Abstract
Background:
Alternanthera sessilis is a medicinal herb which is consumed as vegetable and used as traditional remedies of various ailments in Asia and Africa.
Objective:
This study aimed to investigate the antiglucosidase and antioxidant activity of solvent fractions of A. sessilis leaf and callus.
Materials and Methods:
Leaf and callus methanol extracts were fractionated to produce hexane, chloroform, ethyl acetate, butanol, and water fractions. Antiglucosidase and 1,1-diphenyl-2-picrylhydrazyl scavenging activities as well as total phenolic (TP), total flavonoid (TF), and total coumarin (TC) contents were evaluated. Lineweaver–Burk plot analysis was performed on leaf and callus fractions with the strongest antiglucosidase activity.
Results:
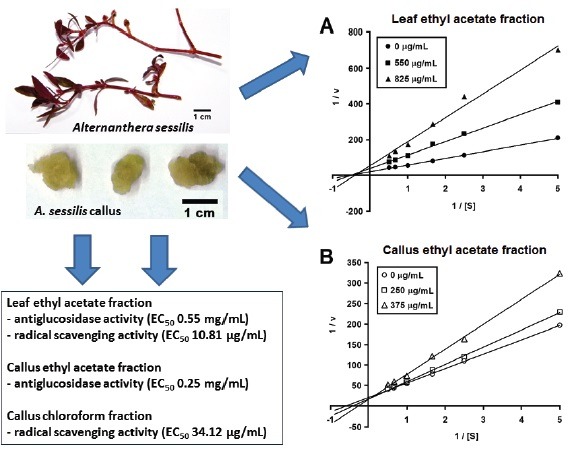
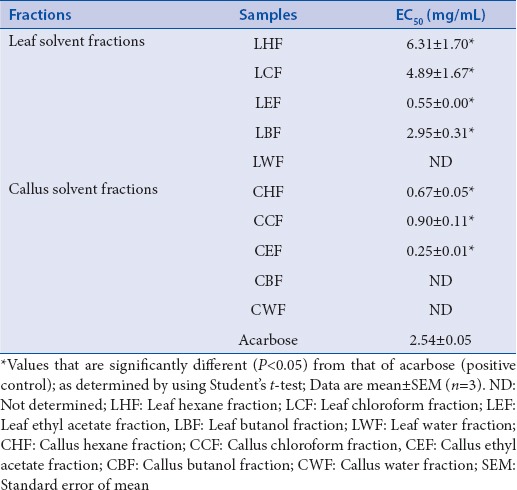
Leaf ethyl acetate fraction (LEF) had the strongest antiglucosidase (EC50 0.55 mg/mL) and radical scavenging (EC50 10.81 μg/mL) activity among leaf fractions. Callus ethyl acetate fraction (CEF) and chloroform fraction had the highest antiglucosidase (EC50 0.25 mg/mL) and radical scavenging (EC50 34.12 μg/mL) activity, respectively, among callus fractions. LEF and CEF were identified as noncompetitive and competitive α-glucosidase inhibitors, respectively. LEF and CEF had greater antiglucosidase activity than acarbose. Leaf fractions had higher phytochemical contents than callus fractions. LEF had the highest TP, TF, and TC contents. Antiglucosidase and antioxidant activities of leaf fractions correlated with phytochemical contents.
Conclusion:
LEF had potent antiglucosidase activity and concurrent antioxidant activity. CEF had the highest antiglucosidase activity among all fractions. Callus culture is a promising tool for enhancing production of potent α-glucosidase inhibitors.
SUMMARY
Leaf ethyl acetate fraction (LEF) had the strongest antiglucosidase (EC50 0.55 mg/mL) and radical scavenging (EC50 10.81 μg/mL) activity among leaf fractions
Callus ethyl acetate fraction (CEF) and chloroform fraction had the highest antiglucosidase (EC50 0.25 mg/mL) and radical scavenging (EC50 34.12 μg/mL) activity, respectively, among callus fractions
LEF and CEF were identified as noncompetitive and competitive á-glucosidase inhibitors, respectively
Antiglucosidase and antioxidant activities of leaf fractions correlated with phytochemical contents.
Abbreviations used: LHF: Leaf hexane fraction, LCF: Leaf chloroform fraction, LEF: Leaf ethyl acetate fraction, LBF: Leaf butanol fraction, LWF: Leaf water fraction, CHF: Callus hexane fraction, CCF: Callus chloroform fraction, CEF: Callus ethyl acetate fraction, CBF: Callus butanol fraction, CWF: Callus water fraction, TP: Total phenolic, TF: Total flavonoid, TC: Total coumarin.
Keywords: Alternanthera sessilis, antiglucosidase, antioxidant, callus, leaf
INTRODUCTION
Diabetes is a major health problem worldwide. The global population of people with diabetes was forecasted to rise to 592 million by 2035.[1] Type 2 diabetes accounts for a major portion of diabetes cases worldwide. Postprandial hyperglycemia is a hallmark of Type 2 diabetes and also a key risk factor for diabetes-associated complications. Control of postprandial hyperglycemia is one of the key strategies for the management of diabetes and diabetes-related complications (Aryangat and Gerich, 2010). Currently, oral antihyperglycemic drugs, such as acarbose, miglitol, and voglibose, are used to manage postprandial hyperglycemia. These drugs exert their effects by inhibiting α-glucosidase in the brush border of the small intestine, hence delaying or inhibiting gastrointestinal digestion of oligosaccharides and the subsequent glucose absorption into the bloodstream.[2] Such drugs have undesirable side effects, such as flatulence and diarrhea. This has generated tremendous interest among researchers to search for alternative, potent α-glucosidase inhibitors from natural products, particularly from medicinal plants.[3]
To search for novel sources of natural products with potent antiglucosidase properties from tropical flora, we have investigated the antiglucosidase potential of Alternanthera sessilis. In Asia and Africa, the herb is consumed as vegetable and used as traditional remedies for various ailments, including diarrhea, headache, hepatitis, bronchitis, and asthma.[4] Pharmacological investigations found A. sessilis extracts to have various bioactivities, including antioxidant,[5] antibacterial,[6] and cytotoxic[7] activities. High-performance liquid chromatography analysis of the ethanol extract of A. sessilis revealed the presence of catechin, rutin, ellagic acid, and quercetin.[8] Notably, recent studies demonstrated antihyperglycemic effects of A. sessilis extracts in diabetic rats[9] and glucose-loaded mice.[10] These studies proposed that A. sessilis may contain natural products that can lower blood glucose level in vivo[9,10] The antiglucosidase activity of A. sessilis has never been previously reported in the literature. Meanwhile, phytochemicals with potent α-glucosidase inhibitory activity are abundant in nature, with more than 400 such natural products documented in the literature.[3] This prompted us to speculate that A. sessilis extracts may be a source of potent antiglucosidase agents.
This study was undertaken to explore the antiglucosidase and antioxidant potential of a total of ten solvent fractions prepared from crude methanol extracts of A. sessilis leaf and leaf-derived callus. Characterizing the antioxidant properties of an antidiabetic herb, in addition to its antiglucosidase potential, has practical importance. An antidiabetic herb with potent antioxidant activity may have additional advantages in tackling oxidative stress, which is considered a key mechanism reinforcing the connection between chronic hyperglycemia and diabetes-associated complications.[11] In this study, we compared the antiglucosidase and antioxidant activity of A. sessilis leaf and leaf-derived callus. Although antioxidant activity of A. sessilis leaf extracts have been reported,[5] comparison of antioxidant activity between leaf and leaf-derived callus has not been investigated. Plant tissue culture is a promising technique for mass-producing bioactive secondary metabolites.[12] Field plants and in vitro cultures of even the same plant species are known to produce different phytochemical profiles and levels of bioactivities.[13] Our findings should answer the question whether A. sessilis callus culture can be considered a promising tool for mass-producing potent antiglucosidase and antioxidant natural products.
The aims of this study were (1) to comparatively evaluate the antiglucosidase and antioxidant activity of A. sessilis leaf and callus; (2) to determine the modes of α-glucosidase inhibition exerted by the most active leaf and callus solvent fractions; and (3) to determine the total phenolic (TP), total flavonoid (TF), and total coumarin (TC) contents in the solvent fractions and analyze their correlations with antiglucosidase and radical scavenging activities.
MATERIALS AND METHODS
Plant material
Specimens of A. sessilis (family Amaranthaceae) were collected from the university medicinal plant plots in May 2014. Herbarium voucher was stored at the Faculty of Science for future reference.
Preparation and fractionation of leaf extract
Plant specimens were cleaned under running tap water and blotted dry with tissue. Leaves excised from the plants were oven-dried at 45°C to constant dry weight and then pulverized with a Waring blender. The leaf powder was extracted with methanol (99.9%) at a 1:10 (g of powder: mL of methanol) ratio on an orbital shaker (125 rpm) at room temperature for 48 h. The leaf powder suspension was vacuum-filtered and then centrifuged at 7830 rpm for 5 min. The supernatant obtained was concentrated in vacuo with a rotary evaporator and then oven-dried at 37°C to constant dry weight. The solid residue of leaf methanol extract recovered was fractionated by solvent-solvent partitioning. Solid residue of the extract (3.5 g) was suspended in 50 mL of deionized water, followed by sequential partitioning into hexane, chloroform, ethyl acetate, and n-butanol by using a separatory funnel. All organic fractions were concentrated in vacuo and oven-dried at 37°C to constant dry weight. Water fraction was freeze-dried. The yields of leaf hexane fraction (LHF), leaf chloroform fraction (LCF) leaf ethyl acetate fraction (LEF), leaf butanol fraction (LBF), and leaf water fraction (LWF) were 22.5%, 7.2%, 2.6%, 10.5%, and 22.5% (w/w), respectively.
Callus culture initiation and maintenance
Leaf explants excised from A. sessilis were surface-sterilized for 15 min in 20% (v/v) clorox solution and rinsed three times (10 min per wash) with sterilized distilled water. The explants were cut into small pieces (5 mm × 5 mm) and cultured on the Murashige and Skoog medium[14] supplemented with 0.8% agar as gelling agent, 3% sucrose as a carbon source, and 3 mg/L picloram as growth regulator. Initiated calli were subcultured using the same medium at a 4-week interval. All callus cultures were maintained at 25 ± 1°C with a 16-h photoperiod under fluorescent light (1000 lux).
Preparation and fractionation of callus methanol extract
For preparation of callus methanol extract, 4-week old calli were blotted dry with tissue and oven-dried at 45°C to constant dry weight. Dried calli were pulverized and extracted with methanol as described above for leaf methanol extract preparation. The callus methanol extract obtained was solvent-partitioned as described above to produce callus hexane fraction (CHF), callus chloroform fraction (CCF), callus ethyl acetate fraction (CEF), callus butanol fraction (CBF), and callus water fraction (CWF). The yields of CHF, CCF, CEF, CBF, and CWF were 3.4%, 1.6%, 1.7%, 9.2%, and 64.7% (w/w), respectively.
Determination of α-glucosidase inhibitory activity
α-glucosidase inhibitory activity was carried out as previously described.[15] Acarbose, an oral hypoglycemic drug used clinically in diabetes mellitus treatment,[3] was used as the positive control. EC50 value, defined as the sample concentration required to achieve 50% antiglucosidase activity, was computed using linear regression analysis.
Lineweaver–Burk plot analysis
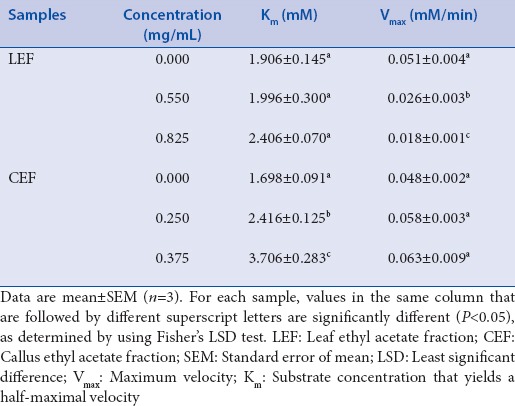
The modes of inhibition of α-glucosidase by LEF and CEF were determined by means of Lineweaver–Burk plot analysis. Briefly, the antiglucosidase assay was carried out in the presence of LEF (0, 550, and 825 µg/mL) and CEF (0, 250, and 375 µg/mL) using 0-2 mM of p-nitrophenyl α-d-glucopyranoside as the substrate (S). The concentrations of LEF and CEF chosen correspond to 0-, 1-, and 1.5-fold of their respective antiglucosidase EC50 values. Double reciprocal plots (1/v vs. 1/[S]) were prepared for LEF and for CEF, which were analyzed by using the Michaelis–Menten kinetics. Maximum velocity (Vmax) and substrate concentration that yields a half-maximal velocity (Km) were determined from the plots.
Determination of 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity
1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging assay was carried out as previously described.[16] Quercetin, a potent antioxidant phytochemical found ubiquitously in vegetables and fruits,[17] was used as the positive control. EC50, defined as the sample concentration required to achieve 50% DPPH scavenging activity, was determined using linear regression analysis.
Determination of total phenolic, flavonoid, and coumarin contents
TP contents of the leaf and callus fractions were determined using the Folin–Ciocalteu colorimetric assay.[18] TP content was expressed as mg gallic acid equivalents/g sample, calculated from a standard curve prepared with gallic acid (0–100 µg/mL). TF content was determined using an aluminum chloride colorimetric assay.[19] TF content was expressed as mg quercetin equivalents/g sample, calculated from a standard curve prepared with quercetin (0–240 µg/mL). TC content was assessed as previously described.[20] TC content was expressed as mg coumarin equivalents /g sample, calculated from a standard curve prepared with coumarin (0–500 µg/mL).
Data analysis
Experiments were performed in triplicates, and data are reported as mean ± standard errors of the mean. Statistical analyses were carried out using SAS (Version 9.3)(SAS, North Carolina, USA). Data were analyzed by one-way ANOVA test and means of significant differences were separated using Fisher's Least Significant Difference (LSD) test or Student's t-test where appropriate, at α = 0.05. Linear regression and correlation analyses were performed using Microsoft Office Excel 2010.
RESULTS
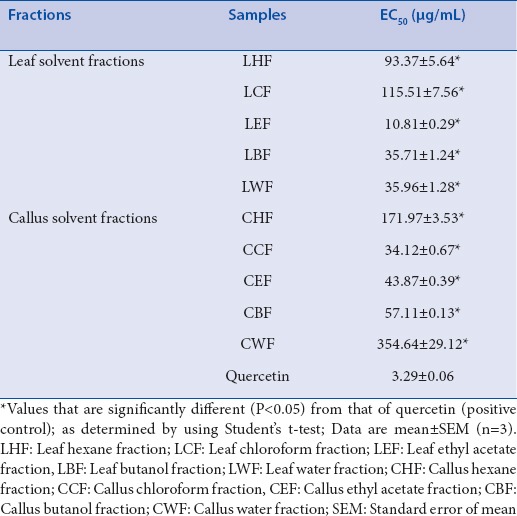
Leaf solvent fractions (EC50 0.55–6.31 mg/mL) exhibited weaker antiglucosidase activity compared with callus solvent fractions (EC50 0.25–0.90 mg/mL) [Table 1]. EC50 of LEF, the most active leaf solvent fraction, was 4.6-fold lower (P < 0.05) when compared with acarbose. Notably, EC50 of CEF, the most active callus solvent fraction, was 10-fold smaller (P < 0.05) than that of acarbose. LWF, CWF, and CBF showed glucosidase-stimulatory activities (data not shown). Thus, EC50 values were not determined for these three samples. Antiglucosidase activity of all other leaf and callus fractions increased in concentration-dependent manner (data not shown).
Table 1.
Antiglucosidase activity of leaf and callus solvent fractions
The modes of inhibition of LEF and CEF (the most active leaf and callus fractions) towards α-glucosidase were investigated. Lineweaver–Burk double reciprocal plots of α-glucosidase activity in the presence of LEF and CEF as inhibitors were established [Figure 1]. From these plots, Km and Vmax values at different levels of LEF and CEF concentrations were computed [Table 2]. For LEF, Km values showed no statistically significant changes (P > 0.05), whereas Vmax values decreased statistically significantly (P < 0.05) with increasing LEF concentration. For CEF, Km values showed a statistically significant rising trend (P < 0.05), whereas Vmax values showed no statistically significant changes (P > 0.05) with increasing CEF concentration.
Figure 1.
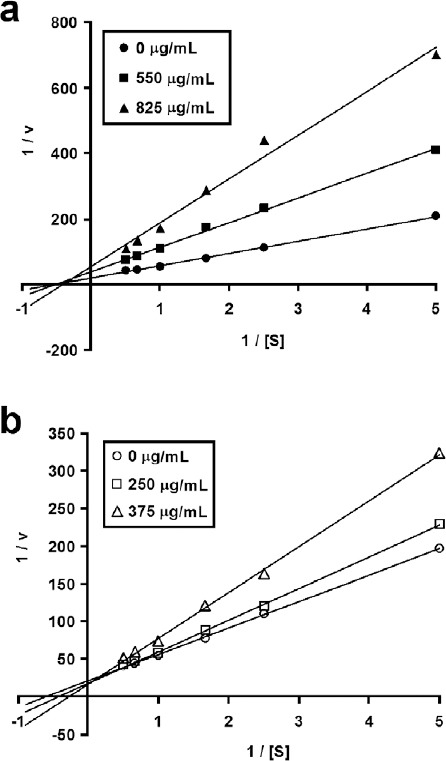
Lineweaver–Burk double reciprocal plots of leaf ethyl acetate fraction (a) and callus ethyl acetate fraction (b). Data points represent mean values of three replicates
Table 2.
Km and Vmax values for α-glucosidase activity in the presence or absence of leaf ethyl acetate fraction and callus ethyl acetate fraction
In this study, DPPH scavenging activity of all leaf and callus fractions increased in concentration-dependent manner (data not shown). Overall, leaf solvent fractions (EC50 10.81-115.51 µg/mL) had stronger DPPH scavenging activity than callus solvent fractions (EC50 34.12-354.64 µg/mL) [Table 3]. EC50 of CCF, the most active callus solvent fraction, was about 3-fold greater compared with EC50 of LEF, the most active leaf solvent fraction. EC50 values of LEF and CCF were 3- and 10-fold higher (P < 0.05) than that of quercetin, respectively.
Table 3.
1,1-diphenyl-2-picrylhydrazyl radical scavenging activities of leaf and callus solvent fractions
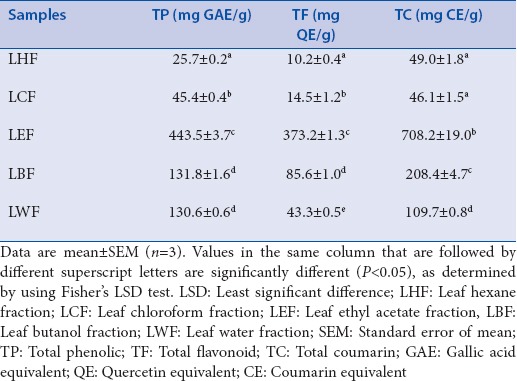
Phytochemical assays found that among leaf solvent fractions, LEF contained the highest TP, TF, and TC contents [Table 4]. Notably, when expressed on the basis of CEs, LEF contained 70% TC by weight. LHF contained the lowest TP and TF contents. The lowest TC contents were detected in LCF.
Table 4.
Selected phytochemical contents of leaf solvent fractions
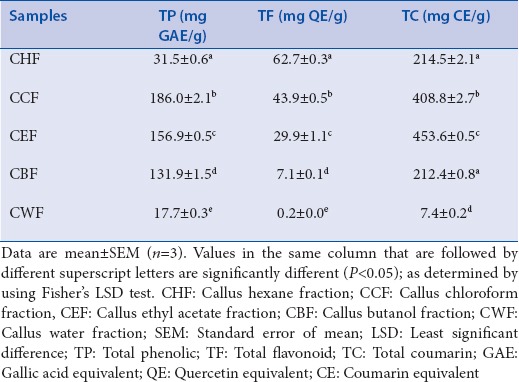
Among the five callus fractions, CCF had the highest TP content [Table 5]. CHF had the highest TF content, whereas CEF had the highest TC content. CWF consistently had the lowest levels of the three phytochemical parameters analyzed, with negligible levels of TF. Overall, when all ten leaf and callus fractions are compared, LEF had the highest levels of all phytochemical parameters examined.
Table 5.
Selected phytochemical contents of callus solvent fractions
We found strong correlation between antiglucosidase activity of leaf solvent fractions and TP (r2 = 0.97, P < 0.05), TF (r2 = 0.98, P < 0.05), and TC (r2 = 0.97, P < 0.05). No statistically significant correlation was found between antiglucosidase activity of callus solvent extracts and the phytochemical parameters. DPPH scavenging activity of leaf solvent fractions correlated statistically significant (P < 0.05) with TP (r2 = 0.99), TF (r2 = 0.97), and TC (r2 = 0.96). For callus solvent fractions, statistically significant correlation (P < 0.05) was found between DPPH scavenging activity and TP (r2 = 0.98) and TC (r2 = 0.69).
DISCUSSION
This study demonstrated for the first time the antiglucosidase activity of active fractions prepared from leaf and callus of A. sessilis. A previous study found that grape skin extract which inhibited in vitro α-glucosidase activity can also suppress in vivo postprandial hyperglycemia in diabetic mice.[21] Thus, α-glucosidase inhibition may account for, at least in part, the antihyperglycemic effects of A. sessilis in obese Type 2 diabetic rats,[9] which should be verified in future. Specifically, LEF derived from leaf methanol extract had the strongest antiglucosidase activity among all leaf fractions. Importantly, LEF exhibited much stronger antiglucosidase activity than acarbose, an antihyperglycemic drug. Our finding concords with previous observation that in diabetic rats, ethyl acetate fraction derived from crude ethanol extract possessed the most potent antihyperglycemic effect.[9] Analysis of changes in Km and Vmax values indicated that LEF was a noncompetitive inhibitor of α-glucosidase. Thus, active principles in LEF potentially acted primarily by binding to a site different from the substrate-binding site of α-glucosidase, changing the enzyme structure, and consequently repressing formation of enzyme-substrate complex and catalytic product.[22] The mode of α-glucosidase inhibition by A. sessilis natural products has not been previously reported. However, natural products that noncompetitively inhibit α-glucosidase have been reported in other plant species.[23,24] Interestingly, the mechanism of α-glucosidase inhibition exerted by LEF differs from that by antihyperglycemic drugs acarbose, miglitol, and voglibose, which are competitive inhibitors of α-glucosidase.[25]
Among the five leaf fractions, LEF is the most interesting because in addition to potent antiglucosidase activity, LEF had the strongest radical scavenging activity. Although antioxidant activity of A. sessilis has been reported,[5,26] our finding of LEF being a potent dual-function antiglucosidase agent with concurrent antioxidant activity is novel and notable. Concurrent antiglucosidase and antioxidant properties of LEF are advantageous as antioxidant therapy is known to mitigate oxidative damage associated with diabetic complications.[27] Hence, LEF may be a promising source of natural products for the development into antidiabetic therapy or an adjunct therapy for the former in future.
TP, TF, and TC contents correlated with antiglucosidase and DPPH scavenging activity of leaf solvent fractions. This finding suggests that antiglucosidase and antioxidant activities in A. sessilis leaf solvent fractions may both be attributed to flavonoids and coumarins. Rutin, catechin, and quercetin, which are bioactive compounds found in A. sessilis,[8] possess both antiglucosidase and antioxidant activities.[3,28] Coumarins and their derivatives also possess antiglucosidase and antioxidant activities.[3,29] There is still no report of isolation and identification of coumarins from A. sessilis, although coumarins are recognized as key bioactive constituents in the Alternanthera species.[30] Our findings provide a rationale for future research to explore for coumarins with potential antiglucosidase and antioxidant properties from A. sessilis leaf.
With the exceptions of CBF and CWF, other callus solvent fractions were found to have potent antiglucosidase activity in addition to concurrent antioxidant activity. Callus solvent fractions generally exhibited stronger antiglucosidase activity than leaf solvent fractions, as indicated by the ranges of antiglucosidase activity EC50 values determined from these samples [Table 1]. Importantly, CHF, CCF, and CEF all showed considerably higher antiglucosidase activity than positive control acarbose. Moreover, CEF had an EC50 value half as small as that of LEF, indicating that CEF was a more powerful α-glucosidase inhibitor than LEF. Our results therefore suggest that plant tissue culture may be a promising technique for mass-producing α-glucosidase inhibitory phytochemicals of A. sessilis. Unlike results obtained for leaf solvent fractions, antiglucosidase activity of callus fractions did not correlate with TP, TF, or TC contents. This suggests that the active α-glucosidase inhibitors of CEF apparently were not phenolic constituents, but this should be verified in future phytochemical investigations. Lineweaver–Burk plot analysis revealed that that unlike LEF, CEF was a competitive inhibitor of α-glucosidase. As competitive inhibitors, active principles in CEF probably inhibited α-glucosidase activity mainly by competing with the substrate for active site of the enzyme.[22] The different modes of α-glucosidase inhibition and the aforementioned discrepancy between outcomes of correlation analyses suggest that the most potent α-glucosidase inhibitors produced by A. sessilis callus were chemically dissimilar to those produced by in vivo leaf tissue.
Among callus solvent fractions, CCF had the strongest antioxidant activity. CCF, nevertheless, was clearly a weaker antioxidant than LEF. Thus, although A. sessilis callus remained as a concurrent source of antiglucosidase and antioxidant natural products, it appeared to be a suboptimal source of natural antioxidants compared with in vivo A. sessilis leaf. In this study, CCF also had lower levels of TP, TF, and TC compared with LEF. Our results are consistent with a recent report of lower antioxidant activity as well as lower TP and TF contents in callus culture of red clover, when compared with in vivo plants.[31] We also found antioxidant activity to be correlated with TP and TC contents of callus solvent fractions, but not with TF content as was observed for leaf solvent fractions. Taken together, it is possible that the profiles of antioxidant phytochemicals produced by A. sessilis callus differed qualitatively and quantitatively from those produced by in vivo leaf tissue.
CONCLUSION
Our findings demonstrated that both leaf and callus of A. sessilis were sources of natural α-glucosidase inhibitors more potent than antidiabetic drug acarbose. Callus solvent fractions were generally stronger α-glucosidase inhibitors than leaf solvent fractions. The most active fractions, LEF and CEF, have been identified as noncompetitive and competitive α-glucosidase inhibitors, respectively. Our results have offered potent antiglucosidase activity of A. sessilis as a possible explanation for the antihyperglycemic effects of the herb. Our findings also highlight the potential of callus culture as a promising tool for mass-producing natural α-glucosidase inhibitors of A. sessilis. In this study, leaf solvent fractions generally had higher antioxidant activity than callus solvent fractions. Our results suggest that the prominent antiglucosidase and antioxidant compounds in A. sessilis leaf and callus are potentially dissimilar, qualitatively, and quantitatively. The identities of such bioactive compounds should be pursued in future research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dr. Tsun-Thai Chai
Dr. Tsun-Thai Chai, obtained his PhD degree from the University of Western Australia in 2010. He is currently an associate professor in the biochemistry program of Universiti Tunku Abdul Rahman, Kampar, Malaysia. His research interests include bioactivity and phytochemistry of tropical ferns and freshwater macrophytes, marine bioactive peptides, and plant physiology.
Acknowledgement
We thank Mr. Si-Nan Tan for his technical assistance in the initiation and propagation of the A. sessilis callus culture.
REFERENCES
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Saraf S. Type 2 diabetes mellitus – Its global prevalence and therapeutic strategies. Diabetes Metab Syndr Clin Res Rev. 2010;4:48–56. [Google Scholar]
- 3.Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-glucosidase inhibitors isolated from medicinal plants. Food Sci Hum Wellness. 2014;3:136–74. [Google Scholar]
- 4.Jansen PC. Alternanthera sessilis (L.) DC. Record from PROTA4U. In: Grubben GJ, Denton OA, editors. PROTA (Plant Resources of Tropical Africa/Ressources Végétales de l’Afrique Tropicale) Wageningen, Netherlands: PROTA; 2004. [Last accessed on 2015 April 30]. Available from: http://www.prota4u.org/search.asp . [Google Scholar]
- 5.Tukun AB, Shaheen N, Banu CP, Mohiduzzaman M, Islam S, Begum M. Antioxidant capacity and total phenolic contents in hydrophilic extracts of selected Bangladeshi medicinal plants. Asian Pac J Trop Med. 2014;7S1:S568–73. doi: 10.1016/S1995-7645(14)60291-1. [DOI] [PubMed] [Google Scholar]
- 6.Ullah MO, Haque M, Urmi KF, Zulfiker AH, Anita ES, Begum M. Anti-bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac J Trop Biomed. 2013;3:1–7. doi: 10.1016/S2221-1691(13)60015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George S, Bhalerao SV, Lidstone EA, Ahmad IS, Abbasi A, Cunningham BT. Cytotoxicity screening of Bangladeshi medicinal plant extracts on pancreatic cancer cells. BMC Complement Altern Med. 2010;10:52. doi: 10.1186/1472-6882-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondal H, Saha S, Awang K, Hossain H, Ablat A, Islam MK. Central-stimulating and analgesic activity of the ethanolic extract of Alternanthera sessilis in mice. BMC Complement Altern Med. 2014;14:398. doi: 10.1186/1472-6882-14-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan KK, Kim KH. Alternanthera sessilis red ethyl acetate fraction exhibits antidiabetic potential on obese type 2 diabetic rats. Evid Based Complement Alternat Med 2013. 2013:845172. doi: 10.1155/2013/845172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain AI, Faisal M, Rahman S, Jahan R, Rahmatullah M. A preliminary evaluation of antihyperglycemic and analgesic activity of Alternanthera sessilis aerial parts. BMC Complement Altern Med. 2014;14:169. doi: 10.1186/1472-6882-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de M Bandeira S, da Fonseca LJ, da S Guedes G, Rabelo LA, Goulart MO, Vasconcelos SM. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci. 2013;14:3265–84. doi: 10.3390/ijms14023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korkina L, Kostyuk V. Biotechnologically produced secondary plant metabolites for cancer treatment and prevention. Curr Pharm Biotechnol. 2012;13:265–75. doi: 10.2174/138920112798868692. [DOI] [PubMed] [Google Scholar]
- 13.Grzegorczyk I, Matkowski A, Wysokińska H. Antioxidant activity of extracts from in vitro cultures of Salvia officinalis. L. Food Chem. 2007;104:536–41. [Google Scholar]
- 14.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97. [Google Scholar]
- 15.Chai TT, Elamparuthi S, Yong AL, Quah Y, Ong HC, Wong FC. Antibacterial, anti-glucosidase, and antioxidant activities of selected highland ferns of Malaysia. Bot Stud. 2013;54:55. doi: 10.1186/1999-3110-54-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chai TT, Wong FC. Whole-plant profiling of total phenolic and flavonoid contents, antioxidant capacity and nitric oxide scavenging capacity of Turnera subulata. J Med Plants Res. 2012;6:1730–5. [Google Scholar]
- 17.Boots AW, Haenen GR, Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–37. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Waterhouse AL. Determination of total phenolics. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, et al., editors. Current Protocols in Food Analytical Chemistry. New York: John Wiley and Sons, Inc; 2001. pp. I1–8. [Google Scholar]
- 19.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colometric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 20.Amorim E, Castro V, Melo J, CorreÌa A, Peixoto Sobrinho TJ. Standard operating procedures (SOP) for the spectrophotometric determination of phenolic compounds contained in plant samples. In: Akyar I, editor. Latest Research into Quality Control. Rijeka: Croatia InTech; 2012. pp. 47–66. [Google Scholar]
- 21.Zhang L, Hogan S, Li J, Sun S, Canning C, Zheng SJ. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chem. 2011;126:466–71. [Google Scholar]
- 22.Hacker M, Bachmann K, Messer W. Principles and Practice. Burlington, MA: Academic Press; 2010. [Google Scholar]
- 23.Ha TJ, Lee JH, Lee MH, Lee BW, Kwon HS, Park CH. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012;135:1397–403. doi: 10.1016/j.foodchem.2012.05.104. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Luo J, Kong L. Phenylethyl cinnamides as potential α-glucosidase inhibitors from the roots of Solanum melongena. Nat Prod Commun. 2011;6:851–3. [PubMed] [Google Scholar]
- 25.Derosa G. P Maffioli, α-glucosidase inhibitors and their use in clinical practice. Arch Med Sci. 2012;8:899–906. doi: 10.5114/aoms.2012.31621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho YL, Huang SS, Deng JS, Lin YH, Chang YS, Huang GJ. In vitro antioxidant properties and total phenolic contents of wetland medicinal plants in Taiwan. Bot Stud. 2012;53:55–66. [Google Scholar]
- 27.Hill MF. Emerging role for antioxidant therapy in protection against diabetic cardiac complications: Experimental and clinical evidence for utilization of classic and new antioxidants. Curr Cardiol Rev. 2008;4:259–68. doi: 10.2174/157340308786349453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–23. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Kostova I, Bhatia S, Grigorov P, Balkansky S, Parmar VS, Prasad AK. Coumarins as antioxidants. Curr Med Chem. 2011;18:3929–51. doi: 10.2174/092986711803414395. [DOI] [PubMed] [Google Scholar]
- 30.Taiwanese Native Medicinal Plants: Phytopharmacology Therapeutic Values. Boca Raton, Florida, USA: CRC Press; 2006. [Google Scholar]
- 31.Khorasani Esmaeili A, Mat Taha R, Mohajer S, Banisalam B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (red clover) Biomed Res Int 2015. 2015:643285. doi: 10.1155/2015/643285. [DOI] [PMC free article] [PubMed] [Google Scholar]


