly assigned to treatment with BIL (n = 691) or glargine (n = 678), with similar demographic/baseline characteristics between groups (Table 1). Metformin use during the study was similar between groups: 69.5% BIL, 68.5% glargine. Baseline data were similar between groups except for laboratory FSG values (Table 2). Patient disposition was similar in the two groups (Figure S2, Supporting Information).
Table 1.
Patient demographic and baseline characteristics.
| Glargine (n = 678) | BIL (n = 691) | |
|---|---|---|
| Age, years | 57.8 ± 9.2 | 57.4 ± 9.2 |
| Men, n (%) | 404 (59.6) | 376 (54.4) |
| Race, n (%) | ||
| American‐Indian or Alaskan Native | 2 (0.3) | 2 (0.3) |
| Asian | 30 (4.4) | 25 (3.6) |
| Black or African‐American | 50 (7.4) | 42 (6.1) |
| Multiple | 9 (1.3) | 5 (0.7) |
| Native Hawaiian or Other Pacific Islander | 2 (0.3) | 2 (0.3) |
| White | 585 (86.3) | 615 (89.0) |
| Hispanic or Latino ethnicity, n (%) | 78 (11.5) | 74 (10.7) |
| Weight, kg | 95.8 ± 19.5 | 96.1 ± 19.8 |
| Body mass index, kg/m2 | 33.0 ± 5.6 | 33.3 ± 5.7 |
| Duration of diabetes, years | 14.2 ± 7.8 | 14.1 ± 7.0 |
| HbA1c ≤8.5%, n (%) | 396 (58.4) | 426 (61.6) |
| Basal insulin at baseline, n (%) | ||
| Glargine | 329 (49) | 345 (50) |
| NPH (Isophane) | 90 (13) | 87 (13) |
| Detemir | 97 (14) | 90 (13) |
| Insulin premix | 121 (18) | 136 (20) |
| Other | 2 (<1) | <1 (<1) |
| Baseline number of insulin injections, n (%) | ||
| 1 | 160 (23.6) | 171 (24.7) |
| 2 | 134 (19.8) | 136 (19.7) |
| ≥3 | 384 (56.6) | 384 (55.6) |
| Bolus insulin dosing plan, n (%) | ||
| Carbohydrate counting | 93 (14) | 104 (15) |
| Preprandial action plan with fixed diet | 172 (25) | 167 (24) |
| Pattern adjustment plan | 413 (61) | 420 (61) |
| Lipid‐lowering medications, n (%) | ||
| Statins | 421 (62.1) | 429 (62.1) |
| Non‐statin lipid lowering medications | 124 (18.3) | 122 (17.7) |
| Hypertension, n (%) | 577 (85.1) | 590 (85.4) |
BIL, basal insulin peglispro; HbA1c, glycated haemoglobin.
Data are mean ± standard deviation, unless otherwise noted.
Table 2.
Baseline values and treatment outcomes at 26 weeks.
| Baseline* | 26 weeks | ||||
|---|---|---|---|---|---|
| Outcome | Glargine (N = 678) | BIL (N = 691) | Glargine (N = 678) | BIL (N = 691) | p |
| HbA1c, %† | 8.47 ± 0.04 | 8.38 ± 0.04 | 6.97 ± 0.04 | 6.76 ± 0.04 | <0.001 |
| Change from baseline | — | — | −1.45 ± 0.04 | −1.66 ± 0.04 | |
| LS mean difference (95% CI) | — | −0.21 (−0.31, −0.11) | |||
| FSG, mmol/l† | 9.1 ± 0.1 | 8.7 ± 0.1 | 7.3 ± 0.1 | 7.0 ± 0.1 | 0.015 |
| Nocturnal hypoglycaemia rate‡ | 0.90 ± 0.08 | 0.84 ± 0.08 | 0.92 ± 0.05 | 0.51 ± 0.03 | <0.001 |
| Nocturnal hypoglycaemia incidence, n (%) | 163 (24.1) | 158 (22.9) | 500 (74.0) | 410 (59.5) | <0.001 |
| Documented symptomatic nocturnal hypoglycaemia rate‡ | 0.30 ± 0.04 | 0.38 ± 0.05 | 0.48 ± 0.04 | 0.24 ± 0.02 | <0.001 |
| Documented symptomatic nocturnal hypoglycaemia incidence, n (%) | 65 (9.6) | 81 (11.8) | 327 (48.4) | 252 (36.6) | <0.001 |
| Total hypoglycaemia rate‡ | 3.58 ± 0.20 | 3.10 ± 0.18 | 5.42 ± 0.19 | 5.97 ± 0.20 | 0.053 |
| Total hypoglycaemia Incidence, n (%) | 390 (57.7) | 374 (54.3) | 653 (96.6) | 656 (95.2) | 0.26 |
| Documented symptomatic hypoglycaemia rate‡ | 1.02 ± 0.09 | 1.14 ± 0.10 | 2.60 ± 0.15 | 2.80 ± 0.16 | 0.34 |
| Documented symptomatic hypoglycaemia incidence, n (%) | 169 (25.0) | 190 (27.6) | 562 (83.1) | 575 (83.5) | 0.95 |
| Daytime hypoglycaemia rate‡ | 2.68 ± 0.17 | 2.26 ± 0.14 | 4.53 ± 0.17 | 5.47 ± 0.20 | <0.001 |
| Daytime hypoglycaemia incidence, n (%) | 340 (50.3) | 327 (47.5) | 643 (95.1) | 651 (94.5) | 0.72 |
| Severe hypoglycaemia rate¶ | 3.59 ± 3.59 | 0 ± 0.0 | 4.72 ± 1.91 | 5.28 ± 1.34 | 0.81 |
| Severe hypoglycaemia incidence, n (%) | 1 (0.1) | 0 (0.0) | 10 (1.5) | 16 (2.3) | 0.15 |
| Anti‐BIL treatment‐emergent antibody response§, n (%) | — | — | 161 (24.0) | 152 (22.3) | 0.33 |
| Systolic blood pressure†, mm Hg | 133 ± 0.6 | 134 ± 0.6 | 135 ± 0.5 | 136 ± 0.5 | 0.51 |
| Diastolic blood pressure†, mm Hg | 77 ± 0.4 | 78 ± 0.4 | 78 ± 0.4 | 78 ± 0.4 | 0.93 |
| Triglycerides†, mmol/l | 1.66 ± 0.04 | 1.69 ± 0.04 | 1.60 ± 0.03 | 1.91 ± 0.03 | <0.001 |
| HDL cholesterol†, mmol/l | 1.24 ± 0.01 | 1.25 ± 0.01 | 1.23 ± 0.01 | 1.20 ± 0.01 | <0.001 |
| LDL cholesterol†, mmol/l | 2.47 ± 0.03 | 2.46 ± 0.03 | 2.56 ± 0.03 | 2.53 ± 0.03 | 0.47 |
| Total cholesterol†, mmol/l | 4.45 ± 0.04 | 4.47 ± 0.04 | 4.52 ± 0.03 | 4.58 ± 0.03 | 0.14 |
| ALT†, IU/l | 27.8 ± 0.6 | 27.8 ± 0.5 | 27.2 ± 0.5 | 35.4 ± 0.5 | <0.001 |
| AST†, IU/l | 24.1 ± 0.4 | 24.7 ± 0.4 | 24.3 ± 0.4 | 28.7 ± 0.4 | <0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BIL, basal insulin peglispro; FSG, fasting serum glucose; HbA1c, glycated haemoglobin; s.e., standard error.
Baseline values were not significantly different between groups (p > 0.05), except for FSG (p = 0.026).
Least squares mean ± s.e.
Events/patient/100 years; aggregated rate ± standard deviation.
Events/patient/30 days; group mean ± s.e.
Treatment‐emergent antibody response defined as change from baseline to post‐baseline in the anti‐BIL antibody level either from undetectable to detectable, or from detectable to the value with at least 130% relative increase from baseline.
The primary objective of non‐inferiority of BIL compared with glargine for change in HbA1c at week 26 was achieved with an LS mean difference of −0.21% (95% CI −0.31 to −0.11%), with p < 0.001 indicating statistical superiority of BIL versus glargine with multiplicity adjustment (Table 2 and Figure 1A). The results of multiple sensitivity analyses, including per‐protocol analysis for HbA1c, were consistent with the primary analysis results.
Figure 1.
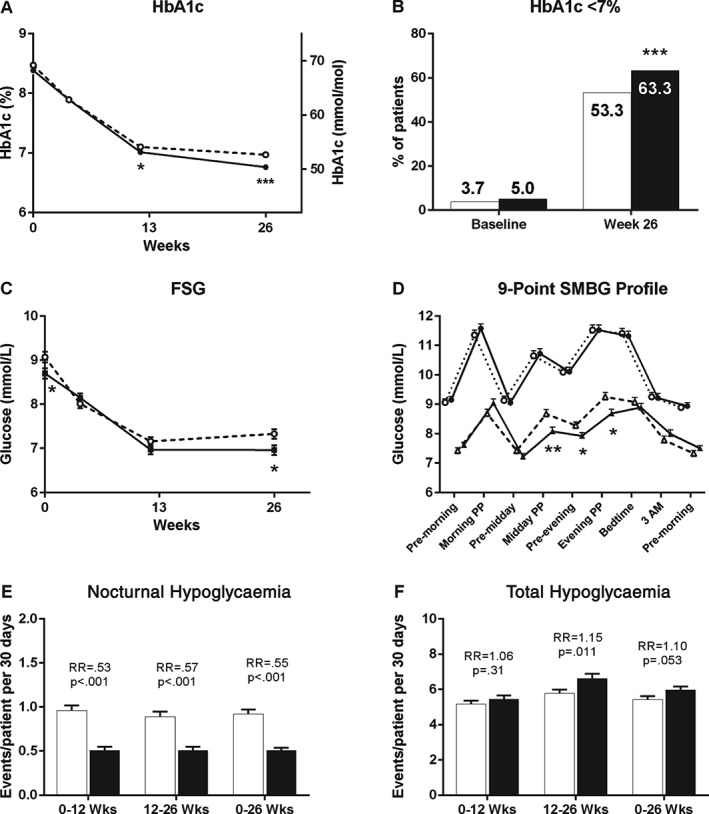
Glycaemic and hypoglycaemia outcome measures over 26 weeks of treatment for basal insulin peglispro (BIL)‐ and glargine‐treated patients. (A) Glycated haemoglobin (HbA1c) over time [least squares (LS) mean ± standard error (s.e.)]. (B) Proportion of patients with HbA1c <7% at baseline and 26 weeks. (C) Fasting serum glucose (FSG) over time (LS mean ± s.e.). (D) Nine‐point SMBG profiles by treatment at baseline and 26 weeks (LS mean ± s.e.). (E) Nocturnal hypoglycaemia rate (group mean ± s.e.). (F) Total hypoglycaemia rate (group mean ± s.e.). White markers or bars/dashed line = glargine; black markers or bars/solid line = BIL. PP, 2‐h postprandial; RR, relative rate; SMBG, self‐monitored blood glucose. *p < 0.05; **p < 0.01; ***p < 0.001 for differences between treatments.
Treatment with BIL resulted in more patients achieving the HbA1c goal of <7.0% than with glargine [63.3% vs 53.3%; p < 0.001 (Figure 1B)] and more patients achieving the HbA1c goal of ≤6.5% (44.4% vs 32.6%; p < 0.001) at week 26. More BIL‐ than glargine‐treated patients reached the HbA1c goal of <7.0% at week 26 without any episodes of nocturnal hypoglycaemia between weeks 0 and 26 (24.4% vs 12.2%; p < 0.001). BIL‐treated patients also had lower laboratory FSG values than glargine‐treated patients at week 26 (LS mean difference −0.37 mmol/l (95% CI −0.67 to −0.07; Table 2 and Figure 1C).
Fasting blood glucose, assessed using SMBG values, was not significantly different between the BIL and glargine groups at week 26 [7.8 ± 0.1 mmol/l vs 7.5 ± 0.1 mmol/l; p = 0.056 (Figure 1D)]. Nine‐point SMBG profiles at week 26 showed lower SMBG values at midday post‐meal and evening pre‐/post‐meal with BIL than with glargine (Figure 1D). At week 26, the BIL group also had a smaller magnitude of overnight glucose excursion compared with glargine from bedtime to pre‐morning meal (LS mean difference 0.6 mmol/l; p = 0.005).
Between‐day glucose variability, as measured by fasting blood glucose standard deviation 7 days before the visit, was lower with BIL than with glargine at week 26 (1.6 ± 0.04 mmol/l vs 1.9 ± 0.04 mmol/l; p < 0.001). Within‐day glucose variability, as measured by nine‐point SMBG profile standard deviation, was also lower with BIL than with glargine at week 26 (2.5 ± 0.1 mmol/l vs 2.7 ± 0.1 mmol/l; p = 0.023).
Nocturnal hypoglycaemia and documented symptomatic nocturnal hypoglycaemia rates and incidences were significantly lower with BIL than with glargine throughout the treatment period (Table 2 and Figure 1E). From weeks 0 to 26, the relative rate of nocturnal hypoglycaemia for BIL:glargine was 0.55 (95% CI 0.47, 0.65; p < 0.001).
The total hypoglycaemia relative rate from weeks 0 to 26 for BIL:glargine was 1.10 (95% CI 1.00, 1.21; p = 0.053), driven by a higher total hypoglycaemia rate with BIL from weeks 12 to 26, as there was no significant difference between groups from weeks 0 to 12 (Table 2 and Figure 1F). Total hypoglycaemia incidence and documented symptomatic hypoglycaemia rate/incidence from 0 to 26 weeks were similar (Table 2). The daytime hypoglycaemia relative rate was higher with BIL than with glargine [1.21 (95% CI 1.09, 1.34); p < 0.001 (Table 2)]. The percentage of symptomatic hypoglycaemia episodes (BIL 46%; glargine 46%) and mean SMBG associated with symptomatic hypoglycaemia (BIL 3.3 mmol/l; glargine 3.3 mmol/l) were not different (p = 0.90). Cumulative total hypoglycaemia events/100 patients over 26 weeks were not different (Figure S3A, Supporting Information). Cumulative nocturnal hypoglycaemia events/100 patients over 26 weeks were lower with BIL than with glargine (Figure S3B, Supporting Information). There were no significant differences between the BIL and glargine groups in the rate or incidence of severe hypoglycaemia (Table 2).
The basal insulin dose (units/day and units/kg/day) was significantly higher with BIL from weeks 3 to 26 (p < 0.05; Figure 2A). At week 26, the mean basal insulin dose for BIL was 67.6 ± 1.2 units/day (0.68 ± 0.01 units/kg/day) and for glargine was 60.3 ± 1.2 units/day (0.60 ± 0.01 units/kg/day; p < 0.001). At week 26, the mean bolus (BIL 61.1 ± 1.7 units/day vs glargine 62.8 ± 1.7 units/day) and total insulin (BIL 126 ± 2.6 units/day vs glargine 121 ± 2.6 units/day) doses were not statistically significantly different between the groups. There was no statistically significant difference between groups in investigator adherence to the basal insulin algorithm over 26 weeks. Weight increased with both treatments, and at week 26, patients in the BIL group had significantly less weight gain compared with baseline than those in the glargine group [LS mean change1.3 kg vs 2.2 kg; p < 0.001 (Figure 2B)].
Figure 2.
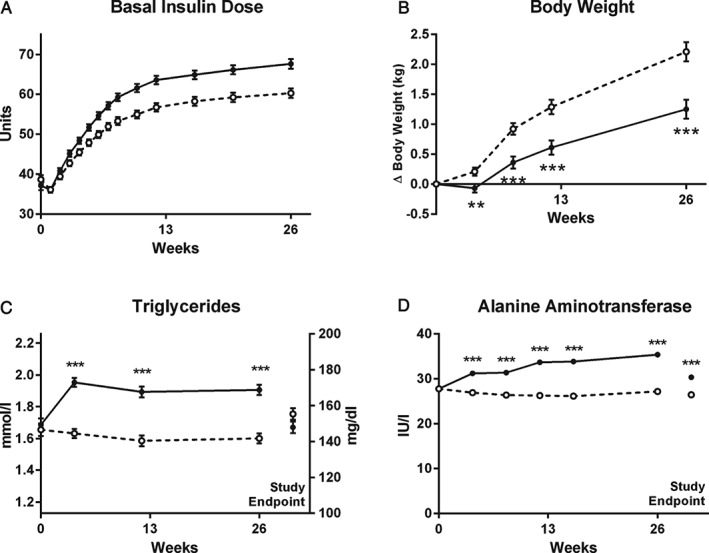
Insulin dose, body weight, serum triglyceride, and alanine aminotransferase (ALT) change over 26 weeks of treatment. (A) Basal insulin dose. Treatment difference was statistically significant (p < 0.05) from weeks 3–26. (B) Change in body weight. (C) Serum triglyceride. (D) ALT. All data are least squares mean ± standard error. White markers or bars/dashed line = glargine; black markers or bars/solid line = basal insulin peglispro (BIL). Study endpoint indicates last visit completed including 4‐week follow‐up visit. *p < 0.05; **p < 0.01; ***p < 0.001 for differences between treatments.
With the pre‐specified gate‐keeping strategy for the primary and six key secondary objectives, the superiority of BIL versus glargine at 26 weeks with regard to nocturnal hypoglycaemia rate, proportion of patients achieving HbA1c <7% without nocturnal hypoglycaemia, HbA1c change, proportion of patients with HbA1c <7%, and FSG was statistically significant with multiplicity adjustment. The sixth objective of superiority of BIL versus glargine for total hypoglycaemia rate from 0 to 26 weeks did not meet the gate‐keeping test for multiplicity.
Subgroup analyses for the stratification factor of baseline number of insulin injections/day (1, 2, ≥3; Table 1) were also performed. There were no significant treatment by subgroup interactions (p > 0.1) for HbA1c change or nocturnal or total hypoglycaemia rate from 0 to 26 weeks. BIL‐treated patients had higher rates of total hypoglycaemia in all three subgroups than glargine‐treated patients; however, patients with one baseline insulin injection had the highest relative risk (BIL:glargine) of 1.23, and patients with ≥3 baseline injections had the lowest relative risk of 1.05 from 0 to 26 weeks.
Overall, treatment‐emergent adverse events and severe adverse events were similar in the two treatment groups (Table S1, Supporting Information). Eleven (1.6%) BIL‐ versus no glargine‐treated patients experienced prospectively defined injection site reaction treatment‐emergent adverse events, primarily lipohypertrophy (n = 8, 1.2%). There was no difference in treatment‐emergent anti‐BIL antibody response between treatments (Table 2).
Adjudicated major adverse cardiovascular events (MACE), including non‐fatal myocardial infarction, non‐fatal stroke and cardiovascular death, were similar for BIL and glargine [1.01 and 0.89%, hazard ratio 1.14 (95% CI 0.38, 3.40) p = 0.81]. MACE+, which included unstable angina hospitalization, were similar for BIL and glargine (1.45 and 1.18%, hazard ratio 1.23 (95% CI: 0.48, 3.10) p = 0.67]. Six deaths occurred; four (0.58%) in the BIL‐ and two (0.30%) in the glargine‐treated group. There were no treatment differences in blood pressure from week 0 to week 26 (Table 2).
Mean triglyceride levels increased from week 0 (baseline) to week 4 and remained stable overall up to week 26 in the BIL group, with 13% higher triglyceride levels with BIL than with glargine at week 26 and a LS mean difference (BIL‐glargine) 0.30 mmol/l (p < 0.001) at week 26 (Table 2 and Figure 2C). At study endpoint (including 4 weeks after discontinuation of BIL), triglyceride levels returned to baseline with BIL and were similar in the two groups. Nine patients (1.3%) in the BIL group and six (0.9%) in the glargine group met the discontinuation criteria of triglycerides >6.8 mmol/l. HDL cholesterol decreased from weeks 0 to 4 with BIL and remained stable overall up to week 26, resulting in a statistically significant treatment difference of −0.03 mmol/l at week 26 (Table 2 and Figure S4B, Supporting Information). At study endpoint, HDL cholesterol levels returned to baseline with BIL and were higher with BIL than with glargine. There were no statistically significant treatment differences in LDL cholesterol or total cholesterol at week 26 (Table 2 and Figure S4C and D, Supporting Information). There were no significant group differences in use (Table 1) or changes to lipid‐lowering medications.
With BIL treatment, mean alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels increased from baseline to week 26 by 27% and 16%, respectively, and there were LS mean treatment differences of 8.2 IU/l and 4.4 IU/l, respectively, at week 26. Mean ALT and AST levels decreased at study endpoint, including after discontinuation of BIL, but remained statistically significantly different from baseline and between groups (Table 2 and Figure 2D). Mean ALT and AST levels remained within or slightly above the central laboratory reference ranges. Twelve patients (1.8%) in the BIL group and five (0.7%) in the glargine group experienced ALT elevation ≥3 × upper limit of normal (ULN) during treatment (p = 0.14), with one additional patient in each group experiencing ALT >3 × ULN during the post‐treatment follow‐up period. Among the 12 BIL‐treated patients, 11 patients had a reduction in ALT to <3 × ULN during the study with nine patients continuing treatment and two patients discontinuing BIL and completing study visits. No patients in the BIL group and two in the glargine group experienced elevations in total bilirubin ≥2 × ULN.
Discussion
In this double‐blind, basal‐bolus study of patients with T2D previously treated with insulin, treatment with BIL compared with insulin glargine resulted in a statistically significantly greater reduction in HbA1c at 26 weeks. Both treatment groups had significant improvement in HbA1c during the study, with 26‐week mean HbA1c values of 6.8% with BIL versus 7.0% with glargine, indicating that the glargine group overall had effective insulin titration to reach glycaemic goals. More BIL‐treated patients were able to reach target HbA1c of <7.0% and had greater reduction in FSG versus glargine‐treated patients. Although the HbA1c treatment difference was modest and not clinically significant in the present study, the BIL phase III programme included six studies in patients with T1D or T2D and resulted in 0.2–0.5% greater HbA1c reductions versus glargine or NPH over 26, 52 and 78 weeks 21, 22, 23, 24, 25. These results may be related to reduced glucose variability and the time–action profile of BIL, which allowed optimization of basal insulin uptitration.
The reduction in HbA1c with BIL was accompanied by a 45% relative rate reduction in nocturnal hypoglycaemia, a key barrier in the ability to titrate basal insulin to reach glycaemic goals. In addition, more BIL‐treated patients reached target HbA1c <7% without any episodes of nocturnal hypoglycaemia over 26 weeks. Basal insulin dose was ∼11% higher with BIL than with glargine at week 26, suggesting that BIL dosing may be optimized to reach glycaemic targets due to a reduced risk of nocturnal hypoglycaemia and less glucose variability. Greater reduction in afternoon/evening SMBG levels when the effects of bedtime dosing of glargine may be waning, as well as longer duration of action, may have also contributed to the greater improvement in glycaemic control with BIL. The total hypoglycaemia rate was 10% higher with a 21% increase in daytime hypoglycaemia rate with BIL versus glargine. Approximately 25% of patients were treated with one insulin injection/day at baseline, and these patients had an increased relative risk of total hypoglycaemia with the transition to basal‐bolus therapy compared with patients previously treated with basal‐bolus therapy. Although basal insulin dosing was higher and BIL had a greater effect on glycaemic variables, there was statistically significantly less weight gain with BIL compared with glargine.
Bolus dosing was not significantly different between groups in this 26‐week study; however, in the 52‐week double‐blind IMAGINE 3 study in patients with T1D, there was a 30% decrease in bolus insulin dose and a 29% increase in basal insulin dose with BIL versus glargine 25. In this T1D study, when bolus insulin dosages were adjusted over longer time periods, total and daytime hypoglycaemia rates did not differ between treatments from 26 to 52 weeks. In addition, in T2D basal insulin‐only studies, including a double‐blind study, nocturnal hypoglycaemia rates were reduced by 26–59% with no significant differences in total or daytime hypoglycaemia rates with BIL versus glargine 21, 24. It is possible that bolus requirements in patients with T2D could also be lower when used in combination with BIL.
With BIL, there was an increase in mean triglycerides accompanied by a decrease in HDL cholesterol and no significant difference in LDL cholesterol versus glargine. Switching from insulin glargine to BIL may have led to reduced suppression of peripheral lipolysis and increased hepatic triglyceride re‐esterification and very low density lipoprotein secretion. The triglyceride and HDL levels remained stable up to 26 weeks, and returned towards baseline after discontinuation of BIL. These triglyceride and HDL cholesterol findings are consistent with other BIL studies in patients with T1D or T2D previously treated with insulin 17, 21, 24, 25, 26, 27. In contrast, in the IMAGINE 2 study of insulin‐naïve patients with T2D, triglyceride levels were essentially unchanged with initiation of BIL and decreased with glargine over 26 weeks 21. A decrease in triglycerides with insulin glargine and other conventional insulins has been described 28, 29, 30. Across the BIL phase II and III programme, there were no significant differences in incidence rates of MACE+, MACE, or all‐cause death between BIL and the comparator 31. In the present study and additional BIL phase III T2D studies, there were no significant differences in blood pressure with BIL versus glargine 21, 24.
Increased mean ALT levels were observed in the BIL group, with a 26‐week treatment difference of 8 IU/l. Mean ALT levels remained within or slightly above laboratory reference ranges, and decreased toward baseline after discontinuation of BIL. More patients experienced ALT ≥3 × the ULN with BIL than with glargine, but no cases were associated with increases in total bilirubin to ≥2 × the ULN. The aetiology of the increase in ALT with BIL is unknown, but could reflect reduced peripheral suppression of lipolysis and an increase in free fatty acid delivery to the liver, which may also be associated with the increase in serum triglycerides compared with conventional insulins 32, 33.
Liver fat content (LFC) was not assessed in the present study, but was evaluated by MRI 34 in subsets of patients in two other BIL phase III T2D studies, and overall the findings parallel the changes in triglycerides over 26 weeks in these two studies. In insulin‐naïve patients (IMAGINE 2), mean LFC decreased from baseline with glargine, but was unchanged with BIL over 52 weeks 21. Previous studies in insulin‐naïve patients with T2D have shown decreases in LFC with initiation of currently available basal and pre‐mixed insulins, similar to those reported in IMAGINE 2 35, 36. By contrast, in the IMAGINE 5 study of patients with T2D previously treated with basal insulin (primarily glargine), mean LFC remained unchanged with glargine and increased from baseline at 26 weeks with stable levels from 26 to 52 weeks after switching to BIL 24. Changes in LFC may also be a potential aetiology of the mean ALT increase with BIL; however, mean increases in ALT with BIL were also observed in insulin‐naïve patients in whom there was no change in LFC.
The strengths of the IMAGINE 4 study include its double‐blind design, the large sample size of >1300 patients (with power to evaluate not only glycaemic efficacy with HbA1c but also nocturnal hypoglycaemia), as well as the global nature of the study, involving 25 countries including North/South America, Europe and Asia. The use of the e‐diary allowed wireless, direct capture of SMBG levels and hypoglycaemia events 18. In addition, the treat‐to‐target SMBG fasting blood glucose levels were not significantly different in the BIL and glargine groups. Potential limitations include the 26‐week duration of the study, the inability to translate the findings outside of a clinical trial setting, and that the findings are limited to the population included. It is also unknown from the present study if a reduction in bolus dosing in patients with T2D would decrease the daytime hypoglycaemia rate with BIL. In addition, although this was a multinational study, the majority of the study population was white.
This 26‐week, double‐blind study in patients with T2D previously treated with insulin demonstrates that treatment with BIL compared with glargine, in combination with prandial insulin lispro, provides superior glycaemic efficacy with a reduced risk of nocturnal hypoglycaemia, lower glucose variability and less weight gain. Increases in daytime hypoglycaemia, ALT, AST and triglycerides in comparison with glargine were observed.
Conflict of Interest
T. B. has served on the advisory panel for Eli Lilly and Company and Sanofi‐Aventis, has received research support from Novo Nordisk, Eli Lilly and Company, Merck and Co., Sanofi‐Aventis, Janssen Pharmaceuticals and Mylan, and has served on the speaker's bureau for Boehringer Ingelheim, Eli Lilly and Company, Sanofi‐Aventis, Astra Zeneca and Merck and Co. T. R. P. has served on the advisory panel for Eli Lilly and Company, Novo Nordisk, Bristol Myers Squibb and Roche Diagnostics, has served as a consultant for Eli Lilly and Company, and has served on the speaker's bureau for Novo Nordisk and Astra Zeneca. G. C. V. has served on the advisory panel for BMS Diabetes Advisory Board and Eli Lilly and Company and has served on the speaker's bureau for Astra Zeneca and Eli Lilly and Company. S. Z. is an employee and shareholder of Eli Lilly and Company. E. J. B. was an employee of Eli Lilly and Company during the trial and is currently a consultant to Viacyte, Inc., San Diego, CA, USA. A. M. C. is an employee and shareholder of Eli Lilly and Company.
T. B., T. R. P. and G. C. V. participated as trial investigators and in the discussion of the research, and reviewed/edited the manuscript. S. Z. contributed to the study design, designed and conducted the statistical analyses and participated in the interpretation of the research and in writing the manuscript. E. J. B. participated in the study design, the conduct of the study, the data analysis and interpretation of the research, and reviewed/edited the manuscript. A. M. C. was responsible for medical oversight during the trial and contributed to the study design, the data analysis and interpretation of the research and in writing the manuscript. All authors approved the final manuscript to be published.
Supporting information
Appendix S1. Investigator list by country.
Appendix S2. Study inclusion and exclusion criteria.
Appendix S3. Randomization and blinding.
Appendix S4. Lipid and hepatic criteria for study insulin discontinuation.
Appendix S5. Insulin initiation and insulin dose algorithms.
Figure S1. IMAGINE 4 study design.
Figure S2. Patient disposition.
Figure S3. Cumulative hypoglycaemia.
Figure S4. Lipid profile.
Table S1. Percent of patients experiencing treatment‐emergent adverse events and serious adverse events from randomization to the end of the study.
Acknowledgements
The authors would like to thank the study participants, and the investigators, nurses and study coordinators who cared for them. The authors thank Caryl Antalis, PhD, (Eli Lilly and Company, Indianapolis, IN, USA) for writing and editorial assistance. This study was funded by Eli Lilly and Company.
References
- 1. Halban PA, Polonsky KS, Bowden DW et al. Beta‐cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014; 37: 1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. U.K. Prospective Diabetes Study Group . U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 1995; 44: 1249–1258. [PubMed] [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 4. UK Prospective Diabetes Study (UKPDS) Group , Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999; 281: 2005–2012. [DOI] [PubMed] [Google Scholar]
- 5. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 6. Shah VN, Moser EG, Blau A, Dhingra M, Garg SK. The future of basal insulin. Diabetes Technol Ther 2013; 15: 727–732. [DOI] [PubMed] [Google Scholar]
- 7. Ratner RE, Gough SC, Mathieu C et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab 2013; 15: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritzel R, Roussel R, Bolli GB et al. Patient‐level meta‐analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab 2015; 17: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen RJ, Cutler GB, Vick A et al. LY2605541: leveraging hydrodynamic size to develop a novel basal insulin. Diabetes 2012; 61(Suppl. 1): A228. [Google Scholar]
- 10. Sinha VP, Choi SL, Soon DK et al. Single‐dose pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 in healthy subjects. J Clin Pharmacol 2014; 54: 792–799. [DOI] [PubMed] [Google Scholar]
- 11. Sinha VP, Howey DC, Choi SL, Mace KF, Heise T. Steady‐state pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 dosed once‐daily in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014; 16: 344–350. [DOI] [PubMed] [Google Scholar]
- 12. Mudaliar S, Henry RR, Ciaraldi TP et al. Basal insulin peglispro (BIL) demonstrates hepato‐preferential action vs. Insulin glargine (GL) in patients with type 1 diabetes mellitus (T1DM). Diabetes 2015; 64(Suppl. 1A): LB22–LB23. [Google Scholar]
- 13. Henry RR, Mudaliar S, Ciaraldi TP et al. Basal insulin peglispro demonstrates preferential hepatic versus peripheral action relative to insulin glargine in healthy subjects. Diabetes Care 2014; 37: 2609–2615. [DOI] [PubMed] [Google Scholar]
- 14. Morrow LA, Hompesch M, Jacober SJ, Choi SL, Qu Y, Sinha VP. Glucodynamics of long‐acting basal insulin peglispro (BIL) compared with insulin glargine at steady state in subjects with type 1 diabetes: substudy of a randomized crossover trial. Diabetes Obes Metab 2016; May 12 [Epub ahead of print] doi:10.1111/dom.12691. [DOI] [PubMed] [Google Scholar]
- 15. Moore MC, Smith MS, Sinha VP et al. Novel PEGylated basal insulin LY2605541 has a preferential hepatic effect on glucose metabolism. Diabetes 2014; 63: 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 17. Rosenstock J, Bergenstal RM, Blevins TC et al. Better glycemic control and weight loss with the novel long‐acting basal insulin LY2605541 compared with insulin glargine in type 1 diabetes: a randomized, crossover study. Diabetes Care 2013; 36: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bastyr EJ III, Zhang S, Mou J et al. Performance of an electronic diary system for intensive insulin management in Global Diabetes Clinical Trials. Diabetes Technol Ther 2015; 17: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dmitrienko A, Tamhane AC, Wiens BL. General multistage gatekeeping procedures. Biom J 2008; 50: 667–677. [DOI] [PubMed] [Google Scholar]
- 20. Qu Y, Luo J. Estimation of group means when adjusting for covariates in generalized linear models. Pharm Stat 2015; 14: 56–62. [DOI] [PubMed] [Google Scholar]
- 21. Davies MJ, Russel‐Jones D, Selam J‐L et al. Basal insulin peglispro vs insulin glargine in insulin‐naïve type 2 diabetes: IMAGINE 2 randomized trial. Diabetes Obes Metab 2016. [Submitted Paper]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grunberger G, Chen L, Rodríguez Á et al. A Randomized Clinical Trial of Basal Insulin Peglispro versus NPH in Insulin‐Naïve Patients with Type 2 Diabetes: IMAGINE 6. Diabetes Obes Metab 2016. [Submitted Paper]. [DOI] [PubMed] [Google Scholar]
- 23. Garg SK, Dreyer M, Jinnouchi H et al. A Randomized Clinical Trial Comparing Basal Insulin Peglispro and Insulin Glargine, in combination with Prandial insulin Lispro, in Patients with Type 1 Diabetes: IMAGINE 1. Diabetes Obes Metab 2016. [Submitted Paper]. [DOI] [PubMed] [Google Scholar]
- 24. Buse JB, Rodbard HW, Trescoli Serrano C et al. Randomized clinical trial comparing basal insulin peglispro and insulin glargine in patients with Type 2 diabetes previously treated with basal insulin: IMAGINE 5. Diabetes Care 2016; 39: 92–100. [DOI] [PubMed] [Google Scholar]
- 25. Bergenstal RM, Lunt H, Franek E et al. A randomized, double‐blind clinical trial comparing basal insulin peglispro and insulin glargine, in combination with prandial insulin lispro, in patients with type 1 diabetes: IMAGINE 3. Diabetes Obes Metab 2016. doi: 10.1111/dom.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bergenstal RM, Rosenstock J, Arakaki RF et al. A randomized, controlled study of once‐daily LY2605541, a novel long‐acting basal insulin, versus insulin glargine in basal insulin‐treated patients with type 2 diabetes. Diabetes Care 2012; 35: 2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ginsberg H, Cariou B, Orchard T et al. Lipid changes during basal insulin peglispro, insulin glargine, or NPH treatment in 6 IMAGINE trials. Diabetes Obes Metab 2016. [Submitted Paper]. [DOI] [PubMed] [Google Scholar]
- 28. Chaudhuri A, Rosenstock J, DiGenio A et al. Comparing the effects of insulin glargine and thiazolidinediones on plasma lipids in type 2 diabetes: a patient‐level pooled analysis. Diabetes Metab Res Rev 2012; 28: 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gerstein HC, Yale JF, Harris SB et al. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes on either no oral glucose‐lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) study. Diabet Med 2006; 23: 736–742. [DOI] [PubMed] [Google Scholar]
- 30. Yki‐Jarvinen H, Kauppinen‐Makelin R, Tiikkainen M et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 2006; 49: 442–451. [DOI] [PubMed] [Google Scholar]
- 31. Hoogwerf BJ, Lincoff AM, Rodriguez A et al. Major adverse cardiovascular events with basal insulin peglispro versus comparator insulins in patients with type 1 or type 2 diabetes: a meta‐analysis. Cardiovasc Diabetol 2016; 15: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donnelly KL, Smith CI, Schwarzenberg SJ et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vatner DF, Majumdar SK, Kumashiro N et al. Insulin‐independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci 2015; 112: 1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mashhood A, Railkar R, Yokoo T et al. Reproducibility of hepatic fat fraction measurement by magnetic resonance imaging. J Magn Reson Imaging 2013; 37: 1359–1370. [DOI] [PubMed] [Google Scholar]
- 35. Juurinen L, Tiikkainen M, Hakkinen AM, Hakkarainen A, Yki‐Jarvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 292: E829–E835. [DOI] [PubMed] [Google Scholar]
- 36. Lingvay I, Raskin P, Szczepaniak LS. Effect of insulin‐metformin combination on hepatic steatosis in patients with type 2 diabetes. J Diabetes Complications 2007; 21: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Investigator list by country.
Appendix S2. Study inclusion and exclusion criteria.
Appendix S3. Randomization and blinding.
Appendix S4. Lipid and hepatic criteria for study insulin discontinuation.
Appendix S5. Insulin initiation and insulin dose algorithms.
Figure S1. IMAGINE 4 study design.
Figure S2. Patient disposition.
Figure S3. Cumulative hypoglycaemia.
Figure S4. Lipid profile.
Table S1. Percent of patients experiencing treatment‐emergent adverse events and serious adverse events from randomization to the end of the study.


