Abstract
Background:
Myoporum bontioides A. Gray is a commonly used medicinal plant in China. Recently, the chemical and bioactive investigations to the endophytic fungi of this plant have led to several new compounds with antimicrobial and cytotoxic activities. To find out more active molecules, the metabolites of an endophytic fungus, Trichoderma sp. 09 from the root of Myoporum bontioides were investigated.
Materials and Methods:
The metabolites were isolated by column chromatography on silica gel, and their structures were elucidated on the basis of spectroscopic analysis[one-dimensional (1D), two-dimensional (2D)-nuclear magnetic resonance (NMR), Mass spectrometry (MS)], and by comparison with the published data. The dilution method was used for the evaluation of antifungal activity.
Results:
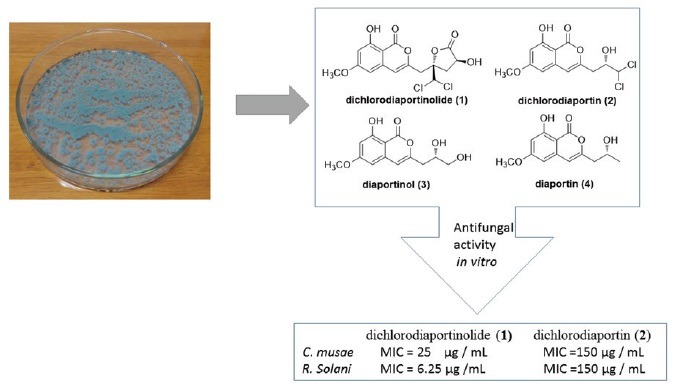
Four metabolites were isolated and identified as: dichlorodiaportinolide (1), dichlorodiaportin (2), diaportinol (3), and diaportin (4). Compounds 1 and 2 showed weak to high antifungal activities against Colletotrichum musae (Berk. and M. A. Curtis) Arx and Rhizoctonia solani Kühn, as compared with the positive control.
Conclusions:
Compound 1 was a new isocoumarin being worthy of consideration for the development and research of antifungal agents.
SUMMARY
A new isocoumarin named dichlorodiaportinolide, along with dichlorodiaportin, diaportinol, and diaportin were isolated from the endophytic fungus Trichoderma sp. 09 of the root of Myoporum bontioides.
Dichlorodiaportinolide and dichlorodiaportin showed weak to high antifungal activities against musae and R. solani (MIC values from 6.25 to 150 μg/mL).
Dichlorodiaportinolide and dichlorodiaportin were inactive to P. italic and F. graminearum (MIC values > 200 μg/mL).
Abbreviations used: IR: Infrared Radiation, HR-ESI-MS: High resolution electrospray ionization mass spectroscopy, LCMS-IT-TOF: Liquid chromatography mass spectroscopy-Ion trap-Time-of-flight, UV: Ultraviolet-visible, HMBC: Heteronuclear multiple bond correlation, NOE: Nuclear Overhauser effect.
Keywords: Antifungal activity, isocoumarin, Myoporum bontioides, trichoderma sp. 09
INTRODUCTION
Myoporum bontioides A. Gray is a small evergreen shrub distributed along the coastal regions of southern Kyushu islands through Okinawa, Taiwan, southern China, and Indochina.[1] In China, the decoction of Myoporum bontioides is used as a folk medicine for antidermatosis, and as antipyretic and antipsychotic.[2] The previous studies reported that this plant contained sesquiterpenoids, iridoids, monoterpenes, phenylethanoids, and flavonoids etc.[3,4,5] Endophytes, residing in the tissues of the living plants, serve as an important source of bioactive compounds with novel structures.[6] Recently, our chemical and bioactive investigations to the endophytic fungi of Myoporum bontioides, have led to several new compounds with antimicrobial[7] and cytotoxic activities.[8,9] In the study, the metabolites of an endophytic fungus, Trichoderma sp. 09 isolated from the root of Myoporum bontioides were investigated. As a result, a novel chlorine-containing isocoumarin named dichlorodiaportinolide (1), along with three known analogues, dichlorodiaportin (2), diaportinol (3),[10] and diaportin (4) [Figure 1],[11] were isolated. The isolation, structural elucidation, and the antifungal activity against several plant pathogenic fungi of the metabolites are reported herein.
Figure 1.
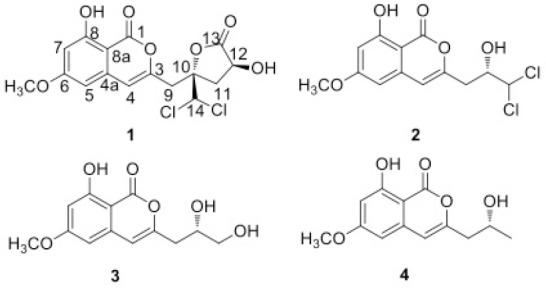
Chemical structures of compounds 1-4
MATERIALS AND METHODS
General
IR spectra was obtained with a Nicolet 5DX-Fourier transformer infrared spectrophotometer (Thermo Electron Corporation, Madison, USA). All NMR experiments were recorded in a Bruker AVIII 600MHz NMR spectrometer (Bruker BioSpin GmbH company, Rheinstetten, Germany), using deuterated dimethyl sulfoxide, or acetone, or chloroform as solvent and the residual solvent resonance as internal standard. The coupling constants (J) were in Hz.HR-ESI-MS, and MS was performed on LCMS-IT-TOF (Shimadzu, Japan) mass spectrometer. The optical rotations were measured on a Jasco P-1020 digital polarimeter. Chromatography was carried out on a silica gel column (200-300 mesh; Qingdao haiyang chemicals Co., Ltd., Qingdao, China). All other reagents used were of analytical grade.
Fungus and cell material
The Trichoderma sp. 09 strain was isolated from the root of Myoporum bontioides A. Gray collected in Leizhou Peninsula, Guangdong Province, China. Its species was identified by comparing with the morphological characteristics with that of Trichoderma sp. 09[12] This fungus is deposited in College of Materials and Energy (Formerly College of Science), South China Agricultural University, Guangzhou, China. A small scrap of agar slice with mycelium was added into a 500 mL Erlenmeyer flask containing 250 mL of GYT medium (1% glucose, 0.1% yeast extract, 0.2% peptone, 0.2% crude sea salt) aseptically, and incubated at 28 °C, 180 rpm for 6 days as seed culture. The fermentation was obtained with 100 Erlenmeyer flasks (the size of each was 1 L) and each containing a rice medium (100 mL water, 100 g rice, 0.3 g crude sea salt). 4 mL seed culture was added into each flask and then cultivated at room temperature for 30 days under static conditions.
Extraction and isolation
[200] mL methanol was added into each of the flasks and extracted for 72 h at room temperature. The solvent was concentrated to 3L with the rotary evaporator in vacuo in a 50°C water bath and extracted three times in sequence with the equal volume of ethyl acetate. The extracts were evaporated to dryness in vacuo and combined. The combined extract was chromatographed repeatedly on silica gel column using gradient elution to obtain (1), (2), (3), and (4), from the petroleum ether/ethyl acetate 80:20, 75:25, 70:30, and 85:15 (v/v) fractions, respectively.
Antifungal activity
The following four phytopathogenic fungi were used for bioassay: Colletotrichum musae (Berk. and M. A. Curtis) Arx. (C. musae), Fusarium graminearum Schw.(F. graminearum), Penicillium italicumWehme (P. italicm), and Rhizoctonia solani Kühn (R. solani). They were obtained from College of Agriculture, South China Agricultural University. The quantitative tests of the antimicrobial activities of the pure compounds were performed by the broth dilution method, as described in the previous report to determine the minimum inhibitory concentration (MIC).[7,13] The carbendazim was used as the positive control of growth inhibition, and the negative control of solvent influence was detected parallely. The results were presented as mean values of three measurements.
RESULTS
Dichlorodiaportinolide (1)
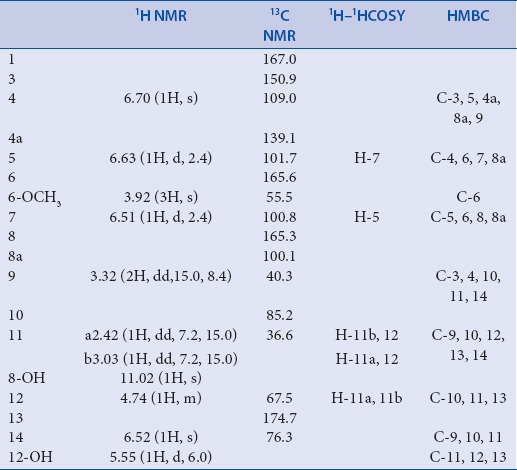
White needles; mp 185-187°C; [α]D25 +17° (c 0.04, acetone); IR (KBr) cm-1 3382, 2810, 1760, 1681, 1625, 1511, 1302. UV λmax(CH3OH) nm 330, 274, 245. 1H (600 MHz, CD3COCD3) and 13C-NMR (150MHz, CD3COCD3) data, see [Table 1]. HR-ESI-MS m/z: 389.0184 ([M+H]+, C16H14Cl2O7, calcd.389.0188)
Table 1.
NMR Data for compound 1 (600/150MHz, CD3COCD3, δ/ppm, J/Hz)
DISCUSSION
Compound 1 was obtained as white needles and it has a molecular formula of C16H14Cl2O7 as determined by HR-ESI-MS m/z: 389.0184 ([M+H]+, calcd.389.0188), indicating nine degrees of unsaturation. Its UV spectrum with absorption maxima at 245, 274 and 330 nm, and IR spectrum with absorption bands at 1681, 1625, and 1511 cm-1 revealed the presence of typical isocoumarin moiety in comparison with known isocoumarins.[14,15] The 1H and 13C NMR spectral data [Table 1] of 1 were closely comparable to those of 2 and 3 which displayed similar NMR data,[10] and suggested that a hydroxyl (δH 11.02), a methoxyl (δH 3.92, δc 55.5), and a methine (δH 3.32, δc 40.3) were connected to the isocoumarin moiety at C-8 (δC 165.3), C-6 (δC 165.6), and C-3 (δC 150.9), respectively, in the same manner as 2 and 3. The isocoumarin group, along with a carbonyl (δC 174.3), accounted for 8 of the 9 elements of unsaturation in 1. It suggested that the remaining one unit of unsaturation was due to another monocyclic ring. Moreover, the HMBC correlations [Table 1] from H-9 (δH 3.32) to C-3 (δC 150.9), C-4 (δC 109.0), C-10 (δC 85.2), and C-11 (δC 36.6), from H-11 (δH 2.42) to C-9 (δC 40.3), C-10 (δC 85.2), C-12 (δC 67.5), and C-13 (δC 174.7), from H-12 (δH 4.47) to C-10 (δC 85.2), C-11 (δC 36.6), and C-13 (δC 174.7), and from 12-OH (δH 5.55) to C-11 (δC 36.6), C-12 (δC 67.5), and C-13 (δC 174.7), indicated that an α-hydroxyl-γ-lactone ring was formed by ring closure involving the oxygen atom bridged to C-10 (δC 85.2) and C-13 (δC 174.7), and its location at C-9 (δC 40.3). Thus, the only position left to place the remaining chlorine atoms was at C-14. The HMBC correlations from H-14 (δH 6.52) to C-9 (δC 40.3), C-10 (δC 85.2), and C-11 (δC 36.6), further demonstrated the connectivity of C-14 and C-10. Therefore, the planar structure of compound 1 was established as shown in [Figure 1].
The relative configuration of compound 1 was inferred as 10S* and 12S* from the NOE correlations between H-14 (δH 6.52) and H-11b (δH 3.03), between 12-OH (δH 5.55) and H11b (δH 3.03), and between 12-OH (δH 5.55) and H14 (δH 6.52), as well as no NOE correlations observed between H-14 and H-11a or between 12-OH and H11a in the NOESY spectrum. Compound 1 was named as dichlorodiaportinolide, according to its similar structure with dichlorodiaportin.[10]
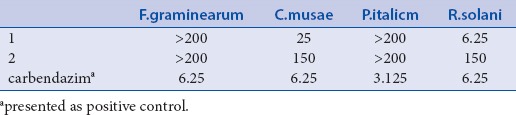
The antifungal activity of the isolated compounds was assessed quantitatively by the broth dilution method to determine the minimum inhibitory concentration (MIC).[7,13] The results are summarized in [Table 2]. According to the results, compound 1 showed significant antifungal activity against R. solani with a MIC value of 6.25 μg mL-1 which was the same as the positive control carbendazim and moderate activity against C. musae with a MIC value of 25 μg mL-1. However, compound 2 only exhibited weak activities toward these two fungi with MIC values of 150 μg mL-1. Neither of them was active to P. italic and F. graminearum (MICs > 200 μg mL-1). The activities of compounds 3 and 4 were not detected due to their small amounts. These results suggested that compound 1, could be valuable for a development of a new fungicide or lead compound for the treatment of some fungal infections.
Table 2.
The antifungal activity of the compounds by the MIC values (μg/mL)
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (21102049), the Natural Science Foundation of Guangdong Province (2015A030313405, 9451064201003751), the Science and Technology Project for public welfare research and capacity building of Guangdong Province (2016A020222019), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry([2015]311), and the Innovation Experiment Program for University students of Guangdong Province(201510564196).
Conflicts of interest
There are no conflicts of interest
ABOUT AUTHOR

Chunyuan Li
Dr. Chunyuan Li, Obtained his Ph. D. degree in 2006 from School of Chemistry and Chemical Engineering, Sun Yat-sen University, China. Currently, he is positioned as associate professor of College of Materials and Energy, South China Agricultural University, China. Dr. Li is working in the area of Pharmacognosy and Chemistry of Natural Products, mainly in isolation, structure elucidation and bioactivity evaluation of natural products from marine plant endophytic fungi.
REFERENCES
- 1.Deng Y, Yang Z, Yu Y, Bi X. Inhibitory activity against plant pathogenic fungi of extracts from Myoporum bontioides. A. Gray and indentification of active ingredients. Pest Management Science. 2008;64:203–7. doi: 10.1002/ps.1491. [DOI] [PubMed] [Google Scholar]
- 2.Huang LL, Li JW, Ni CL, Gu WX, Li CY. Isolation, crystal structure and inhibitory activity against Magnaporthe Grisea of (2R,3R)-3,5,7-trihydroxyflavanone 3-acetate from Myoporum Bontioides A. Gray. Chin J Struct Chem. 2011;30:1298–304. [Google Scholar]
- 3.Wang QG, Ma CL, Zhai JJ. Furanoeudesmane-B, a new eudesmane sesquiterpenoid from Myoporum bontioides. Acta Cryst C. 2000;56:e569. [Google Scholar]
- 4.Kanemoto M, Matsunami K, Otsuka H, Shinzato T, Ishigaki C, Takeda Y, et al. Chlorine-containing iridoid and iridoid glucoside, and other glucosides from leaves of Myoporum bontioides. Phytochemistry. 2008;69:2517–22. doi: 10.1016/j.phytochem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Li XZ, Li CY, Wu LX, Yang FB, Gu WX. Chemical constituents from leaves of Myoporum bontioides. Chin Tradit Herb Drugs. 2011;42:2204–7. [Google Scholar]
- 6.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiology and Molecular Biology Reviews. 2003;67:491–02. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JH, Ding WJ, Wang RM, Du YP, Liu HL, Kong XH, et al. Identification and bioactivity of compounds from the mangrove endophytic fungus Alternaria sp. Marine Drugs. 2015;13:4492–504. doi: 10.3390/md13074492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JH, Cox DG, Ding WJ, Huang GH, Lin YC, Li CY, et al. Three new resveratrol derivatives from the mangrove endophytic fungus Alternaria sp. Marine Drugs. 2014;12:2840–50. doi: 10.3390/md12052840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CY, Gong B, Cox DG, Li CL, Wang JH, Ding WJ, et al. Dichlorodiaportinol A, a new chlorine-containing isocoumarin from an endophytic fungus Trichoderma sp. from Myoporum bontioides A. Gray and its cytotoxic activity. Pharmacognosy magazine. 2014;10:s153–8. doi: 10.4103/0973-1296.127367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen TO, Breinholt J. Dichlorodiaportin, diaportinol, and diaportinic acid: three novel isocoumarins from Penicillium nalgiovense. Journal of Natural Products. 1999;62:1182–4. doi: 10.1021/np990066b. [DOI] [PubMed] [Google Scholar]
- 11.Mantle PG. Biosynthesis of diaporthin and orthosporin by Aspergillus ochraceus. Phytochemistry. 2001;57:165–9. doi: 10.1016/s0031-9422(01)00004-8. [DOI] [PubMed] [Google Scholar]
- 12.Amaresan K, Bhagat N, Madhuri S, Srivastava K. RCIsolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian Journal of Microbiology. 2012;52:137–44. doi: 10.1007/s12088-011-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng CL, Ma YM. Isolation and anti-phytopathogenic activity of secondary metabolites from Alternaria sp. FL25, an endophytic fungus in Ficus carica [J] Chin J Appl Environ Biol. 2010;16:76–8. [Google Scholar]
- 14.Ichihara A, Hashimoto M, Hirai T, Takeda I, Sasamura Y, Sakamura S, et al. Structure, synthesis, and stereochemistry of (+)-orthosporin, a phytotoxic metabolite of Rhynchosporium orthosporium. Chem Lett. 1989;18:1495–8. [Google Scholar]
- 15.Yang SX, Gao JM, Zhang Q, Laatsch H. Toxic polyketides produced by Fusarium sp., an endophytic fungus isolated from Melia azedarach. Bioorganic and Medicinal Chemistry Letters. 2011;21:1887–9. doi: 10.1016/j.bmcl.2010.12.043. [DOI] [PubMed] [Google Scholar]


