Abstract
Background
Data linkage studies find that depression before or after a breast cancer diagnosis predicts reduced survival. This study aimed to determine whether depression or bipolar recorded in routine hospital admission data independently predicts survival in English breast cancer patients and whether onset in relation to cancer diagnosis is significant.
Methods
Data on 77 173 women diagnosed with breast cancer (ICD‐10 C50) in South East England, 2000–2009, were included. Of these, 131 women had a diagnosis of bipolar affective disorder (ICD‐10 F31) and 955 of depression (either depressive episodes (ICD‐10 F32) or depressive disorder (ICD‐10 F33)) recorded in Hospital Episode Statistics between 3 years before and a year following cancer diagnosis. Kaplan–Meier plots were used to examine overall survival. Cox regression analyses were carried out overall and separately for mood disorder diagnoses before and after the cancer diagnosis and adjusted for confounding variables.
Results
A record of depression was a predictor of worse overall survival in breast cancer patients (adjusted HR = 1.33, 95% CI: 1.20–1.48, p < 0.001), while the effect of bipolar was not statistically significant (adjusted HR = 1.33, 95% CI: 0.97–1.82, p = 0.079). New recordings of depression and bipolar diagnoses following a cancer diagnosis appeared better predictors of overall survival than a prior history of either.
Conclusions
There is evidence that English breast cancer patients with depression and bipolar recorded in routine hospital data have worse overall survival than those without these mood disorders. Further work exploring the concordance of records within administrative health data with clinical diagnosis and cause‐specific death within these patient groups is needed. © 2015 The Authors. Psycho‐Oncology Published by John Wiley & Sons Ltd.
Background
For many years, patients and clinicians have been trying to understand the link between physiological and psychological health. Cancer patients often attribute psychological factors to exceeding prognosis expectations, believing that these play a key role in lengthening survival 1, but research to support this is contradictory and inconclusive 2, 3, 4.
Breast cancer is the most common cancer among women in the UK, accounting for 30% of new cases of cancer in women and affecting 49 936 women in 2011 5. Breast cancer survival is determined by a number of factors including age 6, 7, comorbidity 8, socio‐economic factors and ethnicity 9, 10, stage of the cancer at presentation 11 and treatment 12, 13.
Depression either prior to or post‐breast cancer diagnosis has been shown to be a predictor of reduced survival 4, 14, 15, 16, 17. Researchers have suggested that this finding may be due to patients with mood disorders presenting at a later stage, receiving different treatments or having a greater number of comorbidities 18, 19, 20. However, studies adjusting for comorbidities have suggested that this is not the case 21, 22, raising the possibility that depression may be an independent predictor of a worse prognosis. One study in the Netherlands has also linked bipolar with an increased risk of dying from cancer, although these results were not statistically significant and length of survival was not reported 16. As bipolar disorder has elements of both low mood (depression) and elevated mood (mania), examining the association with breast cancer survival may help determine the mechanism of mood disorders on cancer survival. Studies have also shown that mental illnesses including bipolar disorder are a risk factor for cancer onset 23, 24; with this suggested link in incidence, it is thus important to establish its impact on outcome.
Much research investigating the impact of mood disorders on cancer survival has faced methodological limitations, such as small sample sizes 14, 15, 17, 25. Many studies have used cancer mortality as an outcome measure, making it hard to draw conclusions about the length of survival after the diagnosis 17, 20, 26, and no studies of bipolar disorder and breast cancer survival could be identified. In addition, few studies have investigated the timing of onset of the mood disorder in relation to the cancer diagnosis 14, 22.
This study aims to determine whether mood disorders, defined as depression or bipolar routinely recorded during hospital admissions, are independent predictors of survival in breast cancer patients in South East England. It also aims to determine whether the timing of the onset of the disorder in relation to the cancer diagnosis is of significance.
Methods
This study used cancer registration data collected for patients diagnosed with breast cancer between 2000 and 2009 and who were resident in the areas of London, Kent, Surrey and Sussex in South East England covered by the former Thames Cancer Registry (TCR). TCR received information from National Health Service (NHS) hospitals about the clinical and pathological features of patients' cancers and on the deaths of patients from the Office for National Statistics via the NHS Central Register. Trained cancer registration officers extracted further demographic and tumour details, and information on the surgical, radiotherapy and chemotherapy treatment patients received within the first 6 months from medical records. Data records were quality assured as they were added to a central database and duplicate cases eliminated. All inpatient and day‐case Hospital Episode Statistics (HES) episodes for cancer patients were obtained from the Health and Social Care Information Centre.
Hospital Episode Statistics data include information on the clinical diagnoses of all patients admitted to English NHS hospitals and are the basis of all analyses of hospital activity and of contract payments made to hospitals for patient care. This information is based on the data entered to the Patient Administration System in each hospital by clinical coding staff, a short period after patients are discharged. Coding staff undergo well‐defined training to be able to extract and code diagnoses using ICD‐10 from the clinical records. Coders do not make any inferences about diagnoses but use only the information written in the records and in typed discharge summaries by the responsible clinicians 27. These data have been used to describe patterns of admission for adult psychiatric illness 28, possible links between bipolar and physical illness 29 and case finding for audits of dementia care 30. A systematic review of their accuracy has suggested that this is improving because of a greater focus on payment and increased understanding by clinicians of how the information they include can be used 31. These episodes were linked to the registry data as part of the National Cancer Data Repository using a rule‐based linkage algorithm based on NHS number, date of birth, sex, postcode of residence and date of death.
All diagnoses listed in the HES episodes for the cancer patients were examined. The mood disorders used in the analysis were bipolar affective disorder (ICD‐10 F31), at least one depressive episode (ICD‐10 F32) and recurrent depressive disorder (ICD‐10 F33) where these were recorded within HES in the 3 years before and the year following the cancer diagnosis. Diagnoses of mood disorders were included regardless of the reason for the patient's admission to hospital.
Breast cancer patients with any of the mental health codes in the 4 years specified were classified into two main groups: ‘depression’ and ‘bipolar’. Patients with a diagnosis of either a recurrent depressive disorder or a depressive episode were grouped together as ‘depression’ (n = 955). Of these patients, 422 had a prior record of a diagnosis of depression, and 533 had a new record of depression after the cancer diagnosis. Patients with a diagnosis of bipolar were also grouped together in a ‘bipolar’ group (n = 131): 68 of these patients had a record of a diagnosis before their cancer diagnosis, and 63 had a new record of bipolar made after the cancer diagnosis. There were 94 patients who had a record of depression both before and after their cancer diagnosis and 27 who had a record of bipolar both before and after their cancer diagnosis.
Other variables included in the analysis were age at cancer diagnosis (which was grouped into 10‐year age groups from age 30 to 90 years) and self‐assigned ethnicity data from HES data (categorized as White, Black, Asian, Chinese/other and not known for this analysis). Based on postcode of residence at cancer diagnosis, each patient was assigned to a socio‐economic deprivation quintile using the income domain of the Indices of Deprivation 32. Information regarding stage of breast cancer at diagnosis was also included; TCR used a simple four‐level staging system, using information in the patients' notes. This allowed solid tumours to be assigned to categories based on whether the disease (1) was local, (2) had direct extension beyond the organ of origin, (3) had regional lymph node involvement or (4) had metastasized. Treatment given within 6 months of diagnosis including cancer surgery, chemotherapy, radiotherapy and hormone therapy was also considered.
Diagnostic codes within HES for admissions between 2 years before and 3 months after the cancer diagnosis were also used to calculate Charlson comorbidity scores 33 using a coding method developed by Quan et al. 34. These were aggregated into four categories for analysis ranging from 0 (no comorbid conditions) to 3 or more (most severe). For this initial study, death from any cause was chosen as the end point of interest. Historically, cancer registries have not captured information on date of recurrence, nor is there an accepted method for defining this from HES, making disease‐free survival difficult to study.
Statistical analyses
Survival rates 5 years after cancer diagnosis were calculated using the Kaplan–Meier method for patients with either ‘depression’ or ‘bipolar’ compared with patients without these mood disorders recorded. Cox regression was used to estimate hazard ratios for patients with a recorded diagnosis of either ‘depression’ or ‘bipolar’. Further Cox regression was used to find the estimated hazard ratios for both ‘depression’ and ‘bipolar’ in relation to timing of onset. These estimated hazard ratios were then adjusted to account for the effect of confounders including age, ethnicity, deprivation, comorbidities, stage and recorded cancer treatment. We ran post‐analysis power calculations using stpower in Stata (StataCorp LP, College Station, TX, USA) to determine the level of power for the sample size and adjusted hazard ratios found in the study, also taking into account the squared multiple‐correlation coefficient between the mood disorder variable of interest and other variables in the model.
Results
There were 77 173 patients diagnosed with breast cancer in South East England from 2000 to 2009. The numbers of all breast cancer patients and those with a diagnosis of depression or bipolar in each variable category are shown in Table 1. Women with depression were most likely to be aged over 70 years (37%), compared with all breast cancer patients (32%) and women with a diagnosis of bipolar (27%). Ethnicity information was more complete in women with a depression or bipolar diagnosis. However, even taking this into account, a higher proportion of women in these groups were White. Women with depression or bipolar were more likely to live in deprived areas than all breast cancer patients. A lower proportion of breast cancer patients with a diagnosis of depression had no other comorbidity (65%) than those with a diagnosis of bipolar (73%) or all breast cancer patients (72%, or 87% of those where a comorbidity score was known). The stage of disease at diagnosis was very similar in the three groups, with 7–8% of patients with metastatic disease. Recorded treatment was also similar across the groups, although women with depression were slightly less likely to receive different treatment modalities than all breast cancer patients.
Table 1.
Hazard ratios, 95% confidence intervals and p values for breast cancer patients with depression and with bipolar, unadjusted and adjusted for age, ethnicity, deprivation quintile, comorbidity score, stage at diagnosis and treatment received, South East England, 2000–2009
| Total number of women | Women with depression – all diagnoses | Women with bipolar – all diagnoses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | HR | (95% CI) | p | n | % | HR | (95% CI) | p | |||
| Mood disorder | Unadjusted model | 77 173 | 955 | (1.2) | 1.96 | (1.77, 2.17) | <0.001 | 131 | (0.2) | 1.37 | (1.00, 1.88) | 0.049 |
| Fully adjusted model | 1.33 | (1.20, 1.48) | <0.001 | 1.33 | (0.97, 1.82) | 0.079 | ||||||
| Age group | 30–39a | 4650 | 33 | (3.5) | 1.00 | 3 | (2.3) | 1.00 | ||||
| 40–49 | 12 217 | 137 | (14.3) | 0.80 | (0.74, 0.87) | <0.001 | 15 | (11.5) | 0.80 | (0.74, 0.87) | <0.001 | |
| 50–59 | 18 441 | 203 | (21.3) | 0.87 | (0.81, 0.94) | <0.001 | 49 | (37.4) | 0.87 | (0.81, 0.94) | <0.001 | |
| 60–69 | 17 297 | 229 | (24.0) | 1.32 | (1.23, 1.42) | <0.001 | 29 | (22.1) | 1.32 | (1.23, 1.42) | <0.001 | |
| 70–79 | 13 218 | 152 | (15.9) | 2.88 | (2.68, 3.11) | <0.001 | 24 | (18.3) | 2.88 | (2.67, 3.10) | <0.001 | |
| 80–89 | 9181 | 156 | (16.3) | 5.44 | (5.04, 5.87) | <0.001 | 11 | (8.4) | 5.44 | (5.04, 5.86) | <0.001 | |
| 90+ | 2169 | 45 | (4.7) | 10.46 | (9.59, 11.40) | <0.001 | 0 | (0.0) | 10.51 | (9.64, 11.46) | <0.001 | |
| Ethnicity | Whitea | 50 769 | 828 | (86.7) | 1.00 | 115 | (87.8) | 1.00 | ||||
| Asian | 2724 | 27 | (2.8) | 0.91 | (0.83, 1.00) | 0.042 | 3 | (2.3) | 0.91 | (0.83, 0.99) | 0.034 | |
| Black | 2825 | 30 | (3.1) | 1.16 | (1.07, 1.26) | <0.001 | 4 | (3.1) | 1.16 | (1.07, 1.25) | <0.001 | |
| Chinese/other | 2074 | 36 | (3.8) | 0.97 | (0.88, 1.06) | 0.510 | 3 | (2.3) | 0.97 | (0.89, 1.07) | 0.545 | |
| Not known | 18 781 | 34 | (3.6) | 1.48 | (1.41, 1.55) | <0.001 | 6 | (4.6) | 1.48 | (1.41, 1.55) | <0.001 | |
| Deprivation quintile | 1 (least deprived)a | 16 327 | 145 | (15.2) | 1.00 | 13 | (9.9) | 1.00 | ||||
| 2 | 16 213 | 155 | (16.2) | 1.08 | (1.03, 1.13) | 0.001 | 21 | (16.0) | 1.08 | (1.03, 1.13) | 0.001 | |
| 3 | 15 702 | 192 | (20.1) | 1.13 | (1.08, 1.18) | <0.001 | 27 | (20.6) | 1.13 | (1.08, 1.18) | <0.001 | |
| 4 | 16 136 | 216 | (22.6) | 1.18 | (1.13, 1.24) | <0.001 | 33 | (25.2) | 1.19 | (1.14, 1.24) | <0.001 | |
| 5 (most deprived) | 12 795 | 247 | (25.9) | 1.21 | (1.15, 1.26) | <0.001 | 37 | (28.2) | 1.21 | (1.15, 1.27) | <0.001 | |
| Comorbidity score | 0a | 55 386 | 623 | (65.2) | 1.00 | 96 | (73.3) | 1.00 | ||||
| 1 | 6455 | 212 | (22.2) | 1.45 | (1.38, 1.51) | <0.001 | 22 | (16.8) | 1.46 | (1.40, 1.53) | <0.001 | |
| 2 | 1411 | 71 | (7.4) | 2.05 | (1.90, 2.20) | <0.001 | 10 | (7.6) | 2.06 | (1.92, 2.22) | <0.001 | |
| 3+ | 719 | 49 | (5.1) | 2.74 | (2.50, 3.01) | <0.001 | 3 | (2.3) | 2.78 | (2.54, 3.05) | <0.001 | |
| Not known | 13 202 | 0 | (0.0) | 0.50 | (0.47, 0.53) | <0.001 | 0 | (0.0) | 0.50 | (0.47, 0.53) | <0.001 | |
| Stage at diagnosis | 1a | 31 957 | 384 | (40.2) | 1.00 | 51 | (38.9) | 1.00 | ||||
| 2 | 997 | 10 | (1.0) | 1.94 | (1.77, 2.11) | <0.001 | 0 | (0.0) | 1.93 | (1.77, 2.11) | <0.001 | |
| 3 | 17 306 | 218 | (22.8) | 1.59 | (1.53, 1.66) | <0.001 | 33 | (25.2) | 1.59 | (1.52, 1.66) | <0.001 | |
| 4 | 5022 | 79 | (8.3) | 5.06 | (4.84, 5.28) | <0.001 | 10 | (7.6) | 5.06 | (4.84, 5.29) | <0.001 | |
| Not known | 21 891 | 264 | (27.6) | 1.22 | (1.18, 1.27) | <0.001 | 37 | (28.2) | 1.22 | (1.18, 1.27) | <0.001 | |
| Treatment received | Surgery | 51 521 | 597 | (62.5) | 0.43 | (0.42, 0.45) | <0.001 | 91 | (69.5) | 0.43 | (0.42, 0.45) | <0.001 |
| Chemotherapy | 21 543 | 228 | (23.9) | 1.36 | (1.31, 1.42) | <0.001 | 35 | (26.7) | 1.36 | (1.31, 1.42) | <0.001 | |
| Radiotherapy | 22 451 | 210 | (2.0) | 0.76 | (0.74, 0.79) | <0.001 | 31 | (23.7) | 0.76 | (0.74, 0.79) | <0.001 | |
| Hormone therapy | 30 025 | 353 | (37.0) | 0.83 | (0.80, 0.85) | <0.001 | 48 | (36.6) | 0.83 | (0.80, 0.85) | <0.001 | |
Baseline group.
Figure 1 shows that patients with depression had worse survival than patients without depression, with 55% of patients with a record of depression alive 5 years after their cancer diagnosis compared with 75% without depression recorded. Figure 2 shows that patients with a record of a diagnosis of bipolar had initially had similar survival than those without. However, from 2 years after diagnosis, bipolar patients had worse survival, with a 5‐year survival of 64% compared with 74% in patients without a bipolar diagnosis.
Figure 1.
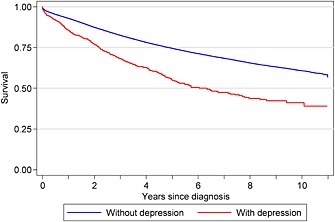
Kaplan–Meier survival for breast cancer patients with and without a record of a diagnosis of depression, South East England, 2000–2009
Figure 2.
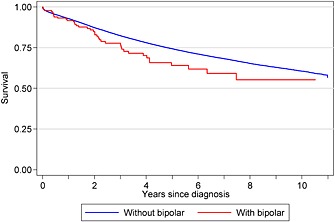
Kaplan–Meier survival for breast cancer patients with and without a record of a diagnosis of bipolar, South East England, 2000–2009
Table 1 shows that patients with a record of depression before or following the breast cancer diagnosis had a higher risk of dying even after adjusting for age, ethnicity, deprivation, comorbidities, stage of disease and treatment (fully adjusted HR = 1.33, 95% confidence interval (CI): 1.20–1.48, p < 0.001). The timing of the onset of depression appeared to be important. Depression recorded prior to the cancer diagnosis had a high unadjusted hazard ratio of 2.29 (95% CI: 1.98–2.64, p < 0.001) (Table 2), but this reduced to 1.23 (95% CI: 1.07–1.42, p = 0.005) after adjustment, with the main attenuating factors being age, comorbidity and stage. Depression after the cancer diagnosis, when no previous history of depression had been recorded, demonstrated a high hazard ratio of 1.69 (95% CI: 1.46–1.95, p < 0.001) but was less affected by adjustment (HR = 1.45, 95% CI: 1.25–1.68, p < 0.001).
Table 2.
Hazard ratios, 95% confidence intervals and p values for breast cancer patients with depression and with bipolar before or after cancer diagnosis, unadjusted and adjusted for age, ethnicity, deprivation quintile, comorbidity score, stage at diagnosis and treatment received, South East England, 2000–2009
| Women with depression | Women with bipolar | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐cancer diagnosis | Post‐cancer diagnosis only | Pre‐cancer diagnosis | Post‐cancer diagnosis only | ||||||||||
| HR | (95% CI) | p | HR | (95% CI) | p | HR | (95% CI) | p | HR | (95% CI) | p | ||
| Mood disorder | Unadjusted model | 2.29 | (1.98, 2.64) | <0.001 | 1.69 | (1.46, 1.95) | <0.001 | 1.37 | (0.90, 2.08) | 0.138 | 1.37 | (0.85, 2.20) | 0.197 |
| Fully adjusted model | 1.23 | (1.07, 1.42) | 0.005 | 1.45 | (1.25, 1.68) | <0.001 | 1.20 | (0.79, 1.82) | 0.402 | 1.54 | (0.96, 2.48) | 0.075 | |
| Age group (years) | 30–39a | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 40–49 | 0.80 | (0.74, 0.87) | <0.001 | 0.80 | (0.74, 0.87) | <0.001 | 0.80 | (0.74, 0.87) | <0.001 | 0.80 | (0.74, 0.87) | <0.001 | |
| 50–59 | 0.87 | (0.81, 0.94) | <0.001 | 0.87 | (0.81, 0.94) | <0.001 | 0.87 | (0.81, 0.94) | <0.001 | 0.87 | (0.81, 0.94) | <0.001 | |
| 60–69 | 1.32 | (1.23, 1.43) | <0.001 | 1.32 | (1.22, 1.42) | <0.001 | 1.32 | (1.23, 1.42) | <0.001 | 1.32 | (1.23, 1.42) | <0.001 | |
| 70–79 | 2.88 | (2.67, 3.10) | <0.001 | 2.88 | (2.67, 3.10) | <0.001 | 2.88 | (2.67, 3.10) | <0.001 | 2.88 | (2.67, 3.10) | <0.001 | |
| 80–89 | 5.44 | (5.04, 5.87) | <0.001 | 5.44 | (5.04, 5.86) | <0.001 | 5.44 | (5.04, 5.87) | <0.001 | 5.44 | (5.04, 5.86) | <0.001 | |
| 90+ | 10.48 | (9.62, 11.43) | <0.001 | 10.49 | (9.62, 11.43) | <0.001 | 10.51 | (9.64, 11.46) | <0.001 | 10.51 | (9.64, 11.45) | <0.001 | |
| Ethnicity | Whitea | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Asian | 0.91 | (0.83, 0.99) | 0.037 | 0.91 | (0.83, 0.99) | 0.037 | 0.91 | (0.83, 0.99) | 0.034 | 0.91 | (0.83, 0.99) | 0.033 | |
| Black | 1.16 | (1.07, 1.25) | <0.001 | 1.16 | (1.07, 1.25) | <0.001 | 1.16 | (1.07, 1.25) | <0.001 | 1.16 | (1.07, 1.25) | <0.001 | |
| Chinese/other | 0.97 | (0.88, 1.07) | 0.528 | 0.97 | (0.88, 1.07) | 0.532 | 0.97 | (0.89, 1.07) | 0.548 | 0.97 | (0.88, 1.07) | 0.538 | |
| Not known | 1.48 | (1.41, 1.55) | <0.001 | 1.48 | (1.41, 1.55) | <0.001 | 1.48 | (1.41, 1.55) | <0.001 | 1.48 | (1.41, 1.55) | <0.001 | |
| Deprivation quintile | 1 (least deprived)a | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 1.08 | (1.03, 1.13) | 0.001 | 1.08 | (1.03, 1.13) | 0.001 | 1.08 | (1.03, 1.13) | 0.001 | 1.08 | (1.03, 1.13) | 0.001 | |
| 3 | 1.13 | (1.08, 1.18) | <0.001 | 1.13 | (1.08, 1.18) | <0.001 | 1.13 | (1.08, 1.18) | <0.001 | 1.13 | (1.08, 1.18) | <0.001 | |
| 4 | 1.19 | (1.13, 1.24) | <0.001 | 1.19 | (1.13, 1.24) | <0.001 | 1.19 | (1.14, 1.24) | <0.001 | 1.19 | (1.14, 1.24) | <0.001 | |
| 5 (most deprived) | 1.21 | (1.15, 1.27) | <0.001 | 1.21 | (1.15, 1.26) | <0.001 | 1.21 | (1.15, 1.27) | <0.001 | 1.21 | (1.16, 1.27) | <0.001 | |
| Comorbidity score | 0a | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 1 | 1.45 | (1.39, 1.52) | <0.001 | 1.45 | (1.39, 1.52) | <0.001 | 1.46 | (1.40, 1.53) | <0.001 | 1.46 | (1.40, 1.53) | <0.001 | |
| 2 | 2.06 | (1.91, 2.22) | <0.001 | 2.06 | (1.91, 2.22) | <0.001 | 2.07 | (1.92, 2.23) | <0.001 | 2.07 | (1.92, 2.23) | <0.001 | |
| 3+ | 2.76 | (2.52, 3.03) | <0.001 | 2.77 | (2.53, 3.04) | <0.001 | 2.78 | (2.54, 3.05) | <0.001 | 2.78 | (2.54, 3.05) | <0.001 | |
| Not known | 0.50 | (0.47, 0.53) | <0.001 | 0.50 | (0.47, 0.53) | <0.001 | 0.50 | (0.47, 0.53) | <0.001 | 0.50 | (0.47, 0.53) | <0.001 | |
| Stage at diagnosis | 1a | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 1.93 | (1.77, 2.11) | <0.001 | 1.94 | (1.77, 2.12) | <0.001 | 1.93 | (1.77, 2.11) | <0.001 | 1.93 | (1.77, 2.11) | <0.001 | |
| 3 | 1.59 | (1.53, 1.66) | <0.001 | 1.59 | (1.52, 1.66) | <0.001 | 1.59 | (1.53, 1.66) | <0.001 | 1.59 | (1.52, 1.66) | <0.001 | |
| 4 | 5.06 | (4.84, 5.28) | <0.001 | 5.06 | (4.85, 5.29) | <0.001 | 5.06 | (4.84, 5.29) | <0.001 | 5.06 | (4.84, 5.29) | <0.001 | |
| Not known | 1.22 | (1.18, 1.27) | <0.001 | 1.22 | (1.18, 1.27) | <0.001 | 1.22 | (1.18, 1.27) | <0.001 | 1.22 | (1.18, 1.27) | <0.001 | |
| Patients receiving different treatments | Surgery | 0.43 | (0.42, 0.45) | <0.001 | 0.43 | (0.42, 0.45) | <0.001 | 0.43 | (0.42, 0.45) | <0.001 | 0.43 | (0.42, 0.45) | <0.001 |
| Chemotherapy | 1.36 | (1.31, 1.42) | <0.001 | 1.36 | (1.31, 1.42) | <0.001 | 1.36 | (1.31, 1.42) | <0.001 | 1.36 | (1.31, 1.42) | <0.001 | |
| Radiotherapy | 0.76 | (0.74, 0.79) | <0.001 | 0.76 | (0.74, 0.79) | <0.001 | 0.76 | (0.74, 0.79) | <0.001 | 0.76 | (0.74, 0.79) | <0.001 | |
| Hormone therapy | 0.83 | (0.80, 0.85) | <0.001 | 0.83 | (0.80, 0.85) | <0.001 | 0.83 | (0.80, 0.85) | <0.001 | 0.83 | (0.80, 0.85) | <0.001 | |
Baseline group.
Table 1 also shows that patients with a record of bipolar had an increased risk of death compared with breast cancer patients without (HR = 1.37, 95% CI: 1.00–1.88, p = 0.049). This association was slightly attenuated and not statistically significant after adjustment, partly because of the small numbers of patients in these groups. The main attenuating factors for bipolar were deprivation, comorbidities and treatment. There was no statistically significant association with survival and a pre‐cancer diagnosis of bipolar (fully adjusted HR = 1.20, 95% CI: 0.79–1.82, p = 0.402) (Table 2). The hazard ratio for post‐cancer onset of bipolar increased after adjustment but also did not reach statistical significance (unadjusted HR = 1.37, 95% CI: 0.85–2.20, p = 0.197, to fully adjusted HR = 1.54, 95% CI: 0.96–2.48, p = 0.075).
There was high power for all the adjusted depression and post‐cancer depression variables (both 0.99), while adjusted pre‐cancer depression had power of 0.59. All the adjusted bipolar results had low power (0.39, 0.12 and 0.43 for all bipolar, pre‐cancer and post‐cancer bipolar, respectively).
Conclusions
Summary of main findings
This study of linked cancer registration and hospital admission data for 77 173 women diagnosed with breast cancer between 2000 and 2009 found that mood disorders appear to indicate worse survival in breast cancer patients. Depression was a clear predictor of reduced survival in breast cancer patients, whereas bipolar showed indications of a similar effect that was not statistically significant. Mood disorders with an onset after the breast cancer diagnosis were more strongly associated with reduced survival than a previous medical history of either depression or bipolar.
Comparison of findings with previous research
Previous research looking solely at the link between depression and breast cancer survival has produced greater hazard ratios for depression in breast cancer patients than observed in this study 14, 35, 36, although these were sometimes underpowered and not statistically significant. Some previous research, however, conflicts with our findings by suggesting no effect of depression on breast cancer survival 15, 37. These differences may be due to the smaller sample sizes and variation in the inclusion and diagnostic criteria used.
Conversely, a meta‐analysis of 12 independent studies analysing the impact of depression on survival of different types of cancer 4 reported a lower but statistically significant adjusted hazard ratio (1.09 compared with 1.33 in this study). Another meta‐analysis examining depression and cancer mortality also found a significant association in breast cancer patients 38. The findings of our study that the worse survival cannot be solely attributed to later stage of presentation are also similar to others 21, 22, 26, 39.
Our finding of worse survival in patients with bipolar is in line with that of Guan et al. 16 who found increased cancer mortality in these patients but not with that of a larger study by Osby et al. 40.
Possible explanations for the link between depression and worse survival include the biological and personal impact of depression. Depression has been linked to a chronic activation of the hypothalamic–pituitary–adrenal axis, which can affect the cellular immune system 41, 42, 43. In addition, abnormalities in diurnal cortisol rhythms 44 have been associated with worse survival. Disruptions to immune and endocrine functions may therefore be mediators of a more rapid disease progression and higher cancer‐related mortality.
Depression could also influence coping styles, behaviour and lifestyle choices with studies reporting an associated increase in risk of relapse or death 45. Patients with depression are characteristically less motivated and have a reduced level of self‐care, which has been linked to poorer compliance with treatment 22, 46. Studies suggest that these patients receive suboptimal treatment and increased waiting times as clinicians face obstacles with eliciting capacity and encouraging uptake and compliance 22, 26, 47. This may be in line with our findings that cancer surgery, radiotherapy and chemotherapy utilization in patients with depression was slightly lower than that in all breast cancer patients, with studies demonstrating their role in reducing recurrence and breast cancer mortality 48, 49. However, without more in‐depth clinical information about the patients' cancer and their management plans, it is difficult to make assumptions that these patients received suboptimal care. Clinicians also have to adjust cancer treatment because of the contraindications and interactions with the medication patients may be taking for their mental illness, as well as their increased number of comorbidities 20. Depression is also associated with somatic symptoms including fatigue, decreased appetite and pain, all of which are determinants of quality of life, an independent predictor of patient prognosis 50.
Depression onset after cancer diagnosis was more strongly associated with worse survival than a prior diagnosis of depression. This could be explained if these diagnoses represent those that were not detected, managed and controlled earlier and may therefore have had a more profound impact on health behaviours, lifestyle choices, and treatment uptake and compliance. These could also be cases previously attributed to low‐mood symptoms ‘expected’ after a cancer diagnosis 51.
Both depression and bipolar have been linked to higher suicide rates and unhealthier lifestyles including smoking and alcohol intake – which are risk factors for reduced survival 40. These factors and the low‐mood component of bipolar may explain the trend that we noticed between bipolar and breast cancer survival.
Strengths and limitations
This linkage study has a larger sample size than many others in this field and is the first to be carried out on breast cancer using routine hospital data in England and to investigate the impact of bipolar. This study was also able to take into account many potential confounders: age, deprivation, ethnicity, comorbidities, disease stage at presentation and recorded cancer treatment. There were several tumour factors that were not available to include in the study, including oestrogen receptor, progesterone receptor and HER‐2 status; performance status; or more detailed staging information. Some variables also had incomplete information: staging information (28%), ethnicity (24%) and comorbidity (17%).
The main limitations relate to potential deficits in the recording and coding of depression and bipolar in routine hospital data. Patients who were not admitted as inpatients or day cases and those whose depression or bipolar diagnoses were not recorded as clinically relevant or went unnoticed would not be included as having a mood disorder in the analyses. Symptoms of low mood, disinterest, fatigue and reduced energy may be common in cancer patients, and the nonspecific nature of the symptoms of depression (particularly the somatic symptoms) makes it hard to distinguish from side effects of treatment like chemotherapy 51. A review by Fann et al. 52 suggested that estimates of depression rates in breast cancer patients who had received surgery varied between 10% and 25%. Studies have reported the issues of under‐recognition and poor oncologist–patient agreement regarding levels of depression 53, which may explain the lower prevalence of depression in the patient population in this study than in the general population. It could be that only the most severely affected patients were therefore included in this analysis. While these cases would be likely to have the greatest impact on survival, the exclusion of other cases may have underestimated the full effect.
The sample size of patients with mood disorders is quite small, partly reflecting the relative rarity of the disease and the way these patients were identified, limiting the study's power. The low power for the bipolar analyses suggests that there may be an effect on survival that we did not have a large enough sample to detect. The association between bipolar and survival in breast cancer patients cannot be ruled out from this study, and indeed, the hazard ratios suggest there may be a similar effect to depression.
Cumulative effects of recurrent episodes or of both depressive and bipolar episodes could also not be investigated. Information on a number of confounders was not available and therefore not adjusted for including lifestyle factors such as smoking status, body mass index, alcohol consumption, treatment for mood disorders, waiting times and social support. Another limitation is the endpoint of cause of death, which in this initial study was not cancer‐specific deaths, and the reduction in survival may be linked to the behavioural patterns associated with depression including unhealthy lifestyle, suicide and the higher risk of other comorbidities. Thus, the reduction in survival may be due to the reduced survival in patients with depression or bipolar, regardless of their cancer diagnosis. From a clinical perspective, if the reduced survival is not cancer specific, it still indicates a need for intervention from a psychiatric and social support perspective. Death certificate data on immediate cause of death can vary between providers, but further careful analysis of these factors would shed light on their possible contribution to the earlier deaths found here. Examining length of disease free survival would also be of interest.
Further research
Further research using cancer registration and hospital data for the rest of England should increase the number of patients with mood disorders and allow more in‐depth analysis of groups of patients with isolated or recurrent depressive episodes and the predominantly depressive or manic bipolar patterns. Analysis of patients by different cancer stage and the treatment they received from different mental health specialists would also be possible with larger numbers of patients. Linkage to general practice data has the potential to capture diagnoses made outside hospital and with wider clinical severity, and it would be interesting to see if the same results can be demonstrated for male patients with breast cancer. Studies of the reliability of the diagnostic coding of depression based on clinician assessment compared with validated scales or standardized interviews would also be an important next step.
Further analysis of patients diagnosed with depression or bipolar after treatment has ended may also be informative. Studies show that patients can develop depression because of fears of recurrence, adjusting to the physical results of surgery 54 and lack of focus once treatment has ended 55. Some medical professionals and family may reduce their support if they do not appreciate that completion of medical treatment does not necessarily mean full recovery 55, 56. A separate analysis of patients with a cancer‐specific death may be useful to determine whether the reduced survival is due to the impact of depression on cancer progression or depression on general survival. It may also be possible to investigate disease‐free survival as better information becomes available through recent expansion of information captured by the National Cancer Registration Service.
Implications for practice
The results from this study could imply that it is the low‐mood aspect of a mood disorder that results in worse survival rather than a general mood disturbance. This may explain why bipolar was not a clear indicator of survival, and results yielded were largely inconclusive. Increased clinical awareness of depression to prevent under‐diagnosis and to direct possible interventions may be beneficial irrespective of whether depression directly impacts on survival or affects survival through its impact on cancer progression. A more active approach to cancer screening and establishing treatment guidelines for patients with mood disorders may also target inequalities in uptake and utilization of these services. Introducing social support or psychological interventions for breast cancer patients from more deprived socio‐demographic backgrounds, older age groups and those presenting with a larger number of comorbidities may target those most at risk and limit or avoid the detrimental effects of depression in these patients.
Ethical approval
The former cancer registries in England had approval from the National Information Governance Board to carry out surveillance using the data they collected on all cancer patients under Section 251 of the NHS Act 2006. Therefore, separate ethical approval was not required for this study.
Acknowledgements
This study was carried out by the former TCR at King's College London, which received funding from the Department of Health. The views expressed in the publication are those of the authors and not necessarily those of the Department of Health. The study was completed with the support of the London Knowledge and Intelligence Team, Public Health England.
Kanani, R. , Davies, E. A. , Hanchett, N. , and Jack, R. H. (2016) The association of mood disorders with breast cancer survival: an investigation of linked cancer registration and hospital admission data for South East England. Psycho‐Oncology, 25: 19–27. doi: 10.1002/pon.4037.
The copyright line for this article was changed on 5 October 2016 after original online publication.
References
- 1. Roud PC. Psychosocial variables associated with exceptional survival of patients with advanced malignant disease. J Natl Med Assoc 1987;79:97–102. [PMC free article] [PubMed] [Google Scholar]
- 2. Cassileth BR, Walsh WP, Lusk EJ. Psychosocial correlates of cancer survival: a subsequent report 3 to 8 years after cancer diagnosis. J Clin Oncol 1988;6(11):1753–1759. [DOI] [PubMed] [Google Scholar]
- 3. Fox BH. The role of psychological factors in cancer incidence and prognosis. Oncology 1995;9:245–253. [PubMed] [Google Scholar]
- 4. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta‐analysis. Cancer 2009;115:5349–5361. [DOI] [PubMed] [Google Scholar]
- 5. Cancer Research UK . Breast Cancer Incidence Statistics. 2014; Accessible at: http://www.cancerresearchuk.org/cancer‐info/cancerstats/types/breast/incidence/ [Last Accessed: April 2015] [Google Scholar]
- 6. Mathew A, Pandey M, Rajan B. Do younger women with non‐metastatic and non‐inflammatory breast carcinoma have poor prognosis? World J Surg Oncol 2004;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Office for National Statistics (ONS) . Cancer survival in England: patients diagnosed 2005–2009 and followed up to 2010. London: Office for National Statistics (ONS) 2011. [Google Scholar]
- 8. Louwman WJ, Janssen‐Heijnen MLG, Houterman S, et al Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: a population‐based study. Eur J Cancer 2005;41(5):779–785. [DOI] [PubMed] [Google Scholar]
- 9. Bouchardy C, Verkooijen HM, Fioretta G. Social class is an important and independent prognostic factor of breast cancer mortality. Int J Cancer 2006;119:1145–1151. [DOI] [PubMed] [Google Scholar]
- 10. Jack RH, Davies EA, Møller H. Breast cancer incidence, stage, treatment and survival in ethnic groups in South East England. Br J Cancer 2009;100(3):545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sant M, Allemani C, Capocaccia R, et al Stage at diagnosis is a key explanation of differences in breast cancer survival across Europe. Int J Cancer 2003;106:416–422. [DOI] [PubMed] [Google Scholar]
- 12. Clarke M, Collins R, Darby S, et al Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005;366(9503):2087–2106. [DOI] [PubMed] [Google Scholar]
- 13. Howard JH, Bland KI. Current management and treatment strategies for breast cancer. Curr Opin Obstet Gynecol 2012;24:44–48. [DOI] [PubMed] [Google Scholar]
- 14. Watson M, Haviland JS, Greer S, et al Influence of psychological response on survival in breast cancer: a population‐based cohort study. Lancet 1999;354(9187):1331–1336. [DOI] [PubMed] [Google Scholar]
- 15. Phillips KA, Osborne RH, Giles GG, et al Psychosocial factors and survival of young women with breast cancer: a population‐based prospective cohort study. J Clin Oncol 2008;26:4666–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan NC, Termorshuizen F, Laan W, et al Cancer mortality in patients with psychiatric diagnoses: a higher hazard of cancer death does not lead to a higher cumulative risk of dying from cancer. Soc Psychiatry Psychiatr Epidemiol 2012;1–7. [DOI] [PubMed] [Google Scholar]
- 17. Vodermaier A, Linden W, Rnic K, et al Prospective associations of depression with survival: a population‐based cohort study in patients with newly diagnosed breast cancer. Breast Cancer Res Treat 2014;143(2):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai MM, Bruce ML, Kasl SV. The effects of major depression and phobia on stage at diagnosis of breast cancer. Int J Psychiatry Med 1999;29(1):29–46. [DOI] [PubMed] [Google Scholar]
- 19. Yancik R, Wesley MN, Ries LG, et al Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 2001;285(7):885–892. [DOI] [PubMed] [Google Scholar]
- 20. Howard LM, Barley EA, Davies E, et al Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol 2010;11(8):797. [DOI] [PubMed] [Google Scholar]
- 21. Burgess CC, Ramirez AJ, Smith P, Richards MA. Do adverse life events and mood disorders influence delayed presentation of breast cancer? J Psychosom Res 2000;48(2):171–175. [DOI] [PubMed] [Google Scholar]
- 22. Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc 2004;52(1):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGinty EE, Yiyi Z, Eliseo G, et al Cancer incidence in a sample of Maryland residents with serious mental illness. Psychiatr Serv 2012;63(7):714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. BarChana M, Itzhak L, Irena L, et al Enhanced cancer risk among patients with bipolar disorder. J Affect Disord 2008;108(1):43–48. [DOI] [PubMed] [Google Scholar]
- 25. Hjerl K, Andersen EW, Keiding N, et al Depression as a prognostic factor for breast cancer mortality. Psychosomatics 2003;44:24–30. [DOI] [PubMed] [Google Scholar]
- 26. Kisely S, Crowe E, Lawrence D. Cancer‐related mortality in people with mental illness. JAMA Psychiatry 2013;70(2):209–217. [DOI] [PubMed] [Google Scholar]
- 27.Royal College of Physicians (2007). Hospital activity data – a guide for clinicians. https://www.rcplondon.ac.uk/sites/default/files/hospital‐activity‐data‐guide‐for‐clinicians‐england_0.pdf [Last Accessed: April 2015]
- 28. Thompson A, Shaw M, Harrison G, et al Patterns of hospital admission for adult psychiatric illness in England: analysis of Hospital Episode Statistics data. Br J Psychiatry 2004;185(4):334–341. [DOI] [PubMed] [Google Scholar]
- 29. Wotton CJ, Goldacre MJ. Record‐linkage studies of the coexistence of epilepsy and bipolar disorder. Soc Psychiatry Psychiatr Epidemiol 2014;49(9):1483–1488. [DOI] [PubMed] [Google Scholar]
- 30. Royal College of Psychiatrists (2012) Second round of the National Audit of Dementia (care in general hospitals) guidance document https://www.rcpsych.ac.uk/pdf/Core%20Audit%20Guidance%20‐%202nd%20round.pdf [Last Accessed: April 2015]
- 31. Burns EM, Rigby E, Mamidanna R, et al Systematic review of discharge coding accuracy. J Public Health 2012;34(1):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noble M, Mclennan D, Wilkinson K, et al The English Indices of Deprivation, 2007. [Google Scholar]
- 33. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 34. Quan H, Sundararajan V, Halfon P, et al Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 35. Watson M, Homewood J, Haviland J, Bliss JM. Influence of psychological response on breast cancer survival: 10‐year follow‐up of a population‐based cohort. Eur J Cancer 2005;41(12):1710–1714. [DOI] [PubMed] [Google Scholar]
- 36. Giese‐Davis J, Collie K, Rancourt KM, et al Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol 2011;29(4):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Groenvold M, Petersen MA, Idler E, et al Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat 2007;105:209–219. [DOI] [PubMed] [Google Scholar]
- 38. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta‐analysis. Psychol Med 2010;40(11):1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang C‐K, Hayes RD, Broadbent MTM, et al A cohort study on mental disorders, stage of cancer at diagnosis and subsequent survival. BMJ Open 2014;4(1):e004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Osby U, Brandt L, Correia N, et al Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001;58(9):844. [DOI] [PubMed] [Google Scholar]
- 41. Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004;5(10):617–625. [DOI] [PubMed] [Google Scholar]
- 42. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8(11):887–899. [DOI] [PubMed] [Google Scholar]
- 43. Spiegel D, Giese‐Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry 2003;54:269–282. [DOI] [PubMed] [Google Scholar]
- 44. Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst 2000;92(12):994–1000. [DOI] [PubMed] [Google Scholar]
- 45. Watson M, Homewood J, Haviland J. Coping response and survival in breast cancer patients: a new analysis. Stress Health 2012;28(5):376–380. [DOI] [PubMed] [Google Scholar]
- 46. Ayres A, Hoon PW, Franzoni JB, et al Influence of mood and adjustment to cancer on compliance with chemotherapy among breast cancer patients. J Psychosom Res 1994;38:393–402. [DOI] [PubMed] [Google Scholar]
- 47. Kissane D. Beyond the psychotherapy and survival debate: the challenge of social disparity, depression and treatment adherence in psychosocial cancer care. Psycho‐Oncology 2009;18:1–5. [DOI] [PubMed] [Google Scholar]
- 48.Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005;365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 49. EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10‐year recurrence and 20‐year breast cancer mortality: meta‐analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383(9935):2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reich M, Lesur A, Perdrizet‐Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat 2008;110(1):9–17. [DOI] [PubMed] [Google Scholar]
- 51. Ganz PA, Rowland JH, Meyerowitz BE, Desmond KA. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res 1998;152:396–411. [DOI] [PubMed] [Google Scholar]
- 52. Fann JR, Thomas‐Rich AM, Katon WJ, et al Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry 2008;30(2):112–126. [DOI] [PubMed] [Google Scholar]
- 53. Passik SD, Dugan W, McDonald MV, et al Oncologists' recognition of depression in their patients with cancer. J Clin Oncol 1998;116(4):1594–1600. [DOI] [PubMed] [Google Scholar]
- 54. Stanton AL, Ganz PA, Rowland JH, et al Promoting adjustment after treatment for cancer. Cancer 2005;104:2608–2613. [DOI] [PubMed] [Google Scholar]
- 55. Schnipper HH. Life after breast cancer. J Clin Oncol 2001;19:3581–3584. [DOI] [PubMed] [Google Scholar]
- 56. Lethborg CE, Kissane D, Burns WI, Snyder R. “Cast adrift”: the experience of completing treatment among women with early stage breast cancer. J Psychosoc Oncol 2000;18:73–90. [Google Scholar]


