Abstract
Background:
Eugenia pruniformis is an endemic species from Brazil. Eugenia genus has flavonoids as one of the remarkable chemical classes which are related to the improvement of the healing process.
Aims:
To evaluate of wound healing activity of E. pruniformis leaves and to identify and quantify its main flavonoids compounds.
Materials And Methods:
Wound excision model in rats was used to verify the hydroethanolic and ethyl acetate extracts potential. The animals were divided in four groups of six and the samples were evaluated until the 15° day of treatment. Hydroxyproline dosage and histological staining with hematoxilin-eosin and Sirius Red were used to observe the tissue organization and quantify the collagen deposition, respectively. Chemical compounds of the ethyl acetate extract were identified by chromatographic techniques and mass spectrometry analysis and total flavonoids content was determined by spectrophotometric method. The antioxidant activity was determined by oxygen radical absorbing capacity (ORAC) and 2,2-diphenyl-1-picrylhydrazylhydrate radical photometric (DPPH) assays.
Results:
The treated group with the ethyl acetate extract showed collagen deposition increase, higher levels of hidroxyproline, better tissue reorganization and complete remodeling of epidermis. Quercetin, kaempferol and hyperoside were identified as main compounds and flavonoids content value was 43% (w/w). The ORAC value of the ethyl acetate extract was 0.81± 0.05 mmol TE/g whereas the concentration to produce 50% reduction of the DPPH was 7.05± 0.09 μg/mL.
Conclusion:
The data indicate a wound healing and antioxidant activities of E. pruniformis. This study is the first report of flavonoids and wound healing activity of E. pruniformis.
KEY MESSAGES
Eugenia pruniformis extract accelerates wound healing in skin rat model, probably due to its involvement with the collagen deposition increase, higher levels of hidroxyproline, dermal remodelling and potent antioxidant activity. Chemical standardization of the active wound healing extract was done. The total flavonoid content was 43% (w/w) and quercetin, kaempferol and hyperoside were identified as main compounds.
SUMMARY
Wound excision model in rats showed the potential wound healing activity of E. pruniformis by collagen deposition increase, higher levels of hidroxyproline, better tissue reorganization and complete remodeling of epidermis.
Flavonoids are the main compounds of the endemic E. pruniformis and quercetin, kaempferol and hyperoside were identified in ethyl acetate extract by TLC, HPLC-PDA and HRESI-MS analysis.
The ethyl acetate extract of E. pruniformis showed a potent antioxidant activity by ORAC and DPPH assays
Abbreviation used: NC: Negative control, PC: Positive control, CH: Crude hydroethanolic extract, EA: Ethyl acetate extract, TE: Trolox equivalent, mg: Milligram, mM: Millimolar, mL: Milliliter, HPLC-PDA: High performance liquid chromatography with a photodiode array detector, HRESI-MS: High-resolution electrospray ionization mass spectrometry analysis, TLC: Thin layer chromatography, ORAC: Oxygen radical absorbance capacity, w/v: Weight per volume
Keywords: Eugenia pruniformis, flavonoids, myrtaceae, wound healing
INTRODUCTION
The genus Eugenia (family Myrtaceae) was reported as the most used in folk medicine in the Restinga Jurubatiba National Park at Carapebus City, RJ, Brazil, and has flavonoids as remarkable chemical compounds.[1,2] Among the species of the Eugenia genus, there is Eugenia pruniformis Cambess (synonyms: Eugenia mikaniana Berg., Eugenia ollivaceae Berg., and Myrtus quadrisperma Vell.), popularly known as ’azeitoninha-da-praia’, an endemic Brazilian species.[1,3] However, information on the constituents and biological activities of E. pruniformis remains scarce and only the essential oil has been previously reported.[4]
Flavonoids are shown to have biological and pharmacological activities, including antioxidant, antiviral, antimicrobial, anti-inflammatory, antiproliferative, and wound healing activity.[5,6,7] The flavonols, such as quercetin, kaempferol, rutin, and hyperoside, were isolated from the Eugenia genus. Thus, the extracts and the fractions rich in flavonoids from the Eugenia spp. could be used as active constituents of pharmaceutical products for the treatment of skin diseases. Some plant species are also used for the treatment of skin diseases, such as Calendula officinalis and Copaifera sp., which have action in contaminated wounds.[8,9] Wounds are defined as disruptions of the integrity of the skin and mucous membranes, which may differ in size, shape, and depth, and also, as the etiology, mechanism of injury, degree of tissue loss, and contamination.[10,11] Currently, diseases related to skin and subcutaneous tissue infections are a major public health problem, affecting mainly the elderly and patients with diabetes.[12] The mechanisms involved in healing process are distributed in three interrelated phases, which are as follows: inflammatory phase, proliferative, and tissue remodeling. The inflammatory phase is characterized by hemostasis that occurs due to the deposition of fibrin clots and phagocytic migration responsible for removing foreign substances and microorganisms. The proliferative phase involves the proliferation of fibroblasts, endothelial cells, and keratinocytes, as well as the deposition of fibronexus and collagen type III. Finally, in the tissue modeling phase, there is a massive replacement of collagen type III to collagen type I fibers, which have more resistance to large stresses. In tissue modeling phase, an increase of crosslinkings among the collagen fibers monomers in direction of the skin stress lines also occurs.[13] Thus, the therapeutic targeting of substances with healing activity may differ according to the healing process phase. Other mechanisms may be mentioned as adjuvants, such as elimination of the microbial load on the wound site, treatment with epidermal growth factor, and use of enzymes which lyse the contents of necrosis and crusts, facilitating the cleaning of the damaged tissue.[14,15] In this context, this study aims to evaluate the wound healing activity of the extracts from the leaves of E. pruniformis and determinate its main flavonoids.
MATERIALS AND METHODS
Plant material and preparation of the extracts
Leaves (1500 g) of E. pruniformis were collected from the Restinga Jurubatiba National Park (S22°12 40.85 –W41°35 14.61; S22°12 40.85 –W41°35 14.61; S22°12 36.36 –W41°35 20.18; S22°12 34.90 –W41°35 21.04) on November 2, 2013. The identification was performed by Prof. Dr. Marcelo Guerra Santos of State University of Rio de Janeiro (no. of collecting authorization: 032-2008; no. of authorization for activities with scientific purpose: 13659-3; Voucher specimen: M.G. Santos, 2206). Leaves were dried in a forced ventilation oven for 48 h at 35°C. The air-dried leaves (1200 g) were powdered and exhaustively extracted at room temperature with 96% ethanol. After evaporation under reduced pressure (35°C), a portion (112 g) of the crude hydroethanolic extract (143.7 g) was resuspended in 250 mL 90% ethanol and then partitioned with n-hexane (2 250 mL). The ethanol-soluble fraction was evaporated under reduced pressure, suspended in 250 mL distilled water and partitioned with ethyl acetate (EA; 0.25 L 2) and n-butanol (0.25 L 2), successively.[16] Chemical analysis of the EA extract were carried out by high-performance liquid chromatography with a photodiode array detector (HPLC-PDA) and by mass spectrometry using high-resolution electrospray ionization mass spectrometry (HRESI-MS).[16] A previous identification of the main classes of substances by TLC, using specific reagents, was also done.
Identification of substances by TLC
TLC analysis were carried out on precoated silica gel 60 F254 (0.22-μm thickness; Merck KGaA, Darmstadt, Germany), using toluene/EA/formic acid 5:3:1 and ethyl aceate/acetic acid/formic acid/water 100:11:11:25 as developing solvent for the identification of the aglycones flavonoids and the glycosilated flavonoids, respectively. The detections were done by spraying with natural products-polyethylene glycol reagent (1% methanolic solution of 2-aminoethyl diphenylborinate and 5% ethanolic solution of polyethylene glycol) and 10% sulfuric acid solution.[16] Spots on TLC plates were caracterized by Rf-values and color under UV light before and after spraying the reagent solution and used for flavonoids identification in comparison with standard compounds (kaempeferol, quercetin, isoquercetrin, rutin, and hyperoside).
Analytical method development by high-performance liquid chromatography with a photodiode array
HPLC-PDA analyses were carried out on Shimadzu SPD-M10Avp, consisting of a binary pump, a column oven, and a PDA detector. Separations were performed on a reverse-phase column (4.6 × 250 mm, 5.0 μm; Shimadzu Corporation, Kyoto, Japan) equipped with a precolumn (3 × 10 mm). A gradient of acetonitrile in 0.1% aqueous formic acid from 5:95 to 100:0 in 30 min was used.[16] The flow rate was 1.0 mL/min. The sample was dissolved in methanol (extracts and pure flavonoids) at a concentration of 5 mg/mL (extracts) or 1 mg/mL (pure compounds). Twenty microliters of the samples were injected for each analysis. UV spectra were obtained from 210 to 700 nm. Data acquisition and processing were performed using Shimadzu LC Solution 1.25 software.
High-resolution electrospray ionization mass spectrometry analysis
HRESI-MS data were recorded on a mass spectrometer micrOTOF (Bruker Daltonics GmbH, Leipzig, Germany) in negative tube mode with capillary voltage set at 4000 V, nebulizer pressure 0.4 bar, desolvation temperature 180 °C, and scan range 110–1500. The samples were introduced by infusion in MeOH solution.
Determination of the total flavonoids content of the ethyl acetate extract
The determination of total flavonoid content of EA extract was performed according to an adaptation of the method developed by Rolim.[17] The concentration of total flavonoids equivalent in rutin was determined spectrophotometrically in UV region (360 nm) in comparison with the standard curve of rutin absorbance. The concentration range of the standard curve comprised the following concentrations of rutin: 12.5, 25.0, 50.0, 75.0, and 85.0 mg/mL. A mixture of ethanol 95% and acetic acid 0.02M (99:1) was used as solvent in the preparation of all solutions. The readings were performed in duplicate.
ANTIOXIDANT ACTIVITY
Oxygen radical absorbing capacity assay
The evaluation was performed using a 96-well microplate reader (Fluostar Optima – Fluoroluminometer; BMG Labtech GmbH, Offenburg, Germany). The antioxidant activity of the EA extract from the leaves was measured by fluorescence decay of fluorescein (Sigma- Aldrich®, Merck KGaA, Darmstadt, Germany), induced by 2,2’-azobis (2-amidinopropane) dihydrochloride. To estimate antioxidant activity, measurements were done by following time course of the fluorescence decay. Using Trolox standard, a calibration curve was generated using the net area under the curve AUC (AUCTrolox – AUCblank). Oxygen radical absorbing capacity assay (ORAC) values were calculated using the linear regression and expressed as Trolox equivalent (TE). TE = slope regression curve (sample)/slope regression curve (Trolox).[18] The experil ments were realized in triplicate and acceptable R2 was ≥0.95. 2,2-Diphenyl-1-picryl-hydrazylhydrate (DPPH) radical photometric assay
Sample stock solution of EA extract (1.0 mg/mL) was diluted to final concentrations of 250, 125, 50, 25, 12.5, and 6.25 µg/mL in methanol. One milliliter of a 0.3 mM DPPH ethanol solution was added to 2.5 mL of sample solutions of different concentrations, and allowed to react at room temperature. After 30 min, the absorbance values were measured at 518 nm and converted into the percentage antioxidant activity (AA) using the following formula: AA% = 100 – [(Abssample – Absblank) 100/Abscontrol]. Methanol (1.0 mL) plus extract solution (2.5 mL) was used as a blank. DPPH solution (1.0 mL; 0.3 mM) plus methanol (2.5 mL) was used as a negative control (NC). The EC50 value was calculated by linear regression of plots, where the abscissa represented the concentration of evaluated extract and the ordinate was related to average percent of antioxidant activity.[19] The experiments were realized in triplicate with R2 = 0.99 and Trolox was used as antioxidant control.
Evaluation of wound healing activity of leaf extracts from E. Pruniformis
Sprague-Dawley rats of 8 weeks old and each weighing 250–300 g were used in the accomplishment of the full-thickness excisional wound model using the method described by Morton and Malone.[20] In brief, the circular full-thickness excision wound was made with a biopsy punch of 20 mm in diameter after the induction of general anesthesia. The experimental procedure was approved by the Institutional Animal Care and Use Committee (CEUA) of Universidade Estadual da Zona Oeste – UEZO (Rio de Janeiro, Brazil), protocol code CEUA-UEZO-002/2013. Immediately after surgical excision the rats were randomly divided into four groups of each six animals: untreated animals are NC, treated topically with 0.2 g of collagenase ointment as positive control (PC), treated topically with five drops of propylene glycol solution of 5% (w/v) crude hydroethanolic extract (CH), and treated topically with five drops of propylene glycol solution of 5% (w/v) EA extract. The skin wound samples were collected on the day of the incision (day 0; n = 6), and at eighth (n = 3) and 15th (n = 3) day of treatment. The granulation tissue formed on the injury was excised leaving a 5 mm margin of normal skin for determination of hydroxyproline levels and histopathological analysis.
Histopathologic study and dosage of hydroxyproline levels
The granulated tissue, including the adjacent skin, was weighed, fixed, and stained with Harris HE, and examined microscopically using a 40× objective lens of a light microscope (Nikon, Tokyo, Japan) connected to a digital camera (Coolpix 990; Nikon). To estimate the degrees of wound healing, a histological score was used to determine the dermal and epidermal regeneration and granulation tissue formation.[21] Additional sections were stained with Sirius Red for observation of collagen fibers distribution through the calculus of the percentage of the marked area in red (relative to collagen type I) by field, using the Image Pro Plus 4.5.1 (Media Cybernetics, Silver Spring, MD, USA).[22]
The methodology of hydroxyproline dosage was developed by Woessner.[23] The granulation tissue was dried at 60–70°C for 24 h and weighed. After the samples were hydrolyzed and adjusted to pH 7.0, these were subjected to chloramines-T oxidation. The reaction was terminated by addition of 3.15 M perchloric acid and para-dimethylaminobenzaldehyde at 60 C to develop pink color. Absorbance was measured at 557 nm using a spectrophotometer and hydroxyproline content was determined against a standard curve.
Statistical Analysis
One-way analysis of variance was carried out to identify the differences between treated groups and controls. Statistical comparisons between variables were performed with Student's t-test. The level of significance for significant difference between groups was set at P < 0.05 in all analyses.
RESULTS AND DISCUSSION
Preparation of plant extracts
The hydroethanolic extract obtained by maceration presented mass of 143.70 g, with a final yield of 11.98%. The EA extract obtained after the partition showed a yield of 3.19% (38.32 g). The yields of leaves extracts from E. pruniformis, according to the literature, are considered satisfactory, because the speciesEugenia brasiliensis presented a yield of 11.33% for the raw hydroethanolic extract and 2.22% for the EA extract.[24] According to two studies that included the obtaining of raw hydroethanolic extract of Eugenia uniflora leaves, the yields were 3.35% and 2.8%.[25,26]
Substance identification by TLC, HPLC-PDA, and HRESI-MS analysis
The EA extract showed yellow spots on TLC at 365 nm UV light and after it was sprayed with NP/PEG reagent, which suggested the presence of flavonoids. The sample spots showed similar Rf values to standard solutions of quercetin (fluorescent yellow with a Rf of 0.39) and kaempferol (fluorescent green with a Rf of 0.50). Because the flavonoids compounds could not be identified based only on TLC data, HLPC-PDA and HRESI-MS analyses were performed.
On the basis of our previous analytical work, additional systematic HPLC tests for optimization of the analytical method for flavonoids identification were performed.[16] The influence of mobile phase on chromatographic separation of the flavonoids was investigated in detail. A gradient of acetonitrile in 0.1% aqueous formic acid from 5:95 to 100:0 in 30 min was used as start condition and subsequently optimization of mobile phase was performed based on peak symmetry, peak width, and run time. Thus, the gradient of acetonitrile in 0.1% aqueous formic acid from 90:10 to 40:60 in 30 min was found to be satisfactory. After the method optimization, the flavonoids identification was carried out by comparison of the EA extract [Figure 1] and the standard flavonoids chromatograms with the extract fortified with flavonoids standards (kaempeferol, quercetin, and hyperoside) chromatogram. The EA extract chromatogram showed signals with retention time values (18.5 min for hyperoside, 25.3 min for quercetin, and 28.5 min for kaempferol), and UV absorption spectrum similar to the flavonoids standards. The HPLC developed method proved to be very selective and effective for the simultaneous determination of kaempferol, quercetin, and hyperoside in EA extract from E. pruniformis leaves.
Figure 1.
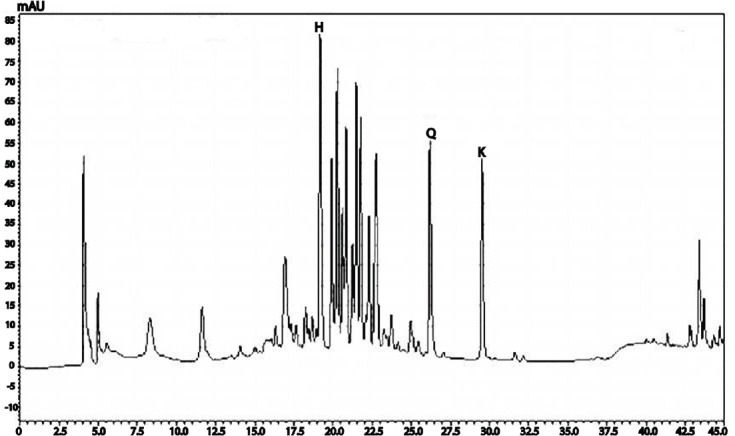
HPLC.PDA analysis of the EA extract from E. pruniformis leaves. UV 360 nm chromatogram. 408 C18 column, MeCN/0.1% aqueous formic acid, 10:90 to 60:40 in 30 min, 1.0 mL/min. Peaks are assigned to 409 the identified compound. H, hyperoside; K, kaempferol; Q, quercetin
The HRESI-MS analysis of the EA extract gave fragment ions at m/z 463.0888 [M-H], 301.0321 [M-H], and 285.0415 [M-H] in agreement with the molecular formula C21H20O12, C15H10O7, and C15H10O6, respectively, and corresponding to hyperoside, quercetin, and kaempferol.
The three identified flavonoids are commonly found in the genus Eugenia. Hyperoside is considered one of the flavonoid gender markers.[27] This same flavonoid is associated with different pharmacological activities, such as antitumoral activity against several cancer cell lines, as well as immunomodulatory and anti-inflammatory activities.[28,29,30] The flavonoids quercetin and kaempferol are quite widespread among angiosperms in general.[5] Quercetin is considered a phytoestrogen, selectively interacting with the type II estrogen receptors, and also has antiproliferative activity through different mechanisms of action.[31,32]
Determination of total flavonoids content of ethyl acetate extract
The total flavonoids content was determined by spectrophotometric method using rutin as a reference standard. The absorbances of a rutin concentrations series were plotted to provide a linear calibration curve (y = 23.834x + 0.07958) with r2 = 0.992. In this study, the total flavonoids content of the EA extract from E. pruniformis leaves was 40%. Although the loss on drying value was equal to 7.84%, so the amount was 43.05% in the dry weight extract. This total flavonoids content was higher than those reported in previous studies for Eugenia spp., such as E. uniflora (8.43%), E. malaccensis (4.07%), E. brasiliensis (5.41%) E. beaurepaireana (11.46%), and E. umbelliflora (3.28%).[24,33] This result may be related to the fact that the species be found in the sandbank vegetation that grows in coastal areas under high luminance, and it is known that flavonoids are secondary metabolites with antioxidant action, protecting the plant tissues from excessive light.[5]
Antioxidant activity
The EA extract of E. pruniformis leaves showed TE value of 0.81 ± 0.05 mmol TE/g. According to the values found in extracts rich in flavonoids and polyphenolics substances from other species, the result obtained for EA extract can be considered as expected, because the ORAC value of hydrophilic leaf extracts were 0.45 and 0.38 mmolTE/g for the species Amaranthus caudatus and Amaranthus hypochondriacus, respectively, whereas the leaf extract in EA of Xylopia ochrantha showed a value of 1.85 mmolTE/g.[30,34]
Regarding DPPH radical assay, the EC50 is the antioxidant concentration required to obtain a 50% radical inhibition. The EC50 values of the EA extract of E. pruniformis and the positive control Trolox were 7.05 ± 0.09 µg/mL and 2.95 ± 0.05 µg/mL. The EA extract of E. pruniformis showed higher antioxidant activity compared to other EA extracts from Myrtaceae species as Myrcia splendens (EC50 of 8.44 µg/mL) and M. palustris (EC50 of 17.83 µg/mL) and Campomanesia adamantium (EC50 of 7.77 µg/mL).[35,36] The potent antioxidant activity may be related to the high flavonoids content of the extract, which is known as antioxidant compounds.[5]
Evaluation of wound healing activity of leaf extracts from E. pruniformis
The HE histological analysis of wound in the treated groups EA and PC at 8th and 15th days showed that wounds displayed better epithelialization and more effective reorganization of the dermis when compared with the others groups [Figure 2]. Interestingly, EA was more efficient because it was possible to observe the complete remodeling of epidermis, indicating the regression of the lesions. These parameters are considered indicative of a healing process improvement, and demonstrate greater efficacy of EA extract to promote the regeneration of the damaged tissue.
Figure 2.
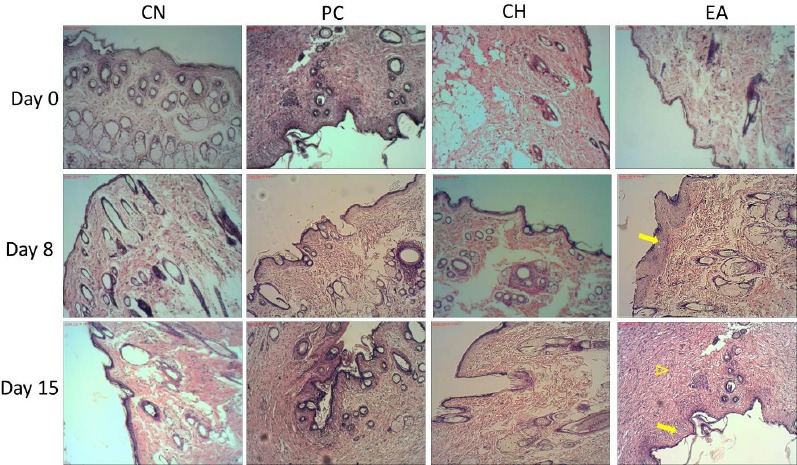
The histological evaluation of the skin flaps revealed by HE coloration (10×). NC is the negative 412 control, PC is treated topically with collagenase ointment as positive control, CH is treated topically with 413 propylene glycol solution of 5% hydroethanolic extract, and EA is treated topically with propylene glycol solution of 5% EA extract. Day 0, 8, and 15 414 days after injury. Microscopic examination of the lesions treated 415 with EA indicated better epithelialization (arrow) and more re-organization of the dermis (arrowhead) 416 compared to the others groups on 8 and 15 days after injury
The evaluation of Sirius Red staining demonstrated a significant increase of collagen distribution in the EA and in the positive control (PC) group when compared to the nontreated and the CH treatment group on the 8th day. The fibers in red, which are related to the dye marked collagen type I, denote a difference among them [Figure 3]. These observations were confirmed by the percentage of area occupied by collagen fibers in each cut [Table 1]. The EA extract was able to increase by about 330% the collagen deposition compared to nontreated group on the 8th day, showing the high result among the groups.
Figure 3.
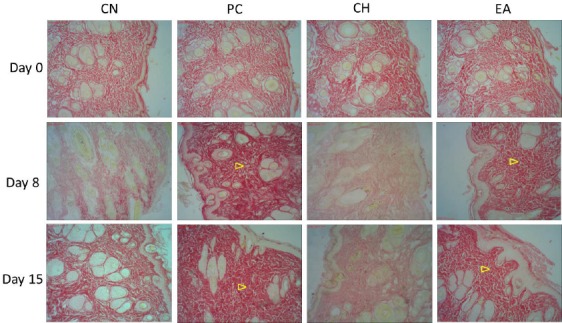
The evaluation of Sirius Red staining was made to recognize the total density of collagen. Each cut 419 was analyzed the percentage of area occupied by collagen fibers. NC is the negative control, PC is treated 420 topically with Collagenase ointment, CH is treated topically with propylene glycol solution of 5% crude 421 hydroethanolic extract, EA is treated topically with propylene glycol solution of 5% EA extract. Day 0, 8, and 15 days after injury. The distribution of collagen was more intense mainly in EA and PC groups on 8 422 and 15 423 days after injury (arrowhead)
Table 1.
Hydroxyproline levels (mg/g) in wound areas and histologic scores (%) of collagen fibers in avaliated groups
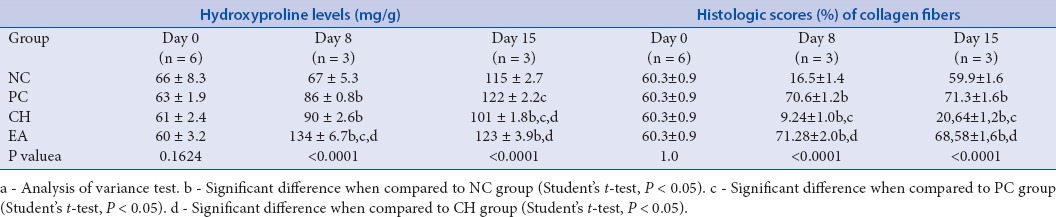
NC is the negative control, PC is treated topically with Collagenase ointment, CH is treated topically with propylene glycol solution of 5% crude hydroethanolic extract, EA is treated topically with propylene glycol solution of 5% EA extract. Values are mean ± standard deviation from at day 0, 8, and 15 days after injury.
Hydroxyproline, one of the basic monomers of collagen, was taken as a marker of collagen synthesis.[37] In the PC and in the groups treated with E. pruniformis extracts (CH and EA), the hydroxyproline content of dry granulation tissue was significantly higher compared to the NC on day 8. The group treated with CH showed an increase of 34%, whereas the EA-treated group was able to increase the levels in the same period by 100%. Comparing to PC, collagenase, an enzymatic debriding agent which showed 28% of hydroxyproline-level augment, the groups treated with E. pruniformis extracts obtained a better performance [Table 1].[38] These results suggest that E. pruniformis extracts accelerate the wound-healing process in the early stages. Moreover, the dosage of hydroxyproline levels shows a higher potential of the EA extract by increasing the collagen deposition at the lesion site, taking into consideration that this extract has a higher concentration of flavonoids, which in turn may be responsible for processes that promote healing.[6,7] This result is similar to histological analysis of collagen distribution, what indicates a more effective result in the group treated with EA extract, and also, to the histological analysis by HE, at which EA demonstrated to promote better reorganization of dermis and complete remodeling of epidermis.
According to data of other works with extracts rich in flavonoids, Sphaeranthus amaranthoides and Martynia annua showed an increase of hydroxyproline levels in 120 and 150%, respectively, when compared to the control group. The flavonoid extract of S. amaranthoides also exhibited an increase of collagen deposition in about three times more than the non-treated group.[6,7] These results are similar to the ones found in this present work, which in turn, reinforces the importance of the flavonoids role in the quality of healing process, and suggests the use of EA extract from E. pruniformis as a healing promoter. Quercetin and hyperoside, one of the main flavonoids present in the extract, have a remarked anti-inflammatory and antimicrobial activity that can be related to a decrease of microbial load in the wound site and abbreviation of early stages of healing, so that, promoting better tissue reorganization and an increase of the re-epithelialization speed.[5,28,29] Furthermore, the high antioxidant values of EA for ORAC and DPPH methods might mean a contribution of antioxidant property in accelerating the healing process by control of oxidative stress, as seen in studies with S. amaranthoides and Clausena anisata, which demonstrated that the extracts with high antioxidant activity also presented significantly improvement in wound healing process.[6,39,40]
CONCLUSION
This work contributes to the chemical and pharmacological knowledge of E. pruniformis and its importance as a wound-healing promoter. Eugenia pruniformis extract accelerates wound healing in skin rat model, probably due to its involvement with the collagen deposition increase, higher levels of hidroxyproline, dermal remodeling, and potent antioxidant activity. Chemical standardization of the active wound-healing extract was carried out, and total flavonoid content was 43% (w/w) and the main compounds were identified as quercetin, kaempferol, and hyperoside. Thus, for the first time, this study describes flavonoids for E. pruniformis that could be used as chemical markers for quality control of the herbal drug. In addition, the use of the E. pruniformis extract may be seen as an alternative for the development of phytopharmaceuticals with wound-healing activity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Adriana Passos Oliveira
Prof. Adriana Passos Oliveira, is a Professor at the Department of Drugs and Medicines, Faculty of Pharmacy, Federal University of Rio de Janeiro (Brazil). She obtained a PhD in Chemistry of Natural Products at the Federal University of Rio de Janeiro in collaboration with the Institute of Pharmaceutical Biology, University of Basel (Switzerland). She has experience in the area of Chemistry of Natural Products and Pharmaceutical Technology, working mainly in phytochemistry, phytopharmaceuticals, quality control and nanoemulsions.
Acknowledgement
The authors thank Dr. Fiorella Fernanda Mazine Capelo for species identification, CAPES and FAPERJ for financial support, and PBV-UFRJ, LaFESP-UEZO e LTPN-UFF for technical support.
REFERENCES
- 1.Santos MG, Fevereiro PCA, Reis GL, Barcelos JI. Recursos vegetais da restinga de Carapebus Rio de Janeiro. BrasilVer Biol Neutrop. 2009;6:35–54. [Google Scholar]
- 2.Haron NW, Moore DM, Harborne JB. Distribution and taxonomic significance of flavonoids in the Genus Eugenia (Myrtaceae) Biochem Syst Ecol. 1992;20:266–8. [Google Scholar]
- 3.The Plant List [Internet]. Version 1.1. [Last accessed on 2015 Jun 2]. Available from http://www.theplantlist.org/
- 4.Albuquerque RDDG, Tietbohl LAC, Fernandes CP, Couteiro PP, Eiriz DN, Santos MG. Chemical and biological study of essential oils from Eugenia pruniformis Cambess., an endemic species from Brazilian Atlantic Forest. Lat Am J Pharm. 2012;31:830–4. [Google Scholar]
- 5.Zuanazzi JAS, Montanha JA, Flavonoides In, Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR. Farmacologia: da planta ao medicamento. 5th ed. Porto Alegre: UFRGS; 2004. pp. 577–614. [Google Scholar]
- 6.Geethalakshmi R, Sakravarthi C, Kritika T, Arul-Kirubakaran M, Sarada DVL. Evaluation of antioxidant and wound healing potentials of Sphaeranthus amaranthoides. Burm.f. Biomed Res Int. 2013;ID 607109:1–7. doi: 10.1155/2013/607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodhi S, Singhai AK. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2013;6:253–9. doi: 10.1016/S1995-7645(13)60053-X. [DOI] [PubMed] [Google Scholar]
- 8.Tabatabai-Naieni A, Miri R, Shafiei N, Tabandeh MR, Oryan A, Nazifi S. Effects of topical application of Calendula officinalis gel on collagen and hydroxyproline content of skin in rats. Comp Clin Pathology. 2010;21:253–7. [Google Scholar]
- 9.Paiva LAF, Alencar-Cunha KM, Santos FA, Gramosa NV, Silveira ER, Rao VSN. Investigation on the wound healing activity of oleo-resin from Copaifera langsdorffi in Rats. Phytother Res. 2002;16:737–9. doi: 10.1002/ptr.1049. [DOI] [PubMed] [Google Scholar]
- 10.Marquez RR, Avaliação da Ferida In, Gogia PP. Feridas: tratamento e cicatrização Rio de. Janeiro: Revinter; 2003. pp. 11–21. [Google Scholar]
- 11.Santos JB, Porto SG, Suzuki LM, Sostizzo LRZ, Antoniazzi JL, Echer IC. Avaliação e tratamento de feridas: orientação aos profissionais de saúde. Porto Alegre: Assessoria de Comunicação Social HCPA; 2011. [Google Scholar]
- 12.Góis ALB, Veras RP. Informações sobre a morbidade hospitalar em idosos nas internações do Sistema Único de Saúde do Brasil. Ciência & Saúde Col. 2010;15:2859–69. doi: 10.1590/s1413-81232010000600023. [DOI] [PubMed] [Google Scholar]
- 13.Isaac C, Ladeira PRS, Rêgo FMP, Aldunate JCB, Ferreira MC. Processo de cura das feridas: cicatrização fisiológica. Rev Med. 2010;9:125–31. [Google Scholar]
- 14.Brown GL, Nanney LB, Griffen J, Cramer AB, Yancey JM, Curtsinger LJ. Enhancement of wound healing by topical treatment with epidermal growth factor. New Engl J Med. 1989;321:76–9. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- 15.Klasen HJ. A review on the nonoperative removal of necrotic tissue from burn wounds. Burns. 2000;26:207–22. doi: 10.1016/s0305-4179(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira AP, Raith M, Kuster RM, Rocha LM, Hamburger M, Potterat O. Metabolite profiling of the leaves of the brazilian folk medicine Sideroxylon obtusifolium. Plant Med. 2012;78:703–10. doi: 10.1055/s-0031-1298269. [DOI] [PubMed] [Google Scholar]
- 17.Rolim A, Maciel CPM, Kaneko TM, Consiglieri VO, Salgado-Santos IMN, Velasco MVR. Validation assay for total flavonoids, as rutin equivalents, from Trichilia catigua Adr. Juss (Meliaceae) and Ptychopetalum olacoides Bentham (Olacaceae) Commercial Extract. J OAOC Int. 2005;88:1015–9. [PubMed] [Google Scholar]
- 18.Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. 2002;50:4437–44. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 19.Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–30. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 20.Morton JJP, Malone MH. Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharm Thérapie. 1972;196:117–9. [PubMed] [Google Scholar]
- 21.Kim SW, Zhang HZ, Guo L, Kim JM, Kim MH. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perini JA, Angeli-Gamba T, Ferreira LC, Nasciutti LE, Machado DE. Topical application of Acheflan on rat skin accelerates wound healing: a histopatological, immunohistochemical and biochemical study. BMC Compl Alt Med. 2015;15:203. doi: 10.1186/s12906-015-0745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 24.Magina MDA, Dalmarco EM, Dalmarco JB, Colla G, Pizzolatti MG, Brighente IMC. Bioactive triterpenes and phenolics of leaves of Eugenia brasiliensis. Quím Nova. 2012;35:1184–8. [Google Scholar]
- 25.Wikantyasning ER, Nurwaini S, Wahyuningtyas N, Mohandani IP, Hapsoro S, Aji W. Effervescent tablet of Sambiloto (Andrographis Paniculata [Burm.F.] Ness) and Dewandaru (Eugenia Uniflora L.) extract: formulation, antidiabetic and antioxidant properties. Int J Pharm Teach Practice. 2013;4:689–94. [Google Scholar]
- 26.Santos KKA, Matias EFF, Tintino SR, Souza CES, BragaMFBM, Miriam-Rólon GMM. Anti-Trypanosoma cruzi and cytotoxic activities of Eugenia uniflora L. Exp Parasitol. 2012;131:130–2. doi: 10.1016/j.exppara.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Maridass M, Ramesh U. Chemosystematics evaluation of Eugenia species based on molecular marker tools of flavonoids constituents. Int J Biol Tech. 2010;1:107–10. [Google Scholar]
- 28.Ku SK, Kwak S, Kwon OJ, Bae JS. Hyperoside inhibits high-glucose-induced vascular inflammation in vitro and in vivo. Inflammation. 2014;37:1389–400. doi: 10.1007/s10753-014-9863-8. [DOI] [PubMed] [Google Scholar]
- 29.Ku SK, Zhou W, Lee W, Han MS, Na MK, Bae JS. Anti-inflammatory effects of hyperoside in human endothelial cells and in mice. Anti-septic activity of hyperoside. Inflammation. 2014 doi: 10.1007/s10753-014-9989-8. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Deng Z, Liu R, Zhu H, Draves J, Marcone M. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J Food Comp Analysis. 2015;37:75–81. [Google Scholar]
- 31.Caltagirone S, Ranelletti RO, Rinelli A, Maggiano N, Colasante A, Musiano P. Interaction with type II estrogen binding sites and antiproliferative activity of tamoxifen and quercetin in human non-small-cell lung cancer. Am J Resp Cell Mol. 1997;17:51–9. doi: 10.1165/ajrcmb.17.1.2728. [DOI] [PubMed] [Google Scholar]
- 32.Behling EB, Sendão MC, Francescato HDC, Antunes LMG, Bianchi MLP. Flavonoide quercetina: aspectos gerais e ações biológicas. Alim Nutr. 2004;15:285–92. [Google Scholar]
- 33.Figueirôa EO, Silva LCN, Melo CML, Neves JKAL, Silva NH, Pereira VRA. Evaluation of antioxidant, immunomodulatory, and cytotoxic action of fractions from Eugenia uniflora L. and Eugenia malaccensis L.: correlation with polyphenol and flavanoid content. Sci World J. 2013;2013:1–7. doi: 10.1155/2013/125027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albuquerque RDDG. Estudo fitoquímico e biológico de folhas da espécie vegetal Xylopia ochrantha (Mart.) [dissertation] NiteróFaculdade de Farmácia, Universidade Federal Fluminense. 2013 [Google Scholar]
- 35.Moresco HH, Pereira M, Bretanha LC, Micke GA, Pizzolatti MG, Brighente IMC. Myricitrin as the main constituent of two species of Myrcia. J App Pharm Sci. 2014;4:1–7. [Google Scholar]
- 36.Pascoal AC, Ehrenfried CA, Eberline MN, Stefanello ME, Salvador MJ. Free radical scavenging activity, determination of phenolic compounds and HPLC-DAD/ESI-MS profile of Campomanesia adamantium leaves. Nat Prod Commun. 2011;6:969–72. [PubMed] [Google Scholar]
- 37.Long KB, Burgwin CM, Huneke R, Artlett CM, Blankenhorn EP. Tight skin 2 mice exhibit delayed wound healing caused by increased elastic fibers in fibrotic skin. Adv Wound Care (New Rochelle) 2014;3:573–81. doi: 10.1089/wound.2014.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakewood NJ. Collagenase, Clostridium histolyticum collagenase (E.C. 3.4.24.3) Worthington enzyme manual, enzymes and related biochemicals. Worthin Biochem Corp. 1993;5:112–20. [Google Scholar]
- 39.Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharm Physiol. 2011;24:113–26. doi: 10.1159/000322643. [DOI] [PubMed] [Google Scholar]
- 40.Agyepong N, Agyare C, Ossei PPS, Boakye YD. Antioxidant and in vivo wound healing activities of Clausena anisata. Eur J Med Plant. 2015;10:1–8. [Google Scholar]


