Abstract
Background:
Obesity causes or aggravates many health problems, both independently and in association with several pathological disorders, including Type II diabetes, hypertension, atherosclerosis, and cancer. Therefore, we screened small compounds isolated from natural products for the development of anti-obesity drugs.
Objective:
The purpose of this study was to investigate the anti-adipogenic activities of platyphylloside, diarylheptanoid isolated from Betula platyphylla, which was selected based on the screening using 3T3-L1 cells.
Materials and Methods:
To evaluate the inhibition of adipocyte differentiation and lipolysis, lipid contents of BPP on were measured using Oil Red O staining in 3T3-L1 cells. The mRNA and protein expression levels of various adipokines were measured by Quantitative real-time PCR and Western blotting analysis, respectively.
Results:
Platyphylloside showed significant inhibitory activity on adipocyte differentiation in 3T3-L1 cells and suppressed adipocyte differentiation even in the presence of troglitazone, a PPARγ agonist. Platyphylloside might suppress adipocyte differentiation through PPARγ, C/EBPα, and SREBP1-induced adipogenesis, which is synergistically associated with downstream adipocyte-specific gene promoters such as aP2, FAS, SCD-1, LPL, and Adiponectin. In addition, platyphylloside affected lipolysis by down-regulating perilipin and HSL and up-regulating TNFα.
Conclusion:
Taken together, the results reveal that platyphylloside has anti-adipogenic activity and highlight its potential in the prevention and treatment of obesity.
SUMMARY
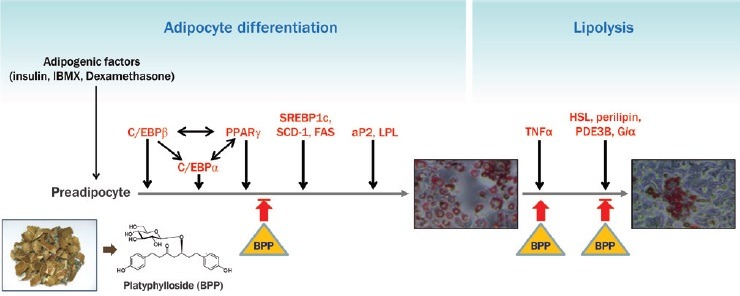
The extract of B. platyphylla bark and its isolate, BPP, had anti-adipogenic activity in 3T3-L1 cells via suppression of adipocyte differentiation from preadipocytes.
Treatment with BPP significantly down-regulated the expression of PPARγ, C/EBP, C/EBPβ, C/EBPδ, SREBP1c, SCD-1, FAS, aP2 and LPL.
BPP induced a lipolytic response in mature adipocytes via up-regulation krof TNFá and down-regulation of HSL, perilipin, PPARγ, PDE3B, and Gia1.
BPP is a novel potential agent in the prevention and treatment of obesity through its anti-adipogenic activities and lipolysis.
Abbreviations used: DMEM: Dulbecco's modified Eagle's medium, FBS: fetal bovine serum, ORO: Oil Red O, PBS: phosphate buffered saline, RT: room temperature, PPAR: peroxisome proliferator-activated receptor, C/EBP: CCAAT/enhancer-binding protein, SREBP1: sterol regulatory element binding protein 1, SCD-1: steroyl-coenzyme A desaturase 1, LPL: lipoprotein lipase, aP2: adipocyte fatty acid binding protein, FAS: fatty acid synthase, HSL: hormone sensitive lipase, Giα1: GPT binding protein, PDE3B: phosphodiesterase 3B, TNFα: tumor necrosis factor α, GAPDH: glyceraldehyde 3-phosphate dehydrogenase, SD: standard deviation, EGCG: epigallocatechin-3-gallate, TZD: thiazolidinediones
Keywords: Adipogenesis, adipocyte differentiation, betula platyphylla, diarylheptanoid, obesity, 3T3-L1 cells, platyphylloside
INTRODUCTION
Obesity has become a globally prevalent health risk and is no longer considered to be only a cosmetic problem affecting certain individuals. In today's society, drugs for weight loss and weight control are becoming common, however the remedies provided by the diet industry have been unsuccessful in the long-term maintenance of weight in obese patients.[1] Obesity can be categorized into two main types, hyperplasic (number of adipocytes increase) and hypertrophic (size of adipocytes increase).[2] In previous studies, increase of adipocyte size preceded increase of adipocyte number in animal studies and this adipose hypertrophy was also an associated risk factor for developing type 2 diabetes.[3,4] At the cellular level, obesity was originally regarded as a hypertrophic condition resulting from an increase in the number or size of individual adipocytes.[5]
Adipose tissues are serious integrators of organismal energy balance through the modulation of food intake and energy expenditure. The adipose tissue mass can be regulated by the suppression of adipogenesis from fibroblastic pre-adipocytes to mature adipocytes.[6] Adipocytes play a pivotal role in regulating adipose mass and lipid metabolism. The transition from undifferentiated fibroblast-like pre-adipocytes into mature adipocytes constitutes the adipocyte life cycle, which includes alteration of cell morphology, growth arrest, clonal expansion and an intricate sequence of changes at the gene level that cause lipid accumulation and finally cell death.[7] The 3T3-L1 pre-adipose 3T3 clonal cell line is an appropriate in-vitro model for both research of the fat cell life cycle and investigation of adipokines that regulate adipogenesis.[8] Triacylglycerol accumulation from dietary sources and endogenous lipogenic signaling increase the size of adipocytes, whereas increased proliferation and differentiation increase the adipocyte number.[6] Therefore, treatments that modulate both the number and size of adipocytes could be a fundamental therapeutic approach for obesity remedies.
Platyphylloside, named BPP, is a well-known diarylheptanoid derived from Betulaceae platyphylla.[9] It has been reported to exert several pharmacological effects such as anti-cancer, anti-arthritis, anti-fibrotic, and hepatoprotective activities.[10] Especially, the protective effects of Betulaceae playphylla and its isolate, BPP, against neurodegeneration and cognitive deficit in scopolamine-activated amnesic mice were mediated through the CREB-BNDF pathway.[10] In this study, we showed that BPP decreases adipocyte differentiation and induces lipolysis in 3T3-L1 cells. Our findings suggest that BPP may be used to overcome obesity by modulating adipogenesis throughout the adipocyte life cycle.
MATERIALS AND METHODS
Plant and phytochemical materials
Betula platyphylla bark was provided by SK E&C (Korea) and a voucher specimen (SNU-797) has been deposited in the Herbarium of the Medicinal Plant Garden, College of Pharmacy, Seoul National University, Koyang, Korea. Dried and pulverized B. platyphylla bark was extracted with 80% methanol using an ultrasonic apparatus at room temperature (RT). Methanolic extract of B. platyphylla bark was concentrated in vacuo to give a crude extract, which was suspended in H2O and partitioned successively in CHCl3, n-butanol, and H2O fractions. BPP was isolated from n-butanol fraction of Betula platyphylla bark.[11] Also, Lee et al. elucidated that BPP is a major constituent in the extract of B. platyphylla bark.[12]
Cell culture and adipocyte differentiation
3T3-L1 mouse embryo fibroblast cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and incubated in Dulbecco's modified Eagle's medium (DMEM) with 10% bovine calf serum until confluence. Two days after confluence, pre-adipocytes were stimulated to differentiate with DMEM, 10% fetal bovine serum (FBS), 0.5 mM 3-isobutyl-1-methyl-xanthine, 10 μg/mL insulin, and 1 μM dexamethasone for 2 days. Cells were then maintained in DMEM/10% FBS and 10 μg/mL M insulin for another 2 days, followed by culturing with DMEM/10% FBS for an additional 4 days. All media contained 100 IU/mL penicillin and 100mg/mLstreptomycin. Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. The purity of each compound was verified as more than 99 % by HPLC-ELSD. Test compounds were dissolved in dimethyl sulfoxide (final concentration: 0.1% in media). The cultures were treated with test samples for the entire culture period (day 0–8).
Measurement of lipid contents
Lipid contents in cells were measured using Oil Red O (ORO) staining.[13] On day 8, culture dishes were washed three times with phosphate buffered saline (PBS) and fixed with 10% formalin for 1 h at RT. After fixation, cells were stained with filtered ORO solution for 30 min at RT and visualized. The lipid contents were quantified by dissolving the stained lipid droplets in 4% Nonidet P-40 in isopropyl alcohol for 5 min. The absorbance was measured at 544 nm by enzyme-linked immunosorbent assay.
Western blot analysis
Cells were lysed in RIPA buffer (Cell Signaling, USA) containing 1% protease inhibitor cocktail, 0.5 mM DTT, and 1 mM PMSF. Supernatants were attained through centrifugation for 15 min at 12,000 × g. The protein assay was conducted using Bradford protein assay kits (Bio-Rad, USA). The mixture of total proteins (20 μg) and sample loading buffer (Biosaesang Co., Korea) was boiled at 100°C for 10 min for Western blot analysis. The denatured proteins were separated through 10% SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, USA). After blocking in TBS-T with 5% non-fat dry milk, the membranes were incubated overnight at 4°C with 1:1000 diluted peroxisome proliferator-activated receptor (PPAR)γ and CCAAT/enhancer-binding protein (C/EBP) α primary antibodies (Cell Signaling, Beverly, USA). After incubation with 1:1000 diluted horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (Santa Cruz Biotechnology, USA) for 1 h at RT, immunoreactive proteins were developed by an enhanced chemiluminescent solution (Thermo Scientific, USA). The density values for the protein bands were expressed as a percentage of the control after normalization to β-actin.[14]
Quantitative real-time PCR
The total RNA was extracted from the 3T3-L1 cells by RNease Plus Kit (QIAGEN Korea Ltd., Seoul, Korea). The cDNA was synthesized from 1 μg of total RNA by QuantiTech Reverse Transcription Kit (QIAGEN Korea Ltd., Seoul, Korea) and then mixed with QuantiFast SYBR Green PCR master mix (QIAGEN Korea Ltd., Seoul, Korea) and specific primers in a total reaction volume of 20 μL. The PCR-specific primers used with the QIAGEN kits for SYBR® Green-based real-time RT-PCR were PPARγ (NM_01146), C/EBPα (NM_007678), C/EBPβ (NM_009883), sterol regulatory element binding protein 1 (SREBP1) (NM_011480), steroyl-coenzyme A desaturase 1 (SCD-1) (NM_009127), lipoprotein lipase (LPL) (NM_008509), adipocyte fatty acid binding protein (aP2) (NM_024406), fatty acid synthase (FAS) (NM_007988), hormone sensitive lipase (HSL) (NM_001039507), GPT binding protein (Giα1) (NM_013818), phosphodiesterase 3B (PDE3B) (NM_011055), perilipin (NM_001113471), tumor necrosis factor α(TNFα) (NM_013639) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (NM_008084) and were obtained from QIAGEN Korea Ltd. Quantitative SYBR Green real-time PCR was performed with an Applied Biosystems 7300 Real-Time PCR System (Life Technologies Corporation, CA, USA). The amplification cycles were carried out at 95°C for 20 s, 60°C for 20 s, and 72°C for 20 s and the last cycle was followed by a final extension step at 72°C for 5 min and analyzed by means of comparative Ct quantification.[15]
Statistical analysis
Statistical analysis was performed using Student's t test. Data were expressed as the means ± standard deviation (SD). Statistical significance is denoted with an asterisk for p values below0.05, with two asterisks for p values below0.01, and three asterisks for p values below0.001.
RESULTS
In the search for anti-adipogenic natural products, we found that methanolic extract of B. platyphylla barks concentrationdependently inhibited adipocyte differentiation in 3T3-L1 pre-adipocytes [Figure 1]. Dried and pulverized B. platyphylla bark was extracted with 80% methanol by using an ultrasonic apparatus. Eighty per cent of methanolic extract of B. platyphylla fruits was suspended in H2O and successively fractioned into CHCl3 and n-butanol fractions. The n-butanol fraction was further subjected to repeated column chromatography to yield BPP, which was identified by comparison to known reference compound [Figure 2].[11]
Figure 1.
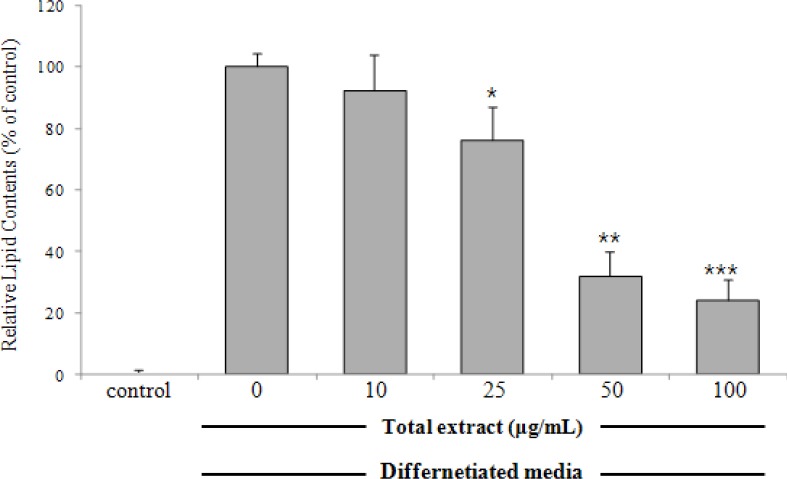
Effects of the methanolic extract of Betula platyphylla on adipocyte differentiation in 3T3-L1 cells3T3-L1 pre-adipocytes were treated with various concentrations of the metabolic extract of B. platyphylla during differentiation (day 0–8). On day 8, cultures were stained with Oil Red O and the lipid contents were quantified spectrophotometrically at 544 nm. Relative lipid contents (%) were calculated as 100 × [(absorbance of sample-treated – absorbance of undifferentiated control)/(absorbance of differentiated control – absorbance of undifferentiated control)]. Results represent the mean ± SD of three independent experiments, each performed in triplicate wells. *p < 0.05, **p < 0.01, ***p < 0.001, versus differentiated cells.
Figure 2.
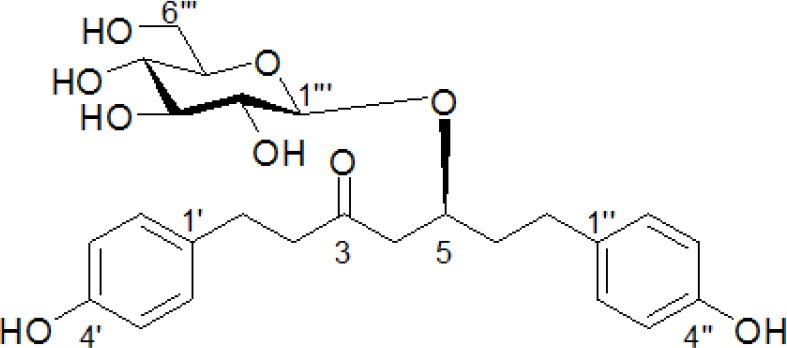
Chemical structure of BPP isolated from Betula platyphylla
To examine the anti-adipogenic effect of BPP isolated from B. platyphylla bark on the differentiation of pre-adipocytes to adipocytes, confluent 3T3-L1 pre-adipocytes were treated with compound at various concentrations during differentiation (days 0–8). On day 8, the lipid drops, stained with ORO, were quantified spectrophotometrically at 544 nm. BPP had potent inhibitory activity on adipocyte differentiation in 3T3-L1 cells with an IC50 value of 14.4 μM, compared to the activity of the positive control epigallocatechin-3-gallate (EGCG) [Table 1].
Table 1.
Effects of BPP on adipocyte differentiation in 3T3-L1 pre-adipocytes
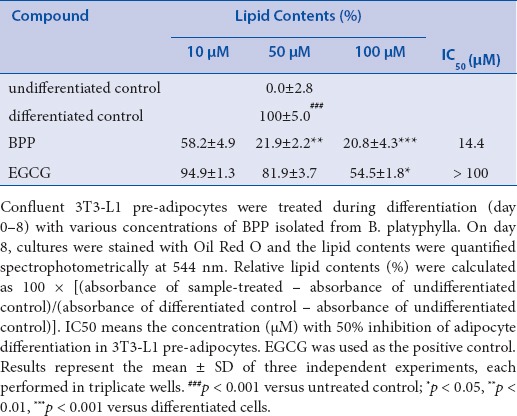
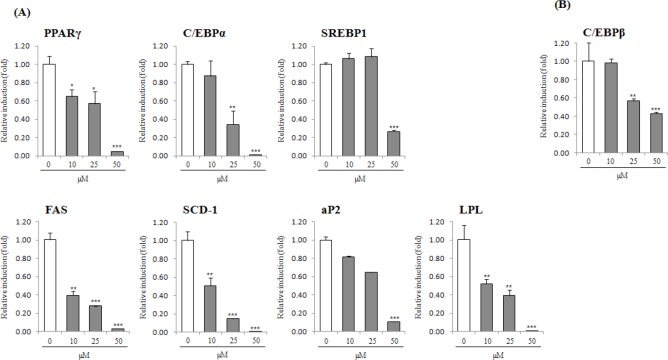
Hence, BPP was further investigated for anti-adipogenic activity in 3T3-L1 cells. Competitive inhibition assays in the process of adipocyte differentiation using a PPARγ agonist, troglitazone (10 μM), were conducted to examine the inhibitory effect on the expression of PPARγ by BPP treatment. Consequently, BPP suppressed adipocyte differentiation even in the presence of troglitazone, as shown by ORO staining. The contents of lipid drops were noticeably reduced by up to 23.0% at a concentration of 100μM, suggesting that BPP suppresses adipocyte differentiation through an antagonistic effect on PPARγ activity [Figure 3]. To determine the expression of adipogenic transcription factor and adipokines associated with adipocyte differentiation, we further investigated the anti-adipogenic mechanism of BPP at concentrations of 10–50 μM using quantitative real-time PCR and/or Western blot analyses [Figs. 4 and 5]. Treatment with BPP noticeably lowered the expression of PPARγ and C/EBP as compared to that from fully differentiated adipocytes in quantitative real-time PCR and Western blot analyses [Figs. 4 and 5A]. In addition, BPP reduced gene expression levels of C/EBPβ and C/EBPδ, which are activated in the initial stage of adipogenesis in pre-adipocytes [Figure 5B]. Moreover, the treatment with BPP suppressed the gene expression level of SREBP1c in a concentration-dependent manner [Figure 5A]. The decrease in gene expression levels of SCD-1 and FAS [Figure 5A], the target genes of SREBP1c, by BPP during adipocyte differentiation imply that the inhibition of adipogenesis is mediated by the regulation of lipogenesis. We also found that BPP significantly reduced the mRNA levels of LPL and aP2.
Figure 3.
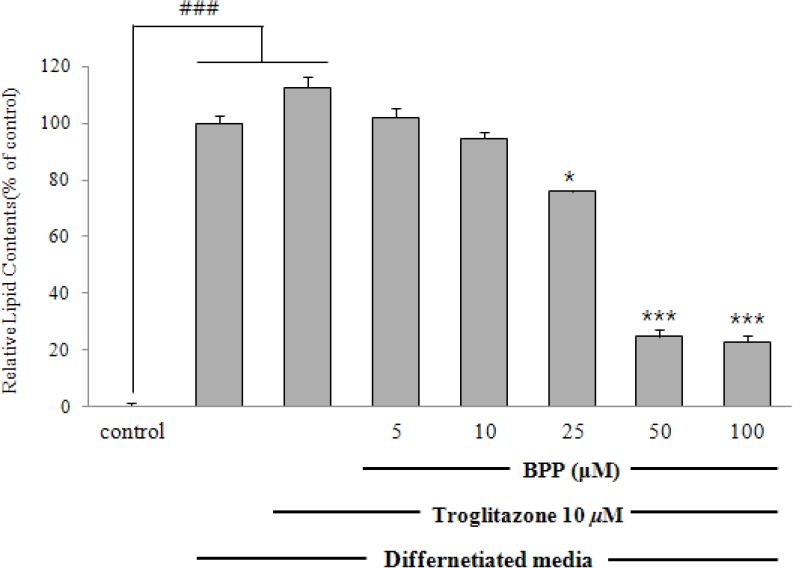
Effect of BPP on PPARγ agonist-induced adipocyte differentiation Confluent 3T3-L1 pre-adipocytes were differentiated into adipocytes with BPP(5, 10, 25, 50, and 100 μM) in the presence of troglitazone (10 μM) for 8 days. On day 8, the lipid contents of cells stained with Oil Red O were quantified. Results represent the mean ± SD of three independent experiments, each performed in triplicate wells. ### p < 0.001 versus untreated control; * p < 0.05, ** p < 0.01, ***p < 0.001 versus differentiated cells.
Figure 4.
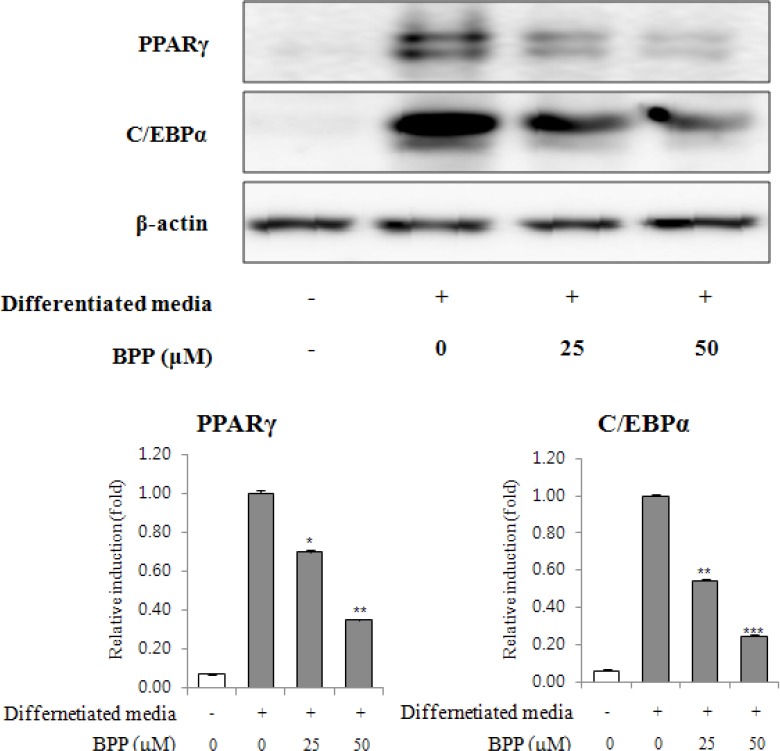
Effects of BPP on PPARγ and C/EBPα expression during 3T3-L1 differentiation. Confluent 3T3-L1 pre-adipocytes were differentiated in the presence of BPP (0, 25, and 50 μM) for 8 days. PPARγ and C/EBPα expression were detected by western blot analysis.
Figure 5.
Effects of BPP on adipokine gene expression in 3T3-L1 by quantitative real-time RT-PCR analysis. Confluent 3T3-L1 pre-adipocytes were differentiated into adipocytes in a medium with BPP (0, 10, 25, and 50 μM) for 2 or 8 days. The mRNA expression levels of PPARγ, C/EBPα SREBP1, SCD-1, FAS, aP2, LPL, and adiponectin on day 8 (A), and C/EBPβ on day 2 (B) was estimated by quantitative real-time RT-PCR analysis. Results represent the mean ± SD of three independent experiments, each performed in triplicate wells. * p < 0.05, ** p < 0.01, ***p < 0.001 versus differentiated cells.
Lipolytic effect of BPP on differentiated adipocytes (8 days) was monitored by ORO staining [Figure 6A]. A significant reduction in ORO content was observed with 50–100 μM BPP [Figure 6B]. The effect of BPP on lipolysis was also assessed by measuring the expression levels of perilipin, PDE3B, Gia1, HSL, and TNFα by real-time PCR upon treatment of mature adipocytes with BPP (post differentiation, 8 days). Treatment with BPP down-regulated the expression of HSL, perilipin, PPARγ, PDE3B, and Gia1, and up-regulated the expression of TNFα [Figure 6C].
Figure 6.
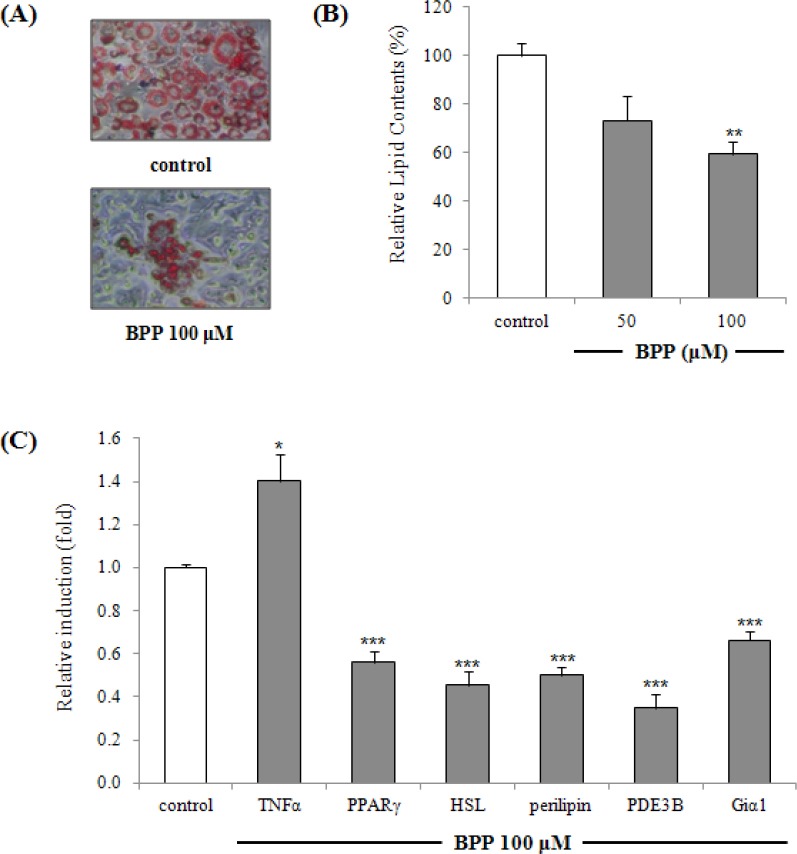
Effects of BPP on expression of lipolysis-associated target genes perilipin, HSL, PDE3B, Giα1, TNFα, and PPARγ in 3T3-L1 cells by quantitative real-time RT-PCR analysis. BPP-treated (48 h, at 50 μM and 100 μM) mature adipocytes (8 days) were stained for intracellular lipids with Oil Red O. Cells stained with Oil Red O were photographed with a phase-contrast microscope (original magnification x 100) (A) and lipid content were quantitated (B). Relative lipid contents (%) were calculated as 100 × (absorbance of sample-treated/absorbance of differentiated control). The mRNA expression levels of lipolysis-associated target genes perilipin, HSL, PDE3B, Giα1, TNFα, and PPARγ were estimated by quantitative real-time RT-PCR analysis (C). Each bar represents the mean ± SD of three independent experiments. * p < 0.05, ** p < 0.01, ***p < 0.001 versus differentiated cells.
DISCUSSION
Mesenchymal stem cell-derived pre-adipocytes have the potential to differentiate into adipocytes.[16] The adipocyte life cycle contains changes in cell shape and growth arrest, clonal amplification and a complex sequence of alteration in gene expression leading to lipid accumulation and finally cell death.[7] Obesity is a condition associated with excessive growth of adipose tissue resulting from an increase in the number and/or size of adipocytes differentiated from pre-adipocytes.[17] Therefore, suppression of pre-adipocyte differentiation to adipocytes, thereby reducing the genesis and development of adipose tissue, may be useful to searching anti-obesity agents.[18] The committed pre-adipocytes undergo initial alterations of cells and subsequent terminal differentiation into adipocytes, as well as changes in gene expression and the storage of lipids, and mature adipocytes can then undergo lipolysis changes under certain conditions.[7,14] The cellular and molecular mechanisms of adipocyte differentiation have been extensively studied using pre-adipocyte culture systems.[18] Thus, the 3T3-L1 cells were used as an in-vitro screening tool to assess the anti-adipogenic activity of natural products. On that basis, we investigated therapeutic anti-obesity agents in 3T3-L1 cells by evaluating their effects on adipocyte differentiation. There is a growing interest in the search for anti-adipogenic phytochemicals from natural products. During a research for naturally occurring anti-adipogenic products from medicinal plants, we observed anti-adipogenic activity of diarylheptanoid isolated from B. platyphylla bark in 3T3-L1 cells.
Cyclic and acyclic diarylhepanoids are abundant in Betula and Alnus species of Betulaceae family.[19] We previously reported the inhibitory effect of cyclic and acyclic diarylheptanoids isolated from leaves of Alnus hirsuta f. sibirica and fruits of A. japonica, respectively, on adipocyte differentiation.[13,15] Moreover, curcumin, a major diarylhepanoid of Curcuma longa, had an anti-adipogenic activity through the regulation of the AMPKα-PPARγ pathway.[20] These previous studies suggest the possible presence of an anti-adipogenic diarylheptanoid, platyphylloside. However, the anti-adipogenic and lipolytic mechanisms of diarylheptanoid isolated from B. platyphylla have not been investigated yet.
The first hallmark of the adipogenic process is alteration in cell shape, paralleled by changes in the expression levels of extracellular matrix components and cytoskeletal components.[21] These proceedings further stimulate the expression of adipogenic transcription factors, including PPARγ, C/EBPα, and SREBP1.[21] Especially, adipogenesis is a complex network modulated via sequential activation of various transcriptional mediators such as PPARγ and C/EBPα depending on the differentiation stage.[22,23] PPARγ, considered the master regulator of adipogenesis, can be activated by thiazolidinediones (TZDs); treatment of pre-adipocytes with TZDs increases both the extent and rate of adipogenesis.[24] BPP significantly blocked the induction of PPARγ and C/EBP expression in differentiated adipocytes, as determined by Western blot and quantitative real-time RT-PCR analysis [Figs. 4 and 5A]. These results suggest that BPP inhibits adipocyte differentiation, in part via inhibition of PPARγ and C/EBPα dependent pathway. Another member of the C/EBP family, C/EBPβ, expressed earlier than C/EBP during adipogenesis in 3T3-L1 cells is responsible for regulating C/EBP expression and transiently enhanced differentiation of pre-adipocytes.[22] To evaluate the effects of BPP on the initial stage of adipocyte differentiation, mRNA levels of C/EBPβ were examined 48 h after adipocyte differentiation by quantitative real-time RT-PCR analysis. As a result, BPP down-regulated C/EBPβ gene expression [Figure 5B]. SREBP is involved with regulation of genes related to the metabolism of lipogenesis and cholesterogenesis.[25] Among identified isomers of SREBP (SREBP1a, SREBP1c, and SREBP2), SREBP1c stimulated lipogenic genes that encode enzymes involved in triglyceride synthesis and desaturation of fatty acids.[9,24] The suppression of SREBPc by BPP might inhibit fatty acid synthesis and may result in the inhibition of lipid accumulation by blocking adipogenesis. This effects of BPP on SREBP1c gene expression has been accompanied by reduction in gene expression of FAS and SCD-1. FAS, highly expressed in liver and adipose tissue, catalyzes the synthesis of saturated fatty acids in cells.[26] Also, SCD-1, which is a key rate-limiting enzyme in the desaturation of cellular lipids into mono-unsaturated fatty acids, is highly expressed in adipose tissue.[27] It was reported that cellular deprivation on the enzymatic activity of SCD-1 can induce down-regulation of SREBP1, resulting in a decrease in lipogenesis.[28] Therefore, treatment with BPP reduced the mRNA levels of SREBP1c, FAS and SCD-1during adipocyte differentiation and these results indicate that inhibition of adipocyte differentiation can be mediated by the down-regulation of these lipogenic genes. PPARγ, C/EBPα, and SREBP1c are induced prior to the transcriptional activation of most adipocyte-specific genes in the early stage of adipocyte differentiation, whereas adipocyte fatty acid binding protein (aP2) and FAS are known as terminal markers of adipocyte differentiation.[9,29]
Breakdown of triglycerides in adipocytes and the release of fatty acids are essential for the regulation of energy homeostasis.[30] Mature adipocytes may also undergo lipolysis and apoptotic cell death under certain conditions.[7] Mature adipocytes treated with BPP (48 h, at 100 μM) were stained for intracellular lipids with ORO [Figure 6A]. A significant reduction in lipid content was observed with 50 μM and 100 μM BPP, indicating a lipolytic effect [Figure 5B]. Effects on lipolysis-associated target genes were assessed by measuring the transcriptional expression of perilipin, PDE3B, Gia1, HSL, and TNFα by real-time PCR upon treatment of mature adipocytes (post differentiation, 8 days) with BPP. BPP down-regulated the expression of HSL, perilipin, PPARγ, PDE3B, and Gia1, and up-regulated the expression of TNFα [Figure 5C]. TNFα increases adipocyte lipolysis and down-regulates the expression of the lipid droplet-associated protein, perilipin, which is thought to modulate the accession of HSL to the surface of the fat droplet.[31] The phosphorylation of HSL and perilipin is critical in lipolysis, and the expression levels of perilipin and HSL are a part of the lipolytic response.[32] TNFα can suppress expression and function of PPARγ, which is known to promote phosphorylation and down-regulation of perilipin.[33,34] The TNFα induction and PPARγ repression caused by BPP affect down-regulation of perilipin. TNFα-induced lipolysis also downregulates anti-lipolytic genes PDE3B and Giα1.[35,36] These data suggest that BPP enhances the lipolytic response in mature adipocytes via modulation of lipolysis-associated gene expression.
CONCLUSION
In conclusion, our study shows that an extract of B. platyphylla bark and its isolate, BPP, had anti-adipogenic activity in 3T3-L1 cells via suppression of adipocyte differentiation from pre-adipocytes. To investigate the inhibitory mechanism of BPP on adipogenesis in 3T3-L1 cells, the effects of BPP on PPARγ and C/EBP expression levels were examined by Western blot and quantitative PCR analyses. Treatment with BPP significantly down-regulated the expression of PPARγ, C/EBP, C/EBPβ, C/EBPδ, SREBP1c, SCD-1, FAS, aP2 and LPL. Moreover, BPP induced a lipolytic response in mature adipocytes via up-regulation of TNFα and down-regulation of HSL, perilipin, PPARγ, PDE3B, and Gia1. Therefore, BPP is a novel potential agent in the prevention and treatment of obesity through its anti-adipogenic activities and lipolysis. Such activities should be assessed further through in-vivo studies in animal models.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (Grant number: NRF-2014R1A1A2056624). Also, this research was supported by Suncheon Research Center for Natural Medicines.
REFERENCES
- 1.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119:688–93. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- 2.Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 4.Lonn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24:326–31. doi: 10.1096/fj.09-133058. [DOI] [PubMed] [Google Scholar]
- 5.Jernas M, Palming J, Sjoholm K, Jennische E, Svensson PA, Gabrielsson BG. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–2. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 6.Roncari DA, Lau DC, Kindler S. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism. 1981;0:425–7. doi: 10.1016/0026-0495(81)90174-8. [DOI] [PubMed] [Google Scholar]
- 7.Rayalam S, Della-Fera MA, Baile CA. Phytochemicals and regulation of the adipocyte life cycle. J Nutr Biochem. 2008;19:717–26. doi: 10.1016/j.jnutbio.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Dave S, Kaur NJ, Nanduri R, Dkhar HK, Kumar A, Gupta P. Inhibition of adipogenesis and induction of apoptosis and lipolysis by stem bromelain in 3T3-L1 adipocytes. PLoS One. 2012;7:e30831. doi: 10.1371/journal.pone.0030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen KH, Li PC, Lin WH, Chien CT, Low BH. Depression by a green tea extract of alcohol-induced oxidative stress and lipogenesis in rat liver. Biosci Biotechnol Biochem. 2011;75:1668–76. doi: 10.1271/bbb.110163. [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Jeong EJ, Huh J, Cho N, Kim TB, Jeon BJ. Cognition-enhancing and neuroprotective activities of the standardized extract of Betula platyphylla bark and its major diarylheptanoids. Phytomedicine. 2012;19:1315–20. doi: 10.1016/j.phymed.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Park JH, Kim TB, Lee HH, Lee KY, Kim YC. Inhibition of antigen-induced degranulation by aryl compounds isolated from the bark of Betula platyphylla in RBL-2H3 cells. Bioorg Med Chem Lett. 2010;20:2824–7. doi: 10.1016/j.bmcl.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 12.Lee M, Park JH, Min DS, Yoo H, Park JH, Kim YC. Antifibrotic activity of diarylheptanoids from Betula platyphylla toward HSC-T6 cells. Biosci Biotechnol Biochem. 2012;76:1616–20. doi: 10.1271/bbb.110887. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Song JY, Chin YW, S SH. Anti-adipogenic diarylheptanoids from Alnus hirsuta f. sibirica on 3T3-L1 cells. Bioorg Med Chem Lett. 2013;23:2069–73. doi: 10.1016/j.bmcl.2013.01.127. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, Lee HH, Lee JK, Ye SK, Kim SH, Sung SH. Anti-adipogenic activity of compounds isolated from Idesia polycarpa on 3T3-L1 cells. Bioorg Med Chem Lett. 2013;23:3170–4. doi: 10.1016/j.bmcl.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Sung SH, Lee M. Anti-adipogenic activity of a new cyclic diarylheptanoid isolated from Alnus japonica on 3T3-L1 cells via modulation of PPARgamma, C/EBPalpha and SREBP1c signaling. Bioorg Med Chem Lett. 2015;25:4648–51. doi: 10.1016/j.bmcl.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Lee KD. Applications of mesenchymal stem cells: an updated review. Chang Gung Med J. 2008;31:228–36. [PubMed] [Google Scholar]
- 17.Roncari DA, Lau DC, Kindler S. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism. 1981;30:425–7. doi: 10.1016/0026-0495(81)90174-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee M, Lee SH, Kang J, Yang H, Jeong EJ, Kim HP. Salicortin-derivatives from Salix pseudo-lasiogyne twigs inhibit adipogenesis in 3T3-L1 cells via modulation of C/EBPalpha and SREBP1c dependent pathway. Molecules. 2013;18:10484–96. doi: 10.3390/molecules180910484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rastogi S, Pandey MM, Kumar Singh Rawat A. Medicinal plants of the genus Betula-traditional uses and a phytochemical-pharmacological review. J Ethnopharmacol. 2015;159:62–83. doi: 10.1016/j.jep.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YK, Lee WS, Hwang JT, Kwon DY, Surh YJ, Park OJ. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J Agric Food Chem. 2009;57:305–10. doi: 10.1021/jf802737z. [DOI] [PubMed] [Google Scholar]
- 21.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 22.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White UA, Stephens JM. Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol. 2010;318:10–14. doi: 10.1016/j.mce.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–307. [PubMed] [Google Scholar]
- 25.Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol. 2002;67:491–8. doi: 10.1101/sqb.2002.67.491. [DOI] [PubMed] [Google Scholar]
- 26.Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J. 1994;8:1248–59. [PubMed] [Google Scholar]
- 27.Yao-Borengasser A, Rassouli N, Varma V, Bodles AM, Rasouli N, Unal R. Stearoyl-coenzyme A desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-gamma responsiveness. J Clin Endocrinol Metab. 2008;93:4431–9. doi: 10.1210/jc.2008-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim E, Lee JH, Ntambi JM, Hyun CK. Inhibition of stearoyl-CoA desaturase1 activates AMPK and exhibits beneficial lipid metabolic effects in vitro. Eur J Pharmacol. 2011;672:38–44. doi: 10.1016/j.ejphar.2011.09.172. [DOI] [PubMed] [Google Scholar]
- 29.Ericsson J, Jackson SM, Kim JB, Spiegelman BM, Edwards PA. Identification of glycerol-3-phosphate acyltransferase as an adipocyte determination and differentiation factor 1- and sterol regulatory element-binding protein-responsive gene. J Biol Chem. 1997;272:7298–305. doi: 10.1074/jbc.272.11.7298. [DOI] [PubMed] [Google Scholar]
- 30.Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27:875–888. doi: 10.1038/sj.ijo.0802326. [DOI] [PubMed] [Google Scholar]
- 31.Ryden M, Arvidsson E, Blomqvist L, Perbeck L, Dicker A, Arner P. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem Biophys Res Commun. 2004;318:168–75. doi: 10.1016/j.bbrc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Ardevol A, Blade C, Salvado MJ, Arola L. Changes in lipolysis and hormone-sensitive lipase expression caused by procyanidins in 3T3-L1 adipocytes. Int J Obes Relat Metab Disord. 2000;24:319–24. doi: 10.1038/sj.ijo.0801130. [DOI] [PubMed] [Google Scholar]
- 33.Xing H, Northrop JP, Grove JR, Kilpatrick KE, Su JL, Ringold GM. TNF alpha-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARgamma without effects on Pref-1 expression. Endocrinology. 1997;138:2776–83. doi: 10.1210/endo.138.7.5242. [DOI] [PubMed] [Google Scholar]
- 34.Arimura N, Horiba T, Imagawa M, Shimizu M, Sato R. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem. 2004;279:10070–6. doi: 10.1074/jbc.M308522200. [DOI] [PubMed] [Google Scholar]
- 35.Rahn Landstrom T, Mei J, Karlsson M, Manganiello V, Degerman E. Down-regulation of cyclic-nucleotide phosphodiesterase 3B in 3T3-L1 adipocytes induced by tumour necrosis factor alpha and cAMP. Biochem J. 2000;346:Pt 2337–43. doi: 10.1042/bj3460337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasic S, Tian B, Green A. Tumor necrosis factor alpha stimulates lipolysis in adipocytes by decreasing Gi protein concentrations. J Biol Chem. 1999;274:6770–5. doi: 10.1074/jbc.274.10.6770. [DOI] [PubMed] [Google Scholar]