Summary
CD101 is a novel echinocandin drug being developed to treat severe fungal infections including invasive candidiasis. We have performed a series of studies to evaluate the antifungal properties of CD101 against both echinocandin‐susceptible and ‐resistant Candida strains. Antifungal susceptibility testing performed on a collection of 95 Candida strains including 30 caspofungin‐resistant isolates containing fks mutations demonstrated comparable antifungal potency of CD101 relative to micafungin (MCF) across different Candida species. Comparable kinetic inhibition of glucan synthase activity was also observed for CD101 and MCF on both wild‐type (WT) and resistant fks mutant Candida strains. Similarly, both drugs yielded nearly identical values for a mutant prevention concentration. In a murine model of invasive candidiasis, CD101 displayed better or at least comparable efficacy relative to MCF in treating WT or fks mutant Candida albicans. An exceptional long‐lived pharmacokinetic profile was observed in mice following a single dose of CD101. Collectively, CD101 has great potential not only in treating invasive Candida infections but also in preventing emergence of resistance to currently approved echinocandin drugs.
Introduction
The echinocandin drugs are the first class of antifungals to target the fungal cell wall (Hector, 1993). These compounds are potent inhibitors of the catalytic subunit of β‐1,3‐D‐glucan synthase, which is responsible for biosynthesis of β‐1,3‐D‐glucan, the major fungal cell wall biopolymer (Denning, 2003). The echinocandins demonstrate in vitro fungicidal activity against most Candida species, including azole‐resistant yeasts, and they are recommended as first‐line therapy for both nonneutropenic and neutropenic patients with candidemia. Echinocandins also are the preferred empiric therapy for suspected candidiasis in nonneutropenic patients in the intensive care unit (Pappas et al., 2009; Pappas et al., 2016). Overall, current frequency of echinocandin resistance remains relatively low (< 1%) with Candida albicans and most other Candida species except Candida glabrata (Castanheira et al., 2010; Pfaller et al., 2011; Pfaller et al., 2013). However, widespread echinocandin usage has been accompanied by reports of emerging multidrug resistance among clinical Candida isolates (Alexander et al., 2013; Fekkar et al., 2014), as well as epidemiological shifts with increased proportion of less susceptible Candida species (Lortholary et al., 2011). Resistance to echinocandins is associated with mutations in two hot spot (HS) regions in the FKS genes that correlate with clinical failure or poor response to therapy (Perlin, 2011; Shields et al., 2012; Beyda et al., 2014).
As a novel echinocandin drug candidate, CD101 has a modified structure (Fig. 1) that confers both superior pharmacokinetics (PK) properties and the potential for an improved safety profile relative to other drugs in the same class (Ong et al., 2015b; Ong et al., 2015a; Rubino et al., 2015). CD101 has demonstrated potent in vitro activity against a broad range of Candida and Aspergillus species including some antifungal‐resistant strains (Castanheira et al., 2014). Presently, a paucity of information exists concerning the antifungal properties of CD101 against well‐defined echinocandin‐resistant clinical isolates. Hence, we have performed a series of studies to comprehensively evaluate antifungal properties of CD101 against both echinocandin‐susceptible and ‐resistant Candida strains, from in vitro susceptibility, enzyme activity assessment, mutant prevention assay, to in vivo PK study and efficacy evaluation against echinocandin‐susceptible and ‐resistant C. albicans strains in a mouse model of invasive candidiasis.
Figure 1.
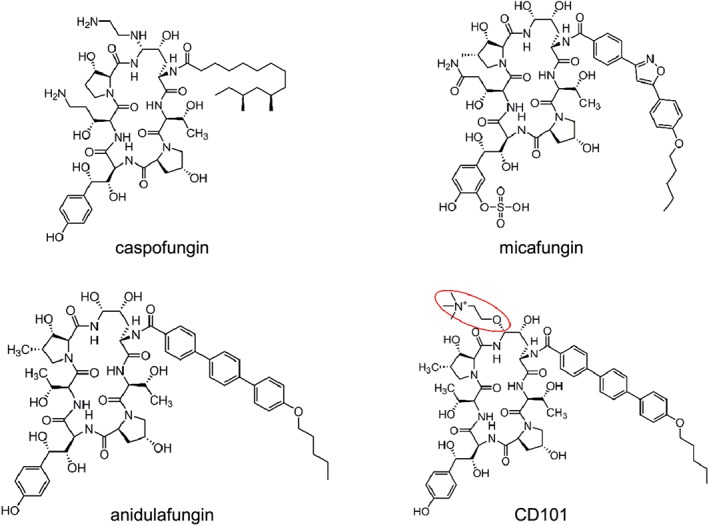
Chemical structure of echinocandin drugs.
Results
Minimal inhibitory concentration (MIC) distributions
Antifungal susceptibility testing was performed for a collection of 95 Candida strains (20 C. albicans, 20 C. glabrata, 2 Candida dubliniensis, 15 Candida krusei, 19 Candida parapsilosis and 19 Candida tropicalis) that included 30 fks mutant isolates showing a caspofungin‐resistant phenotype as per Clinical and Laboratory Standards Institute (CLSI) interpretive criteria (CLSI, 2012). The MIC distributions of the Candida isolates for micafungin (MCF) and CD101 are shown in Table 1. CD101 did not show appreciable differences in MICs for the wild‐type (WT) isolates relative to MCF with the exception of C. krusei isolates, for which MICs were two‐ to fourfold lower for CD101 than MCF. As for caspofungin resistant isolates, CD101 MICs were also generally comparable with that of MCF.
Table 1.
MIC distributions of MCF and CD101 for the Candida isolates included in this study.
| Phenotypea (no. of isolates) | Micafungin | CD101 | |
|---|---|---|---|
| Species | MIC50 mode value [range (µg/ml)] | MIC50 mode value [range (µg/ml)] | |
| C. albicans | WT (10) | ≤0.03 (≤0.03) | ≤0.03 (≤0.03) |
| CR (10) | 1 (0.03–4) | 2 (0.12–2) | |
| C. glabrata | WT (9) | ≤0.03 (≤0.03) | 0.06 (≤0.03–0.06) |
| CR (11) | 0.06/0.25 – 2/4 (0.06–4) | 1 (0.12–4) | |
| C. dubliniensis | WT (1) | 0.03 | 0.03 |
| CR (1) | 0.03 | 0.03 | |
| C. krusei | WT (11) | 0.12 (0.03–0.25) | ≤0.03 (≤0.03–0.06) |
| CR (4) | 0.03 (0.03–8) | ≤0.03–4 (≤0.03–4) | |
| C. parapsilosis | WT (19) | 4 (2–8) | 2 (2–4) |
| C. tropicalis | WT (15) | 0.03 (0.03) | 0.03 (0.03) |
| CR (4) | 2 (1–2) | 2 (0.25–2) |
CR, caspofungin resistant.
IC50s for C. albicans and C. glabrata isolates
To better assess direct inhibition of CD101 on glucan synthase, the kinetic inhibition parameter IC50 (half‐maximal inhibitory concentration) was determined for glucan synthases from WT and fks mutant Candida strains. The inhibition curves for MCF and CD101 against the C. albicans WT isolate showed the typical pattern of β‐1,3‐D‐glucan synthase echinocandin susceptibility reported with other sensitive Candida species (Park et al., 2005; Garcia‐Effron et al., 2009), with mean IC50s of 17.7 and 14.3 ng/ml for MCF and CD101, respectively (supplementary figure and Table 2). Decreased echinocandin susceptibility was observed with the resistant C. albicans F641S, S645P and S645P/S mutant enzymes. The F641S mutant (DPL18) exhibited a 100‐fold and 24‐fold increase in IC50s for MCF and CD101, respectively, compared to the WT. The S645P (DPL20) and S645P/S mutant (DPL22) exhibited 144‐, 14‐fold and 185‐, twofold increase for MCF and CD101 respectively. Whereas the IC50s for MCF and CD101 were similar for the S645P mutant, the IC50 for CD101 was approximately fivefold lower than the MCF IC50 for the F641S mutant. Mean IC50 values for the WT C. glabrata enzyme were 0.5 and 2.6 ng/ml for MCF and CD101, respectively (supplementary figure). The C. glabrata F659del mutant glucan synthase did not exhibit appreciable reductions in activity after treatment with a high concentration (10 000 ng/ml) of either MCF or CD101. However, the S663P mutant exhibited a lower IC50 for MCF compared to CD101.
Table 2.
Half maximal inhibitory concentration (IC50) values for susceptible and resistant C. albicans and C. glabrata isolates used in the study.
| Fks mutation | IC50 (ng/ml)a | ||||
|---|---|---|---|---|---|
| Strain | Species | Fks1p | Fks2p | MCF | CD101 |
| DPL1002 | C. albicans | WT | — | 17.7 | 14.3 |
| DPL18 | C. albicans | F641S | — | 1782.0 | 347.4 |
| DPL20 | C. albicans | S645P | — | 2555.8 | 2641.4 |
| DPL22 | C. albicans | S645P/S | — | 245.5 | 30.5 |
| DPL50 | C. glabrata | WT | WT | 0.5 | 2.6 |
| DPL23 | C. glabrata | WT | F659del | > 10 000 | > 10 000 |
| DPL30 | C. glabrata | WT | S663P | 6772.3 | > 10 000 |
IC50s are the arithmetic mean of three replicate determinations.
Mutant prevention concentration (MPC) determination
The concept of MPC was developed to determine the concentration of antibiotic to prevent the development of resistant bacterial isolates. The MPC is the minimal concentration that suppresses the adapted subpopulation which persists above MIC drug levels, and it is a measure of the susceptibility of the adapted and potentially resistant mutant population (Dong et al., 1999; Zhao and Drlica, 2001). Therefore, administering antibiotic at a dose above the MPC would inhibit the growth of potentially resistant isolates. Using a modified version of the original method, we observed a 3‐ to 5‐log decrease in CFUs around the MICs for MCF and CD101 and a second sharp decrease of recovered colony counts at around 16–32 µg/ml (Fig. 2). We determined the MPC for both MCF and CD101 to be 16 µg/ml against testing WT C. albicans (DPL225) and C. glabrata (BAD55) strains.
Figure 2.
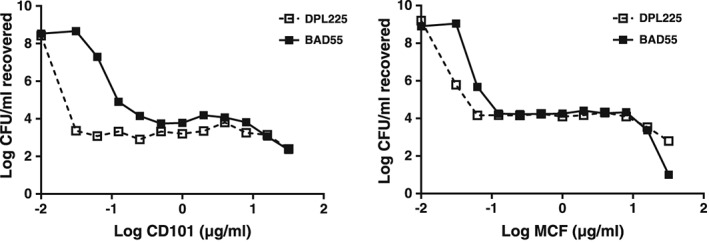
CD101 and MCF MPC determination for wild‐type C. albicans and C. glabrata strains. Fungal cell suspension containing 1 × 107 CFU of WT C. albicans (DPL225) or WT C. glabrata (BAD55) were treated with CD101 or MCF ranging from 0.03 to 32 µg/ml for 24 h. Colonies recovered from treatment were measured by quantitative culture. MPC was defined as the concentration where the second sharp decline of colony counts occurred on the concentration–CFU curve.
In vivo efficacy of CD101 to treat echinocandin resistant C. albicans in a mouse model of invasive candidiasis
To evaluate the in vivo efficacy of CD101 against echinocandin resistant Candida strain, we compared kidney burdens following single dosage of CD101 treatment in neutropenic mice systemically infected with either a WT or resistant C. albicans mutant strain with a heterozygous fks/FKS S645P/S modification (MIC ranged from 0.12 to 0.5 in triplicate testing for both MCF and CD101). CD101 at three increasing dosages of 20, 40 and 60 mg/kg (based on mice PK and human phase 1 clinical trial data, these doses are projected to approximate a range of plasma exposures in mice that would include the actual once‐weekly clinical CD101 dose selected), and MCF at 5 mg/kg, equivalent to human therapeutic dosage, were included in this evaluation. CD101 at all three testing dosages strongly exhibited activity against both WT and fks/FKS mutant strains of C. albicans, as demonstrated by significant kidney burden reduction in all treatment groups at both 24 h and 48 h post‐inoculation time points (P < 0.05) (Figs 3A and 4B). In WT strain infected mice, CD101 exhibited better efficacy than MCF at 24 h post‐inoculation at all three doses (20 mg/kg dose did not achieve statistical significance by post hoc test possibly because of the relatively large variance within the group and small group sample size. Nevertheless, an almost 2 log mean burden difference between 20 mg/kg CD101 and 5 mg/kg MCF was observed). Although the observed superiority of CD101 relative to MCF was only seen with the highest dose (60 mg/kg) at 48 h post‐inoculation, the efficacy of CD101 at 20 mg/kg and 40 mg/kg was still comparable with MCF at 5 mg/kg (Fig. 3A). Regarding the echinocandin resistant fks/FKS mutant strain infected mice, CD101 treatment significantly reduced kidney burdens by over 2 logs at 24 h post‐inoculation compared to vehicle control (P < 0.05). The 24 h burden reduction was not significantly different among the three CD101 dosage groups or MCF treatment group. However, better efficacy of CD101 compared to MCF at 5 mg/kg was observed for all three doses at 48 h post‐inoculation. Burden reduction was comparable between the three CD101 groups (Fig. 3B).
Figure 3.
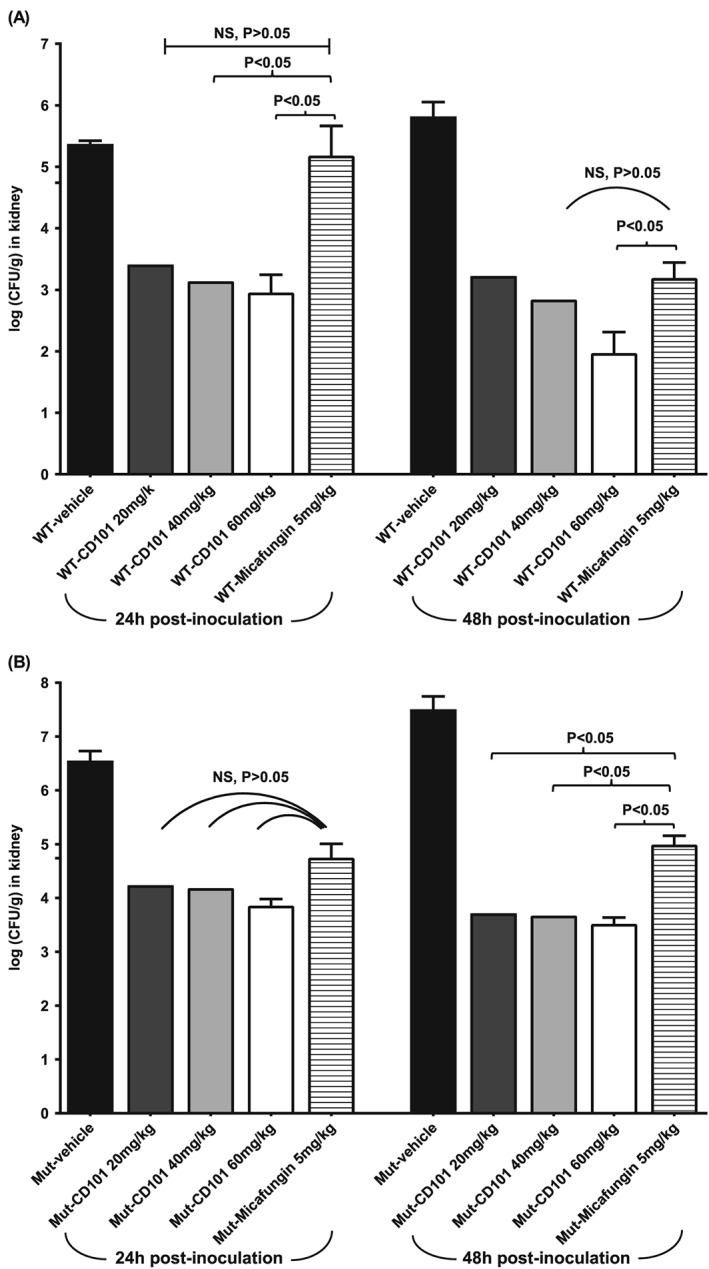
Kidney burden comparison among different treatment groups of mice infected with (A) C. albicans WT strain ATCC 90028 or
(B) C. albicans mutant strain DPL22 S645P/S at 24 h and 48 h post‐infection. Each bar represents the mean burden in the kidneys from five mice. The error bars represent standard deviations.
PK
A single‐dose PK evaluation of CD101 was undertaken in immunocompetent mice following intraperitoneal (IP) doses of 10, 20, 40 and 60 mg/kg of CD101 at 24 h post‐systemic infection with WT C. albicans strain. The time‐course plasma levels of CD101 are shown in Fig. 4. The PK of the drug were relatively linear over the dose range. Maximum plasma concentrations (Cmax) of CD101 were observed at 1 h, 6 h, 6 h and 12 h with mean Cmax values of 23.1, 43.3, 82.3 and 95.8 µg/ml for the doses of 10, 20, 40 and 60 mg/kg respectively. The mean values for AUC0‐t, where t = 48 h post‐dose, were 736, 1250, 2380 and 3300 µg*h/ml for the doses of 10, 20, 40 and 60 mg/kg respectively. The elimination half‐life was long for each dose, ranging from 29.8 to 52.0 h.
Figure 4.
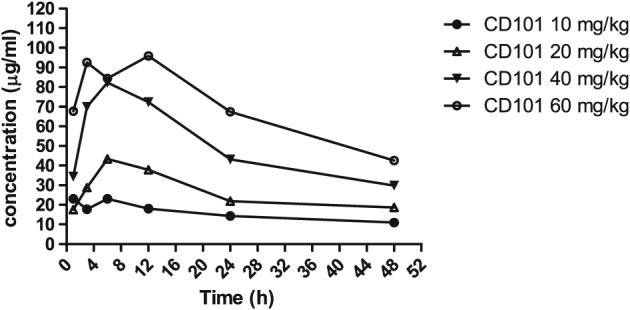
Mean plasma concentration–time profiles of CD101 following single intraperitoneal administration of CD101 at 10, 20, 40 and 60 mg/kg in infected immunocompetent mice. Each symbol represents the mean plasma drug concentration determined for three mice.
Discussion
Previous in vitro susceptibility studies have demonstrated potency of CD101 against a wide spectrum of Candida species and Aspergillus species, variable but overall comparable to anidulafungin and caspofungin (Castanheira et al., 2014). In the current study, we have assessed the in vitro susceptibility profile for CD101 against a well‐characterized panel of caspofungin resistant clinical isolates with fks mutations from a range of Candida species. As expected, CD101 displayed similar activity to MCF against different resistant isolates included in the testing panel, although some slight differences (twofold) between CD101 and MCF were observed in the MIC distribution pattern against C. albicans and C. glabrata ER isolates. In addition, a two to fourfold enhanced activity of CD101 relative to MCF was observed for C. krusei isolates, regardless of FKS genotype. These findings were consistent with previous observations (Hall et al., 2015) and further support the potential of clinical use of CD101.
MIC and FKS genotype status are the key parameters that determine therapeutic response (Perlin et al., 2015). To better understand potential drug–target interactions, we assessed the inhibitory activity of CD101 on its target glucan synthase from 3 C. albicans and 3 C. glabrata isolates with various FKS genotypes. Not surprisingly, the enzyme kinetic profiles were not remarkably different between CD101 and MCF, except that CD101 tends to be slightly more effective than MCF on C. albicans fks mutant enzymes whereas it is not as potent as MCF when tested against C. glabrata fks mutants. These data are consistent with the susceptibility testing results. Whether such enzyme activity variance against different Candida species carrying fks mutations has clinical implications or not will need to be further assessed.
While drug resistance has emerged with echinocandins over the last decade (Arendrup and Perlin, 2014), strategies to efficiently suppress the acquisition of resistance have not been successfully established. A key consideration to this dilemma is that it lacks proper measures of how to overcome development of resistant mutant subpopulations with antifungal drugs. The mutant selection window hypothesis was recently raised to address the critical need of dosing strategy to restrict emergence of resistance to antibacterial agents (Zhao and Drlica, 2001; Drlica and Zhao, 2007; Zhao and Drlica, 2008). This hypothesis postulates that for each antimicrobial–pathogen combination, an antimicrobial range exists in which selective amplification of single‐step, drug‐resistant mutants occurs. More specifically, the lower boundary of the mutant selection window is the lowest drug concentration that eradicates the susceptible cells, and is most likely equal to the MIC99, while the upper boundary of the window is the minimal concentration that inhibits drug‐susceptible mutant subpopulation, a value called the MPC. MPC is a direct measurement of the resistant mutant subpopulation susceptibility. In the present study, MPC was determined for both CD101 and MCF using WT C. albicans and WT C. glabrata strains. For both drugs, concentrations above the MIC level resulted in an adapted cell population that persisted at up to 16 µg/ml of drug level, where decrease of the persisting population was seen with further increase of drug concentration (Fig. 2). From a practical perspective, to maintain drug levels above MPC is often more stringent than necessary and increases risk of toxicity. However, the MPC‐based PK/PD measurement, AUC24/MPC, has been tested to be more accurate than AUC24/MIC in predicting resistance occurrence (Firsov et al., 2006; Olofsson et al., 2006; Drlica and Zhao, 2007), and in vivo experiments also prove the usefulness to control the frequency of resistant mutant development by maintaining drug levels above MPC for certain time of period (Croisier et al., 2004; Etienne et al., 2004). Given the high plasma drug exposure potential and wide safety margin of CD101, and the fact that CD101 and MCF have the same MPC value suggests a possible advantage of CD101 in preventing resistance to currently approved echinocandin drugs.
Despite a growing body of evidence demonstrating robust in vitro antifungal activity of CD101, little was known about the in vivo efficacy of CD101 against echinocandin resistant Candida isolates. In the current study, we partially addressed this critical question by assessing kidney burden reductions post‐antifungal treatment in mice infected with a resistant fks/FKS mutant C. albicans strain. Data acquired from this experiment suggest that CD101 has the potential to achieve efficacy against infections caused by certain Candida strains that are resistant to therapeutic doses of currently approved echinocandin drugs. In another comparison study in mice infected by WT C. albicans strain, the superiority of CD101 was mainly demonstrated by earlier and faster burden reduction compared to MCF (Fig. 3). The burden clearance advantage of CD101 over MCF did diminish at 48 h post‐treatment, yet it is of note that the highest testing dose of CD101 was still significantly better than MCF. The overall testing dosage of CD101 was chosen based on its safety profile and PK data in animals (Ong et al., 2015b; Ong et al., 2015a; Rubino et al., 2015).
Echinocandins kill Candida cells by inhibiting β‐1,3‐D‐glucan synthase in a concentration‐dependent fashion (Douglas et al., 1997; Onishi et al., 2000). From a pharmacokinetic and pharmacodynamic standpoint, drugs that exhibit concentration‐dependent killing and prolonged post‐antibiotic effects are most effective when larger dose levels are administered infrequently (Vogelman et al., 1988; Turnidge et al., 1994; Craig, 1998). In this sense, the long elimination half‐life of CD101, as demonstrated in the current study and others (Ong et al., 2015b; Rubino et al., 2015), as well as its prolonged efficacy and remarkably wide safety margin (Ong et al., 2015b; Ong et al., 2015a), makes CD101 a promising drug candidate that achieves high plasma drug exposure with an extended interval dosing regimen, and may outcompete currently available echinocandin drugs for better prophylactic and treatment efficacy of invasive candidiasis.
In conclusion, CD101 is a novel, long‐acting echinocandin drug that exhibits both in vitro and in vivo strong antifungal activities against a wide spectrum of fungal pathogens. Given its superior PK properties, this drug has the potential to be advantageous for suppressing emergence of resistance to currently approved echinocandin drugs.
Experimental procedures
Strains and antifungal susceptibility testing
All strains used in this study were stocked in the Perlin lab collection. Antifungal susceptibility testing was performed in triplicate for each strain in accordance with the guidelines described in CLSI documents M27‐A3 (CLSI, 2008). C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as quality control strains. CD101 (Cidara Therapeutics, Inc., San Diego, CA, USA) and MCF (Astellas Pharma Inc., Tokyo, Japan) were obtained as standard powders from their manufacturer, and stock solutions were prepared by dissolving the compounds in water (MCF) or 100% dimethyl sulfoxide (DMSO; CD101).
Glucan synthase assay
All testing strains (1 WT and 3 fks C. albicans mutant strains; 1 WT and 2 fks C. glabrata mutant strains) were grown with vigorous shaking at 37°C to early stationary phase in YPD (1% Yeast extract, 2% Peptone, 2% Dextrose) broth, and cells were collected by centrifugation. Cell disruption, membrane protein extraction and partial 1,3‐β‐D‐glucan synthase purification by product‐entrapment were performed as previously described (Garcia‐Effron et al., 2009). Reactions were initiated by the addition of product‐entrapped glucan synthase. Sensitivity to MCF and CD101 was measured in a polymerization assay using a 96‐well 0.65 µm multiscreen HTS filtration system (Millipore Corporation, Bedford, MA) in a final volume of 100 µl, as previously described (Park et al., 2005). Serial dilutions of the drugs (0.01–10 000 ng/ml) were used as calibration standards. MCF was dissolved in water and CD101 was dissolved in 100% DMSO. Inhibition profiles and IC50 values were determined using a normalized response (variable‐slope) curve fitting algorithm with GraphPad Prism, version 6.05, software (Prism Software, Irvine, CA).
MPC determination
As originally described for bacteria, the MPC is determined by spreading a large number of cells (≥ 1011) on agar plates containing increasing concentrations of drug (Dong et al., 1999). However, our attempts to use this method with either C. albicans or C. glabrata resulted in growth on all plates. Alternatively, Candida cells grown overnight in YPD broth with vigorous shaking at 37°C were collected by centrifugation and washed with distilled water. Samples were diluted to 1 × 108 CFU/ml in a total volume of 1.5 ml. One hundred microlitres of fungal cell suspension was added to 0.9 ml of RPMI 1640 medium buffered with MOPS to pH 7.0 with or without drug, providing the starting inoculum of approximately 1 × 107 CFU/ml. The range of CD101 or MCF concentrations tested was 0.03–32 µg/ml. The culture vials were incubated with agitation at 37°C for 24 h. A 100 µl sample was removed from each culture vial and serially diluted with sterile water. Subsequently, 100 µl aliquots of several dilutions were plated on YPD. When colony counts were suspected to be low, 100 µl was taken directly from the culture vials and plated without dilution. Plates were incubated at 37°C for 1–2 days prior to colony counting. MPC was defined as the concentration where the second sharp decline of colony counts occurred on the concentration‐CFU curve (Fig. 2).
Animals
Female 6‐week‐old BALB/c mice (Charles River Laboratories) weighing 18–22 g were used for all animal experiments. Mice were housed in pre‐sterilized filter‐top cages and maintained in accordance with American Association for Accreditation of Laboratory Care criteria. The animal study was approved by Rutgers Institutional Animal Care and Use Committee.
In vivo efficacy of CD101 to treat invasive candidiasis in mice
A well‐established neutropenic disseminated candidiasis murine model was used for this study (Andes, 2005). A total of 100 mice were randomized into 10 different infection/antifungal therapy arms. Sample size of this animal experiment was considered as adequate based on the ‘resource equation’ method (Charan and Kantharia, 2013). Mice were rendered neutropenic by receiving 150 mg/kg and 100 mg/kg of cyclophosphamide via IP injection on day −4 and day −1 prior to infection respectively. The organisms were subcultured in liquid YPD medium at 37°C with shaking overnight. Cells were collected by centrifugation, washed twice with sterile phosphate‐buffered saline (PBS), and counted with a haemocytometer. The inoculum was adjusted to 5 × 106 CFU/ml and 100 µl was used to infect each mouse. Actual infection dose was verified by viable counts on YPD plates spread with proper dilutions of the inoculum and incubated at 37°C for 24 h. On day 0, mice were infected with 5 × 105 CFU of C. albicans FKS WT (DPL1002) (n = 50) or heterozygous mutant strain (DPL22 S645P/S) (n = 50) via lateral tail vein injection. Groups of 10 mice were given single dose of vehicle (provided by Cidara Therapeutics, Inc.), CD101 at 20 mg/kg, 40 mg/kg or 60 mg/kg, or antifungal control (MCF, 5 mg/kg, equivalent to clinical therapeutic dose) at 3 h post‐infection via IP injection. At 24 h post‐inoculation and at the experiment endpoint 48 h post‐inoculation, five mice from each group were euthanized via CO2 inhalation and kidneys were aseptically removed for enumeration of fungal burdens. All graphic data are expressed as means ± SD and were statistically analysed by analysis of variance (ANOVA) using computer Prism software (Prism 5; GraphPad Software, Inc., San Diego, CA). Burden difference between testing and control groups was assessed by post hoc analyses, using Dunnett's or Dunn's multiple comparison test (when group values do not fit Gaussian distribution). A P value of < 0.05 was considered statistically significant.
PK
The PK of CD101 was investigated using an immunocompetent mouse model of disseminated candidiasis (Wiederhold et al., 2011). Mice were infected with 2.0 × 106 CFU of C. albicans WT strain DPL1002 (n = 39) via intravenous injection. Single doses of CD101 at 10, 20, 40 or 60 mg/kg were administered at 24 h post‐infection via IP injection. Blood was collected immediately before, and at 1, 3, 6, 12, 24 and 48 h post‐dose (three mice for pre‐dose, and three mice per dose per post‐dose time point). Plasma samples were prepared within 1 h of collection, stored frozen at −20°C before analysis. CD101 concentration in plasma was measured using liquid chromatography with tandem mass spectrometry (LC/MS/MS). Calibration standards were ranged from 2 to 10 000 ng/ml CD101 in plasma. PK parameters were calculated by non‐compartmental analysis using Phoenix WinNonlin (Pharsight, Mountain View, CA) software.
Conflict of interest
DSP has received support from the US National Institute of Allergy and Infectious Diseases and has received support from Cidara, Astellas, Matinas and Merck, and participates in consult panels for these companies. YZ has received research support from Merck. GH, JBL, KB and VO are employees of Cidara Therapeutics, Inc.
Supporting information
Supplementary figure. Echinocandin inhibition profiles of enriched GS complex from susceptible and resistant C. albicans and C. glabrata isolates. Each strain was tested in triplicate against both CD101 and MCF. Inhibition profiles were generated by using a normalized response (variable‐slope) curve fitting algorithm with GraphPad Prism, version 6.05, software (Prism Software, Irvine, CA)
Supporting info item
Acknowledgements
This work was supported by a research grant from Cidara Therapeutic, Inc. to D.S.P. Cidara Therapeutics, Inc. had a role in study design and review of the manuscript, but did not have a role in the collection, analysis or interpretation of the data. We thank Min‐Hee Lee, Irina Kolesnikova, Enriko Dolgov, George Rasic and Steven Park for their great help with animal experiments.
Zhao, Y. , Perez, W. B. , Jiménez‐Ortigosa, C. , Hough, G. , Locke, J. B. , Ong, V. , Bartizal, K. , and Perlin, D. S. (2016) CD101: a novel long‐acting echinocandin. Cellular Microbiology, 18: 1308–1316. doi: 10.1111/cmi.12640.
References
- Alexander, B.D. , Johnson, M.D. , Pfeiffer, C.D. , Jimenez‐Ortigosa, C. , Catania, J. , Booker, R. , et al. (2013) Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56: 1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andes, D. (2005) Use of an animal model of disseminated candidiasis in the evaluation of antifungal therapy. Methods Mol Med 118: 111–128. [DOI] [PubMed] [Google Scholar]
- Arendrup, M.C. , and Perlin, D.S. (2014) Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyda, N.D. , John, J. , Kilic, A. , Alam, M.J. , Lasco, T.M. , and Garey, K.W. (2014) FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis 59: 819–825. [DOI] [PubMed] [Google Scholar]
- Castanheira, M. , Woosley, L.N. , Diekema, D.J. , Messer, S.A. , Jones, R.N. , and Pfaller, M.A. (2010) Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob Agents Chemother 54: 2655–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanheira, M. , Messer, S.A. , Rhomberg, P.R. , Jones, R.N. , Pfaller, M.A. (2014) Activity of a novel echinocandin biafungin (CD101) tested against most common Candida and Aspergillus species, including echinocandin‐ and azole‐resistant strains. M‐1082..In the 54th Interscience Conference on Antimicrobial Agents and Chemtherapy (ICAAC). Washington D.C.
- Charan, J. , and Kantharia, N.D. (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4: 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts In 3rd ed., M27‐A3. Clinical and Laboratory Standards Institute: Wayne, PA. [Google Scholar]
- CLSI (2012) Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. In M27‐S4. Wayne, PA, Clinical and Laboratory Standards Institute.
- Craig, W.A. (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26: 1–10; quiz 11‐12. [DOI] [PubMed] [Google Scholar]
- Croisier, D. , Etienne, M. , Piroth, L. , Bergoin, E. , Lequeu, C. , Portier, H. , and Chavanet, P. (2004) In vivo pharmacodynamic efficacy of gatifloxacin against Streptococcus pneumoniae in an experimental model of pneumonia: impact of the low levels of fluoroquinolone resistance on the enrichment of resistant mutants. J Antimicrob Chemother 54: 640–647. [DOI] [PubMed] [Google Scholar]
- Denning, D.W. (2003) Echinocandin antifungal drugs. Lancet 362: 1142–1151. [DOI] [PubMed] [Google Scholar]
- Dong, Y. , Zhao, X. , Domagala, J. , and Drlica, K. (1999) Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus . Antimicrob Agents Chemother 43: 1756–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas, C.M. , D'Ippolito, J.A. , Shei, G.J. , Meinz, M. , Onishi, J. , Marrinan, J.A. , et al. (1997) Identification of the FKS1 gene of Candida albicans as the essential target of 1,3‐beta‐D‐glucan synthase inhibitors. Antimicrob Agents Chemother 41: 2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica, K. , and Zhao, X. (2007) Mutant selection window hypothesis updated. Clin Infect Dis 44: 681–688. [DOI] [PubMed] [Google Scholar]
- Etienne, M. , Croisier, D. , Charles, P.E. , Lequeu, C. , Piroth, L. , Portier, H. , et al. (2004) Effect of low‐level resistance on subsequent enrichment of fluoroquinolone‐resistant Streptococcus pneumoniae in rabbits. J Infect Dis 190: 1472–1475. [DOI] [PubMed] [Google Scholar]
- Fekkar, A. , Dannaoui, E. , Meyer, I. , Imbert, S. , Brossas, J.Y. , Uzunov, M. , et al. (2014) Emergence of echinocandin‐resistant Candida spp. in a hospital setting: a consequence of 10 years of increasing use of antifungal therapy? Eur J Clin Microbiol Infect Dis 33: 1489–1496. [DOI] [PubMed] [Google Scholar]
- Firsov, A.A. , Smirnova, M.V. , Lubenko, I.Y. , Vostrov, S.N. , Portnoy, Y.A. , and Zinner, S.H. (2006) Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to daptomycin and vancomycin in an in vitro dynamic model. J Antimicrob Chemother 58: 1185–1192. [DOI] [PubMed] [Google Scholar]
- Garcia‐Effron, G. , Lee, S. , Park, S. , Cleary, J.D. , and Perlin, D.S. (2009) Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3‐beta‐D‐glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53: 3690–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, D. , B.R., Shinabarger, D.L. , Pillar, C.M. (2015) Evaluation of the in vitro activity of CD101, a novel echinocandin, and comparators against recent clinical isolates of Candida spp. M‐850. In Antimicrobial Agents and Chemotherapy (ICAAC) and the International Society of Chemotherapy (ICC) joint meeting. San Diego.
- Hector, R.F. (1993) Compounds active against cell walls of medically important fungi. Clin Microbiol Rev 6: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortholary, O. , Desnos‐Ollivier, M. , Sitbon, K. , Fontanet, A. , Bretagne, S. , and Dromer, F. (2011) Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother 55: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson, S.K. , Marcusson, L.L. , Komp Lindgren, P. , Hughes, D. , and Cars, O. (2006) Selection of ciprofloxacin resistance in Escherichia coli in an in vitro kinetic model: relation between drug exposure and mutant prevention concentration. J Antimicrob Chemother 57: 1116–1121. [DOI] [PubMed] [Google Scholar]
- Ong, V. , Hough, G. , Schlosser, M. , Bartizal, K. , Balkovec, J. , James, K. , Krishnana, R. (2015a) Preclinical evaluation shows CD101, a novel echinocandin, is highly stable with no hepatotoxicity in rats. A‐015. In the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) and the International Congress of Chemotherapy (ICC) joint meeting. San Diego.
- Ong, V. , Miesel, L. , Bartizal, K. , Huang, H.H. , Chien, J.C. (2015b) Prolonged efficacy following one dose of a novel echinocandin, CD101, in a neutropenic mouse model of disseminated candidiasis. F‐761. In the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) and the International Society of Chemotherapy (ICC) joint meeting.
- Onishi, J. , Meinz, M. , Thompson, J. , Curotto, J. , Dreikorn, S. , Rosenbach, M. , et al. (2000) Discovery of novel antifungal (1,3)‐beta‐D‐glucan synthase inhibitors. Antimicrob Agents Chemother 44: 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas, P.G. , Kauffman, C.A. , Andes, D. , Benjamin, D.K., Jr. , Calandra, T.F. , Edwards, J.E., Jr. , et al. (2009) Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48: 503–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas, P.G. , Kauffman, C.A. , Andes, D.R. , Clancy, C.J. , Marr, K.A. , Ostrosky‐Zeichner, L. , et al. (2016) Executive summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62: 409–417. [DOI] [PubMed] [Google Scholar]
- Park, S. , Kelly, R. , Kahn, J.N. , Robles, J. , Hsu, M.J. , Register, E. , et al. (2005) Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother 49: 3264–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin, D.S. (2011) Current perspectives on echinocandin class drugs. Future Microbiol 6: 441–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin, D.S. , Shor, E. , and Zhao, Y. (2015) Update on antifungal drug resistance. Curr Clin Microbiol Rep 2: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller, M.A. , Moet, G.J. , Messer, S.A. , Jones, R.N. , and Castanheira, M. (2011) Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community‐onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob Agents Chemother 55: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller, M.A. , Messer, S.A. , Woosley, L.N. , Jones, R.N. , and Castanheira, M. (2013) Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 51: 2571–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino, C.M. , O.V., Thye, D. , Ambrose, P.G. (2015) Pharmacokinetics–Pharmacodynamics (PK–PD) of a Novel Echinocandin, CD101 (Biafungin), in a Neutropenic Murine Disseminated Candidiasis Model. Slide Session 044. In the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) and the International Society of Chemotherapy (ICC) joint meeting. San Diego.
- Shields, R.K. , Nguyen, M.H. , Press, E.G. , Kwa, A.L. , Cheng, S. , Du, C. , and Clancy, C.J. (2012) The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata . Antimicrob Agents Chemother 56: 4862–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnidge, J.D. , Gudmundsson, S. , Vogelman, B. , and Craig, W.A. (1994) The postantibiotic effect of antifungal agents against common pathogenic yeasts. J Antimicrob Chemother 34: 83–92. [DOI] [PubMed] [Google Scholar]
- Vogelman, B. , Gudmundsson, S. , Turnidge, J. , Leggett, J. , and Craig, W.A. (1988) In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis 157: 287–298. [DOI] [PubMed] [Google Scholar]
- Wiederhold, N.P. , Najvar, L.K. , Bocanegra, R.A. , Kirkpatrick, W.R. , and Patterson, T.F. (2011) Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans . Antimicrob Agents Chemother 55: 3254–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , and Drlica, K. (2001) Restricting the selection of antibiotic‐resistant mutants: a general strategy derived from fluoroquinolone studies. Clin Infect Dis 33(Suppl 3): S147–156. [DOI] [PubMed] [Google Scholar]
- Zhao, X. , and Drlica, K. (2008) A unified anti‐mutant dosing strategy. J Antimicrob Chemother 62: 434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure. Echinocandin inhibition profiles of enriched GS complex from susceptible and resistant C. albicans and C. glabrata isolates. Each strain was tested in triplicate against both CD101 and MCF. Inhibition profiles were generated by using a normalized response (variable‐slope) curve fitting algorithm with GraphPad Prism, version 6.05, software (Prism Software, Irvine, CA)
Supporting info item


