Abstract
The 2D material single‐layer MnPSe3 would be a promising photocatalyst for water splitting, as indicated by the proper positions of band edges, strong absorption in visible‐light spectrum, broad applicability (pH = 0 – 7), and high carrier mobility.
Keywords: 2D materials, carrier mobility, MnPSe3, visible light, water splitting
Since the experimental realization of graphene,1 2D materials have been receiving significant attention due to their unique physical and chemical properties which mainly arise from their topological effects and high surface‐bulk ratios. Graphene has predominated as the most widely studied 2D material. Intractably, lack of a band gap limits its practical applications to high speed switching devices, photocatalysts, etc.2 Nevertheless, the successful preparation of graphene has prompted researchers to investigate more 2D materials such as hexagonal BN,3 transition metal dichalcogenides (TMDs),4 silicene,5 germanane,[[qv: 5b]],6 phosphorene,7 and MXene.8 These 2D materials attract intensive interest due to their novel electronic, mechanical or photocatalytic behaviors,9 making up the shortages of graphene and expanding the applications of 2D materials to field‐effect transistors (FETs)10 and photocatalysts.11
Recently, a new kind of 2D materials (exemplified by MnPSe3) was proposed by Li et al.12 Then, a series of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) were explored by Du et al.13 Particularly, bulk crystals and few‐layer samples of MPX3 (X = S, Se) were obtained and characterized in experiments. The previous reports indicated that the band gaps of these MPX3 bulks range from 1.3 to 3.5 eV,13 suggesting the light absorption at a wide wavelength for photocatalysts.
Hydrogen generation by photocatalytic water splitting would present a promising method for solar energy conversion and play a very important role in solving serious environmental problems.14 However, inability to utilize visible light, low quantum yield, and fast backward reaction limit the practical application of photocatalytic water splitting.15 2D materials with an appropriate band gap (i.e., ≈2–3 eV) would exhibit more efficient use of visible light in the photocatalytic process. Moreover, 2D nature means short distance for the generated electrons and holes to migrate, reducing the possibility of electron–hole recombination, and then giving high quantum yields. Enlightened by some 2D materials which are predicted to be photocatalysts for water splitting under visible light,[[qv: 15b]],16 and especially some proved good photocatalysts in experiments such as g‐C3N4,17 we investigated the band edges and optical properties of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) monolayers to screen more proper photocatalysts for water splitting. Then the carrier mobility was calculated through deformation potential (DP) theory for MnPSe3 monolayer which is a direct‐band‐gap semiconductor and has strong absorption in the visible‐light region. The high carrier mobility of MnPSe3 monolayer (up to 625.9 cm2 V−1 S−1) could be comparable to or even higher than those of many other 2D materials, indicating that the transfer of carriers to reactive sites would be easier in the photocatalytic process. Our results disclose that MnPSe3 monolayer would be a promising photocatalyst for water splitting under visible light.
The structural properties of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) monolayers were explored first. To determine the ground state of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn), both spin‐unpolarized and spin‐polarized computations were performed. The results show that spin‐polarized total energies are less favorable than spin‐unpolarized ones for FePS3, CdPS3, ZnPS3, and FePSe3 monolayers, indicating that these monolayers have nonmagnetic ground states. However, MnPS3, NiPS3, and MnPSe3 monolayers prefer antiferromagnetic (AFM) coupling, which is more stable than the ferromagnetic (FM) state. The structures of these kinds of monolayers are similar, as shown in Figure 1 a.
Figure 1.
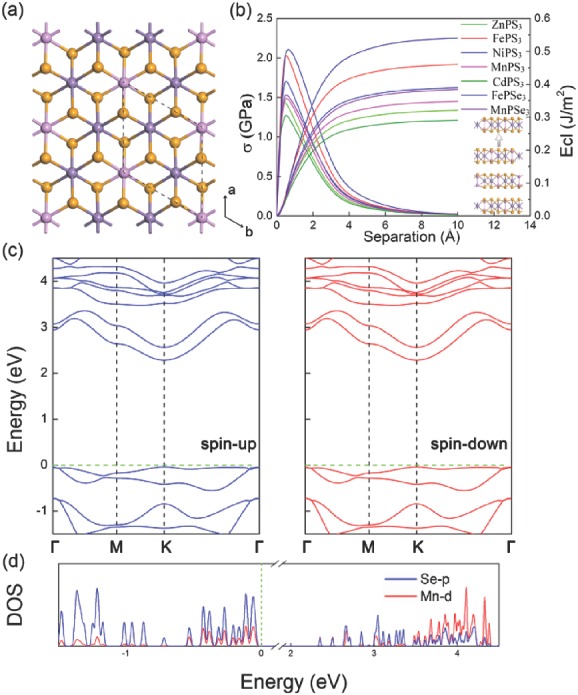
a) Monolayer structure of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn). The pink, orange, and purple balls represent the P, Se/S, and M atoms, respectively. The area circulated by dashes represents the hexagonal primitive cell; b) Cleavage energy E cl (right longitudinal coordinates) and its derivative σ (left longitudinal coordinates) as a function of the separation distance in MPS3 and MPSe3 bulk. Inset: geometry of introduced fracture; c) band structures and d) partial density of states (PDOS) near Fermi level for MnPSe3 monolayer.
To check the stability of the monolayers, the phonon spectra along the high‐symmetry points in Brillouin zone were computed, and are shown in Figure S1 (Supporting Information). There are no imaginary phonon modes in the phonon spectra of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) monolayers, which reveals that these monolayers are dynamically stable configurations and could be realized in experiments.
Another important issue to confirm the stability of 2D materials is whether it can form a freestanding monolayer. Therefore, the in‐plane stiffness should be high enough to withstand its own weight or even external load without substrates. To check this, the in‐plane stiffness was calculated by , where E total, ε, and S 0 represent the total energy per unit cell, uniaxial strain, and the area of the optimized unit cell, respectively. The calculated in‐plane stiffness is shown in Figure S2 and Table S1 of the Supporting Information. The in‐plane stiffness of sulfides is higher than that of the corresponding selenides, which might result from the greater electronegativity of sulfur. Through the elasticity theory, an estimate for the typical out‐of‐plane deformation h/l ≈ (ρgl/C 2D)1/3,18 where l is the length of the monolayer, ρ is the density, and g is the gravitational acceleration. Assuming the length of the monolayer l to be 100 μm, the ratio of the vertical deformation and the length h/l is shown in Table S1 of the Supporting Information, which indicates that MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) monolayers have sufficient rigidity to form freestanding 2D monolayers without substrates.
Then the possibility of mechanical exfoliation from the bulk was checked by calculating the cleavage energy. The relative energy of the bulk increases with the separation distance and converges to the ideal cleavage energy gradually. The optimized lattice constants for the bulk structures are shown in Table S2 of the Supporting Information, which are consistent with the experimental data indicating the credibility of the calculations. As shown in Figure 1b, the cleavage energy (0.29–0.54 J m−2) is comparable to the experimentally estimated value of graphite (≈0.37 J m−2),19 meaning that it is not very difficult to exfoliate monolayers from the bulk in experiments. The theoretical cleavage strength σ could be obtained by computing the maximum derivative of E cl as shown in Figure 1b. The cleavage strength of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) (1.2–2.1 GPa) is even lower than that of graphite (≈2.1 GPa),20 which further indicates that the exfoliation of bulk is feasible in experiments.
We next investigated the electronic properties of the monolayers. The band structures of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) monolayers computed with Heyd–Scuseria–Ernzerhof (HSE06) functional are shown in Figure 1c and Figure 2 .
Figure 2.
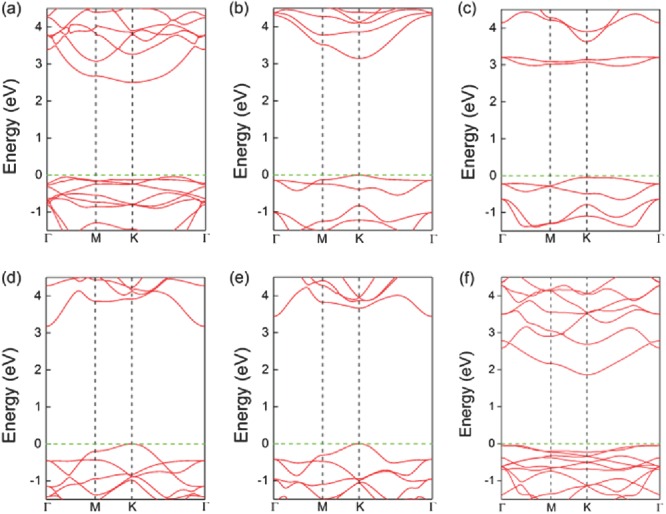
Band structures near Fermi level for a) FePS3, b) MnPS3, c) NiPS3, d) CdPS3, e) ZnPS3, and f) FePSe3 monolayers.
As shown in Table S3 of the Supporting Information, MnPS3 and MnPSe3 monolayers are direct‐band‐gap semiconductors with the band gap of 3.14 and 2.32 eV, respectively, consistent with the previous report.12 The valence band maximum (VBM) and the conduction band minimum (CBM) are both located at the K point. Other monolayers are indirect‐band‐gap semiconductors. More details are provided in the Supporting Information.
To further understand the compositions of VBM and CBM for MPS3 and MPSe3 monolayers, the partial density of states (PDOS) are computed. As shown in Figure 1d and Figure S3 of the Supporting Information, the CBM and VBM of all the monolayers mainly originate from the p states of S/Se and the d states of metal atoms, respectively.
The band structures and PDOS indicate that the band gaps of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) monolayers range from 1.90 to 3.44 eV, which exceed the free energy of water splitting of 1.23 eV. In addition to the magnitude of the band gap, the band edges must straddle the redox potentials of water. To check this, the work functions of these monolayers were calculated and are shown in Table S4 of the Supporting Information.
For the water splitting reaction, the redox potential depends on the pH value.[[qv: 16b]],21 The standard reduction potential for H+/H2 was calculated by and the oxidation potential for O2/H2O was calculated by . Considering that sulfides and selenides might be unstable in an acidic environment, the redox potential for water splitting reaction at neutral environment (pH = 7) was also calculated. The schematic diagram is shown in Figure 3 for the positions of band edges of MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) monolayers for photocatalytic water splitting. Except FePSe3, whose CBM is lower than the reduction potential of H+/H2 at pH = 7, the band edges of the rest compounds straddle the redox potentials of water at pH = 0 and 7. The results indicate that these materials are candidates for water‐splitting photocatalysts without an external bias voltage. More fascinatingly, besides the advantages of suitable positions of band edges at both acidic and neutral environment, MnPS3 and MnPSe3 are direct‐band‐gap semiconductors.
Figure 3.
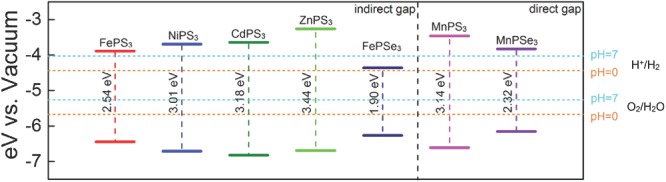
The location of VBM and CBM calculated with HSE06 functional of MPS3 and MPSe3 monolayers. The redox potentials of water splitting at pH = 0 (orange dashed lines) and pH = 7 (cyan dashed lines) are shown for comparison.
Another very important condition for photocatalytic water splitting is that the materials should capture a significant fraction of visible spectrum because it counts for more than 40% of the solar spectrum.
To investigate the performance under light, the optical absorption coefficient was calculated and is shown in Figure 4 . The corresponding imaginary parts ε 2 of the dielectric function is shown in Figure S4 of the Supporting Information. The absorption coefficient is defined as the decay of light intensity spreading in a unit length of medium. FePSe3, MnPSe3, FePS3, and NiPS3 monolayers exhibit obvious optical absorption in visible spectrum and among them, the absorption of FePSe3 and MnPSe3 is stronger. However, FePSe3 might have no photocatalytic activity for water splitting at neutral environment. For MnPSe3, there are two absorption peaks in visible spectrum, indicating the strong optical absorption. Besides, the direct gap would make MnPSe3 more advantageous over other materials. The results indicate that MnPSe3 monolayer would exhibit better performance for photocatalytic water splitting. To investigate the effects of the layer number on the electronic properties and optical absorption of MnPSe3, MnPSe3 bilayer was considered. More details are shown in Figures S5–S7 of the Supporting Information. The results indicate that MnPSe3 bilayer could also exhibit catalytic activity for photocatalytic water splitting under visible light.
Figure 4.
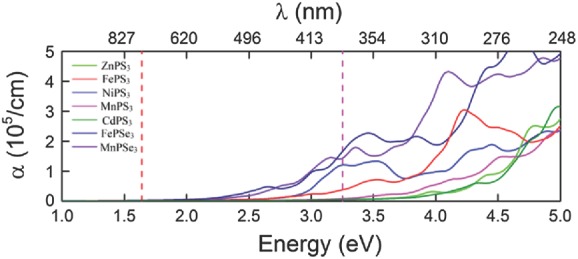
Optical absorption coefficient α for MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn). The area between the red and the purple lines represents the visible range.
To further evaluate the performance of MnPSe3 monolayer as photocatalysts, the carrier effective mass and carrier mobility were investigated for quantitative evaluation on the ability to transfer electron/hole along the specific direction.
The effective masses of electrons (m e*) and holes (m h*) are calculated by fitting parabolic functions to CBM and VBM, respectively. As shown in Table 1 , m e* and m h* along the direction of a (Figure 1a) were calculated, which are in general smaller than those of some common photocatalysts.22 The smaller effective mass indicates that the transfer of carriers to the reactive sites would be easier in the photocatalytic process.
Table 1.
Effective mass |m*| (m e, the mass of free electrons), in‐plane stiffness C 2D (N m−1), DP constant |E 1| (eV), and carrier mobility μ (cm2 V−1 S−1) for electrons and holes along the direction of a (Figure 1a)
| |m*| | C 2D | |E 1| | μ | |
|---|---|---|---|---|
| Electrons (K→Γ) | 0.55 | 60.8 | 2.12 | 625.9 |
| Holes (K→Γ) | 1.22 | 60.8 | 4.08 | 34.7 |
To compute the DP constant E 1, the VBM and CBM position of MnPSe3 monolayer with respect to the vacuum level as a function of the uniaxial strain ε along a direction is shown in Figure S8 of the Supporting Information. On the basis of the obtained |m*|, C 2D, and |E 1|, the calculated carrier mobility of the 2D MnPSe3 monolayer at room temperature (T = 300 K) is shown in Table 1. The electron mobility is 625.9 cm2 V−1 S−1 while the hole mobility is 34.7 cm2 V−1 S−1. The carrier mobility of MnPSe3 monolayer could be comparable to or even higher than that of many other 2D semiconductors, such as MoS2 monolayer (≈200 cm2 V−1 S−1),23 hydrogenated graphene (≈105 cm2 V−1 S−1), fluorinated graphene (≈45 cm2 V−1 S−1), BN (≈487 cm2 V−1 S−1), and BC2N (≈180 cm2 V−1 S−1).24 Moreover, the huge difference between the carrier mobility of electrons and holes indicates that the effective separation of electron–hole pairs and the small probability of recombination for photogenerated carriers. In addition, 2D materials are very promising candidates as photocatalysts, with high specific surface area available for photocatalytic reactions, short distance for the generated electrons and holes to migrate, and further reduced probability in the recombination of photogenerated carriers.[[qv: 15b]]
Overall, MnPSe3 monolayer has many features, strong absorption in visible‐light spectrum, photocatalytic water splitting into H2 and O2 simultaneously, broad applicability (pH = 0–7), high carrier mobility, and feasible synthesis in experiments. Thus, MnPSe3 monolayer is a promising candidate as photocatalysts for water splitting.
In conclusion, the structure and stability of 2D metal phosphorus trichalcogenides monolayers were explored by first‐principles computations, which indicate that these 2D monolayers could be obtained in experiments by exfoliating the corresponding bulk. The calculated band gaps and band edge positions from accurate HSE06 functional predict that MPS3 (M = Fe, Mn, Ni, Cd, Zn) and MPSe3 (M = Fe, Mn) monolayers are promising candidates as photocatalysts for water splitting. Particularly, MnPSe3 monolayer is a direct‐band‐gap semiconductor which exhibits obvious absorption in visible‐light spectrum. Moreover, our calculations of effective mass and carrier mobility for MnPSe3 monolayer illustrate the transfer of carriers to the reactive sites would be easier and the probability of recombination would be lower for photogenerated carriers in the photocatalytic process. These results reflect that 2D MnPSe3 monolayer could be a promising photocatalyst for water splitting.
Experimental Section
Our first‐principles computations based on density functional theory (DFT) were performed with a plan‐wave basis set as implemented in the Vienna ab initio simulation package (VASP).25 The projector augmented wave (PAW) was used to describe the ion–electron interaction.26 The generalized gradient approximation (GGA) expressed by the functional of Perdew, Burke, and Ernzerhof (PBE).27 A 500 eV cutoff was used for the plane‐wave basis set. The DFT‐D3 method with Becke–Jonson damping was adopted to accurately account for the van der Waals force for weak interactions.28 A Monkhorst–Pack k‐point mesh of 7 × 7 × 1 was used for 2D sheet, 7 × 7 × 2 for selenide bulk and 6 × 3 × 5 for sulfide bulk. To study 2D systems under periodic boundary conditions (PBC), a vacuum space with at least 15 Å was inserted between the MPX3 sheets and the periodically repeated images. Moreover, considering that GGA functional systematically underestimates the band gaps,29 we computed the band structures with the HSE06 hybrid functional.30 The computation of the phonon dispersion spectrums was calculated through CASTEP code with finite displacement method as implemented in Materials Studio.31
To investigate the optical absorption, the imaginary part of dielectric function ε 2 was calculated. The expression for ε 2 was given as
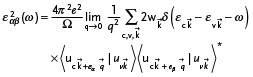 |
(1) |
where the indices c and v refer to the conduction and valence band states, respectively, which were determined by the HSE06 functional here, and is the cell periodic part of the orbital at the k‐point . The absorption coefficient α(ω) was calculated by32
| (2) |
where ε 1 is the real part of dielectric function, which could be obtained from ε 2 by Kramer–Kronig relationship.
For inorganic semiconductors, the electron coherence length is close to the acoustic phonon wavelength, which is much longer than the bonds. As a result, phonon scattering dominates the intrinsic mobility which can be described by the deformation potential theory.33 For 2D materials, the carrier mobility is given by
| (3) |
where T is the temperature, 300 K was adopted in this study, e is the electron charge, and h– is the reduced Planck constant. m* is the effect mass defined as m* = h– 2(∂2 E(k)/∂k 2)−1, where k is the wave vector, and E(k) is the energy corresponding to the wave vector k. E 1 is the DP constant denoting the shift of band edges induced by uniaxial strain, E 1 = ∂E edge/∂ε. C 2D is the in‐plane stiffness as shown in Table S1 of the Supporting Information.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by NSFC (21421001) and MOE Innovation Team (IRT13022) in China. The computations were performed on Magic Cube at Shanghai Supercomputer Center.
Zhang X., Zhao X., Wu D., Jing Y., Zhou Z. (2016). MnPSe3 Monolayer: A Promising 2D Visible‐Light Photohydrolytic Catalyst with High Carrier Mobility. Adv. Sci., 3: 1600062. doi: 10.1002/advs.201600062
References
- 1. Novoselov K. S., Geim A. K., Morozov S., Jiang D., Zhang Y., Dubonos S. A., Grigorieva I., Firsov A., Science 2004, 306, 666. [DOI] [PubMed] [Google Scholar]
- 2. Li X., Dai Y., Li M., Wei W., Huang B., J. Mater. Chem. A 2015, 3, 24055. [Google Scholar]
- 3.a) Jin C., Lin F., Suenaga K., Iijima S., Phys. Rev. Lett. 2009, 102, 195505; [DOI] [PubMed] [Google Scholar]; b) Zeng H., Zhi C., Zhang Z., Wei X., Wang X., Guo W., Bando Y., Golberg D., Nano Lett. 2010, 10, 5049. [DOI] [PubMed] [Google Scholar]
- 4.a) Geim A. K., Grigorieva I. V., Nature 2013, 499, 419; [DOI] [PubMed] [Google Scholar]; b) Wang Q. H., Kalantar‐Zadeh K., Kis A., Coleman J. N., Strano M. S., Nat. Nanotechnol. 2012, 7, 699; [DOI] [PubMed] [Google Scholar]; c) Zeng Z., Yin Z., Huang X., Li H., He Q., Lu G., Boey F., Zhang H., Angew. Chem. Int. Ed. 2011, 50, 11093; [DOI] [PubMed] [Google Scholar]; d) Zhou K.‐G., Mao N.‐N., Wang H.‐X., Peng Y., Zhang H.‐L., Angew. Chem. Int. Ed. 2011, 50, 10839. [DOI] [PubMed] [Google Scholar]
- 5.a) Vogt P., De Padova P., Quaresima C., Avila J., Frantzeskakis E., Asensio M. C., Resta A., Ealet B., Le Lay G., Phys. Rev. Lett. 2012, 108, 155501; [DOI] [PubMed] [Google Scholar]; b) Houssa M., Scalise E., Sankaran K., Pourtois G., Afanas'ev V. V., Stesmans A., Appl. Phys. Lett. 2011, 98, 223107. [Google Scholar]
- 6. Bianco E., Butler S., Jiang S., Restrepo O. D., Windl W., Goldberger J. E., ACS Nano 2013, 7, 4414. [DOI] [PubMed] [Google Scholar]
- 7.a) Reich E. S., Nature 2014, 506, 19; [DOI] [PubMed] [Google Scholar]; b) Churchill H. O. H., Jarillo‐Herrero P., Nat. Nanotechnol. 2014, 9, 330; [DOI] [PubMed] [Google Scholar]; c) Jing Y., Zhang X., Zhou Z., WIRES Comput. Mol. Sci. 2016, 6, 5. [Google Scholar]
- 8.a) Naguib M., Kurtoglu M., Presser V., Lu J., Niu J., Heon M., Hultman L., Gogotsi Y., Barsoum M. W., Adv. Mater. 2011, 23, 4248; [DOI] [PubMed] [Google Scholar]; b) Naguib M., Mochalin V. N., Barsoum M. W., Gogotsi Y.,Adv. Mater. 2014, 26, 992; [DOI] [PubMed] [Google Scholar]; c) Lei J., Zhang X., Zhou Z., Front. Phys. 2015, 10, 276. [Google Scholar]
- 9. Dubertret B., Heine T., Terrones M., Acc. Chem. Res. 2015, 48, 1. [DOI] [PubMed] [Google Scholar]
- 10.a) Ghatak S., Pal A. N., Ghosh A., ACS Nano 2011, 5, 7707; [DOI] [PubMed] [Google Scholar]; b) Late D. J., Liu B., Matte H. R., Dravid V. P., Rao C., ACS Nano 2012, 6, 5635; [DOI] [PubMed] [Google Scholar]; c) Buscema M., Groenendijk D. J., Blanter S. I., Steele G. A., van der Zant H. S., Castellanos‐Gomez A., Nano Lett. 2014, 14, 3347; [DOI] [PubMed] [Google Scholar]; d) Li L., Yu Y., Ye G. J., Ge Q., Ou X., Wu H., Feng D., Chen X. H., Zhang Y., Nat. Nanotechnol. 2014, 9, 372. [DOI] [PubMed] [Google Scholar]
- 11.a) Lee H. U., Lee S. C., Won J., Son B. C., Choi S., Kim Y., Park S. Y., Kim H. S., Lee Y. C., Lee J., Sci. Rep. 2015, 5, 8691; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xiang Q., Yu J., Jaroniec M., J. Am. Chem. Soc. 2012, 134, 6575. [DOI] [PubMed] [Google Scholar]
- 12. Li X., Wu X., Yang J., J. Am. Chem. Soc. 2014, 136, 11065. [DOI] [PubMed] [Google Scholar]
- 13. Du K.‐Z., Wang X.‐Z., Liu Y., Hu P., Utama M. I. B., Gan C. K., Xiong Q., Kloc C., ACS Nano 2016, 10, 1738. [DOI] [PubMed] [Google Scholar]
- 14.a) Gratzel M., Nature 2001, 414, 338; [DOI] [PubMed] [Google Scholar]; b) Zhang J., Qi L., Ran J., Yu J., Qiao S. Z., Adv. Energy Mater. 2014, 4, 1301925; [Google Scholar]; c) Ran J., Zhang J., Yu J., Jaroniec M., Qiao S. Z., Chem. Soc. Rev. 2014, 43, 7787; [DOI] [PubMed] [Google Scholar]; d) Ran J., Ma T. Y., Gao G., Du X.‐W., Qiao S. Z., Energy Environ. Sci. 2015, 8, 3708; [Google Scholar]; e) Rahman M. Z., Kwong P., Davey K., Qiao S., Energy Environ. Sci. 2016, 9, 709. [Google Scholar]
- 15.a) Kudo A., Miseki Y., Chem. Soc. Rev. 2009, 38, 253; [DOI] [PubMed] [Google Scholar]; b) Singh A. K., Mathew K., Zhuang H. L., Hennig R. G., J. Phys. Chem. Lett. 2015, 6, 1087; [DOI] [PubMed] [Google Scholar]; c) Hisatomi T., Kubota J., Domen K., Chem. Soc. Rev. 2014, 43, 7520. [DOI] [PubMed] [Google Scholar]
- 16.a) Liu J., Li X. B., Wang D., Lau W. M., Peng P., Liu L. M., J. Chem. Phys. 2014, 140, 054707; [DOI] [PubMed] [Google Scholar]; b) Zhuang H. L., Hennig R. G., Chem. Mater. 2013, 25, 3232; [Google Scholar]; c) Zhuang H. L., Hennig R. G., J. Phys. Chem. C 2013, 117, 20440; [Google Scholar]; d) Li L., Li P., Lu N., Dai J., Zeng X. C., Adv. Sci. 2015, 2, 1500290; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Zhang X., Li B., Wang J., Yuan Y., Zhang Q., Gao Z., Liu L.‐M., Chen L., Phys. Chem. Chem. Phys. 2014, 16, 25854; [DOI] [PubMed] [Google Scholar]; f) Liu J., Li X.‐B., Wang D., Liu H., Peng P., Liu L.‐M.,J. Mater. Chem. A 2014, 2, 6755. [Google Scholar]
- 17.a) Wang X., Maeda K., Thomas A., Takanabe K., Xin G., Carlsson J. M., Domen K., Antonietti M., Nat. Mater. 2009, 8, 76; [DOI] [PubMed] [Google Scholar]; b) Zheng Y., Lin L., Wang B., Wang X., Angew. Chem. Int. Ed. 2015, 54, 12868; [DOI] [PubMed] [Google Scholar]; c) Zhang J., Chen Y., Wang X., Energy Environ. Sci. 2015, 8, 3092; [Google Scholar]; d) Zheng D., Pang C., Wang X., Chem. Commun. 2015, 51, 17467; [DOI] [PubMed] [Google Scholar]; e) Zheng D., Pang C., Liu Y., Wang X., Chem. Commun. 2015, 51, 9706; [DOI] [PubMed] [Google Scholar]; f) Zheng D., Huang C., Wang X., Nanoscale 2015, 7, 465. [DOI] [PubMed] [Google Scholar]
- 18. Booth T. J., Blake P., Nair R. R., Jiang D., Hill E. W., Bangert U., Bleloch A., Gass M., Novoselov K. S., Katsnelson M. I., Nano Lett. 2008, 8, 2442. [DOI] [PubMed] [Google Scholar]
- 19. Zacharia R., Ulbricht H., Hertel T., Phys. Rev. B 2004, 69, 155406. [Google Scholar]
- 20. Zhao S., Li Z., Yang J., J. Am. Chem. Soc. 2014, 136, 13313. [DOI] [PubMed] [Google Scholar]
- 21. Chakrapani V., Angus J. C., Anderson A. B., Wolter S. D., Stoner B. R., Sumanasekera G. U., Science 2007, 318, 1424. [DOI] [PubMed] [Google Scholar]
- 22.a) Ma X., Dai Y., Guo M., Huang B., ChemPhysChem 2012, 13, 2304; [DOI] [PubMed] [Google Scholar]; b) Li Z., Dai Y., Ma X., Zhu Y., Huang B., Phys. Chem. Chem. Phys. 2014, 16, 3267. [DOI] [PubMed] [Google Scholar]
- 23. Cai Y., Zhang G., Zhang Y.‐W., J. Am. Chem. Soc. 2014, 136, 6269. [DOI] [PubMed] [Google Scholar]
- 24. Bruzzone S., Fiori G., Appl. Phys. Lett. 2011, 99, 222108. [Google Scholar]
- 25. Kresse G., Furthmüller J., Phys. Rev. B 1996, 54, 11169. [DOI] [PubMed] [Google Scholar]
- 26. Kresse G., Joubert D., Phys. Rev. B 1999, 59, 1758. [Google Scholar]
- 27. Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett. 1996, 77,3865. [DOI] [PubMed] [Google Scholar]
- 28.a) Grimme S., Antony J., Ehrlich S., Krieg H., J. Chem. Phys. 2010, 132, 154104; [DOI] [PubMed] [Google Scholar]; b) Grimme S., Ehrlich S., Goerigk L., J. Comput. Chem. 2011, 32, 1456. [DOI] [PubMed] [Google Scholar]
- 29. Kümmel S., Kronik L., Rev. Mod. Phys. 2008, 80, 3. [Google Scholar]
- 30. Heyd J., Scuseria G. E., Ernzerhof M., J. Chem. Phys. 2003, 118, 8207. [Google Scholar]
- 31.a) Segall M., Lindan P. J., Probert M. A., Pickard C., Hasnip P., Clark S., Payne M., J. Phys. Condens. Mater. 2002, 14, 2717; [Google Scholar]; b) Frank W., Elsässer C., Fähnle M., Phys. Rev. Lett. 1995, 74, 1791. [DOI] [PubMed] [Google Scholar]
- 32. Saha S., Sinha T. P., Mookerjee A., Phys. Rev. B 2000, 62, 8828. [Google Scholar]
- 33. Bardeen J., Shockley W., Phys. Rev. 1950, 80, 72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


