Abstract
Background:
Rice (Oryza sativa) is a major cereal crop in many Asian countries and an important staple food source. Rice hulls have been reported to possess antioxidant activities.
Materials and Methods:
In this study, we evaluated the antiinflammatory effects of rice hull extract and associated signal transduction mechanisms in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages.
Results:
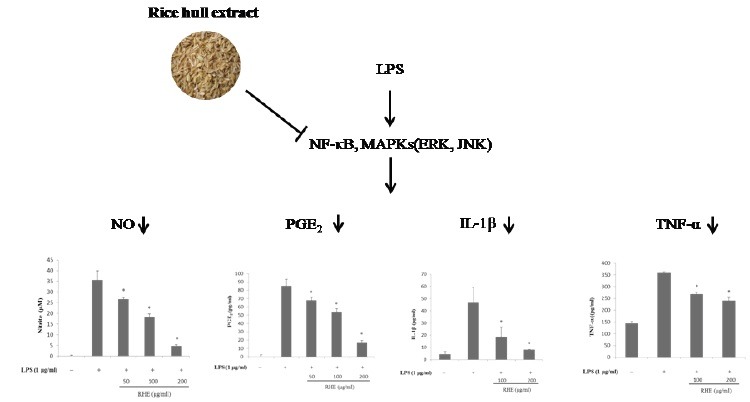
We found that rice hull extract inhibited nitric oxide (NO) and prostaglandin E2 by suppressing the expression of inducible NO synthase and cyclooxygenase-2, respectively. The release of interleukin-1β and tumor necrosis factor-α was also reduced in a dose-dependent manner. Furthermore, rice hull extract attenuated the activation of nuclear factor-kappa B (NF-κB), as well as the phosphorylation of mitogen-activated protein kinases, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), in LPS-stimulated RAW264.7 cells.
Conclusion:
This suggests that rice hull extract decreases the production of inflammatory mediators by downregulating ERK and JNK and the NF-κB signal pathway in RAW 264.7 cells.
SUMMARY
Rice hull extract inhibits the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages.
Rice hull extract inhibited nitric oxide and prostaglandin E2 by suppressing the expression of inducible NO synthase and cyclooxygenase-2, respectively.
Rice hull extract exerted anti-inflammatory effect through inhibition of nuclear factor-kappa B, extracellular signal-regulated kinase and c-Jun N-terminal kinase signaling pathways.
Rice hull extract may provide a potential therapeutic approach for inflammatory diseases.
Abbreviations used: COX-2: cyclooxygenase-2, ERK: extracellular signal-regulated kinase, IκB: inhibitory kappa B, IL-1β: interleukin-1β, iNOS: inducible NO synthase, JNK: c-Jun N-terminal kinase, LPS: lipopolysaccharide, MAPKs: mitogen-activated protein kinases, NF-κB: nuclear factor-κB, NO: nitric oxide, PGE2: prostaglandin E2, RHE: rice hull extract, ROS: reactive oxygen species, TNF-α: tumor necrosis factor-α
Keywords: COX-2, Inflammation, iNOS, Rice hull
INTRODUCTION
The inflammatory response plays an important role in host defense against noxious substances and tissue injury. Usually, inflammation is initiated through the production of specific cytokines or chemokines, characterized by recruitment of leukocytes to the damage site. During inflammation, production of high levels of reactive oxygen species (ROS) assist in the defense against pathogens.[1] However, sustained or excessive inflammation can lead to numerous diseases, due to various cell types expressing and reacting to diverse mediators along a precise sequence of events. Macrophages are key innate immune cells that play an important role in inflammatory disease through the release of factors such as nitric oxide (NO), prostaglandins, and cytokines involved in the immune response. Therefore, inhibition of overactivated macrophages is one strategy for treating inflammatory diseases.
Lipopolysaccharide (LPS), a component of the Gram-negative bacterial cell wall, is frequently used as a model of inflammation due to its ability to activate macrophages. LPS can activate macrophages via multiple signaling pathways, and thus enhance the production of several inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and nitric oxide (NO). The mechanisms by which LPS induces the production of cytokines and inflammatory mediators have been intensively investigated. Several well-characterized signaling pathways are involved. Of these, the mitogen-activated protein kinases (MAPKs) and nuclear factor-κB (NF-κB) pathways may play an essential role because of the rapidity of NF-κB activation and its potency as a transcriptional activator.[2,3]
Rice (Oryza sativa) is a major cereal crop in many Asian countries and an important staple food source. More than one million tons of rice hull are produced annually in South Korea as a byproduct of rice processing. However, rice hulls are wasted or destined for undervalued uses.[4] Rice hulls have been reported to have ROS-scavenging and protective effects against oxidative DNA damage.[4,5] The anti-inflammatory effects of rice hull in macrophages and its underlying mechanisms are not yet well-established. In this study, we used LPS-stimulated murine RAW 264.7 macrophages to investigate the effects of rice hull extract (RHE) on the production of various cytokines and inflammatory mediators. To elucidate the molecular mechanisms underlying these effects, we further determined the effects of RHE on LPS-induced NF-κB and the MAPK-signaling pathways.
MATERIALS AND METHODS
Preparation of rice hull extract
Hulls from rice cultivar (Oryza sativa L.), a Japonica type rice, were purchased from Hwasung, South Korea, in 2010. The hulls were separated in a milling machine, dried, and then ground in a blender. Powdered hulls were extracted with 70% ethanol at room temperature. After filtration, the extracts were dried under vacuum. The lyophilized power was dissolved in dimethyl sulfoxide (Sigma, St. Louis, Missouri, USA).
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, California, USA). LPS was obtained from Sigma Chemical Company (St. Louis, Missouri, USA).
Cell culture
The mouse macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (ATCC, Rockville, Maryland, USA). Cells were cultured in DMEM supplemented with 10% FBS, and 1% penicillin-streptomycin at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was replaced every 3 days.
Measurement of nitric oxide production and cell viability
Nitrite production was measured in RAW 264.7 macrophage supernatants. Briefly, cells (5 × 105) were cultured in 96-well plates and stimulated with LPS (1 μg/ml) for 24 h. The supernatant (50 μl) was harvested and mixed with an equal volume of Griess reagent (1% sulfanilamine, 0.1% naphthylethylene diamine di-hydrochloride, 2% phosphoric acid). After 10 min, the absorbance at 540 nm was measured using a microplate reader. Sodium nitrite was used as a standard to calculate NO2- concentration. Cell viability was assessed using a 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide (MTT) assay.
Measurement of prostaglandin E2
Media were collected and centrifuged 24 h after treatment with LPS (1 μg/ml) in the presence or absence of RHE. Prostaglandin E2 (PGE2) was measured using a competitive enzyme immunoassay kit (PGE2, R&D Systems, Minneapolis, Minnesota, USA), according to the manufacturers protocol.
Measurement of interleukin 1β and tumor necrosis factor-α
IL-1β and TNF-α secreted into culture media were measured by specific ELISA using monoclonal anti-rat IL-1β or TNF-α antibody as a capture antibody and goat biotinylated polyclonal anti-rat IL-1β or TNF-α antibody as a detection antibody (ELISA development reagents; R&D Systems, Minneapolis, Minnesota, USA). Biotinylated anti-IL-1β or anti-TNF-α antibodies were detected by sequential incubation with streptavidin-horseradish peroxidase (HRP) conjugate and chromogenic substrates. The absorbance at 450 nm was measured using a microplate reader.
Western blot analysis
RAW 264.7 cells were seeded in 6-well plates and exposed to LPS (1 μg/ml) in the presence or absence of RHE for various periods. Protein samples from cell extracts were separated by 8% or 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred to nitrocellulose membranes. Membranes were blocked with 5% skim milk and incubated with primary antibodies against inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), phospho-ERK, phospho-JNK, inhibitory kappa B (IκB), and NF-κB p65 (Santa Cruz Biotechnology). After washing with TBST(tris-buffered saline with tween 20), HRP-conjugated, ECL (enhanced chemiluminescence) secondary antibodies were applied. The blots were developed using ECL Western Blotting Detection Reagents (Thermo Scientific, USA).
Statistical analysis
The data were analyzed using Statistical Analysis System software (PRISM). All data are expressed as mean ± SEM (standard error of the mean). Statistical comparisons between treatments were performed using a one way ANOVA with Tukey's multiple comparison posttest. Values of P value less than 0.05 were considered to be statistically significant.
RESULTS
Effect of rice hull extract on lipopolysaccharide-induced nitric oxide production and inducible nitric oxide synthase expression in RAW264.7 cells
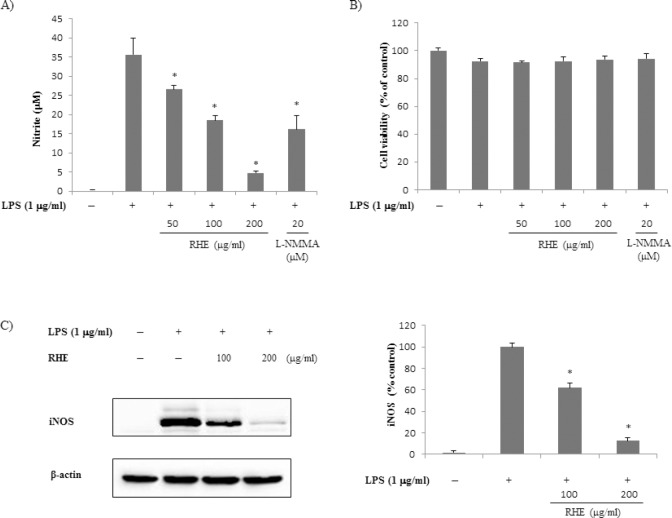
The effect of RHE on LPS-induced NO production was examined using RAW 264.7 macrophages [Figure 1a]. Non-stimulated RAW 264.7 cells secreted basal levels of NO, whereas LPS stimulation resulted in an increase in NO production. Treatment with RHE significantly inhibited LPS-induced NO production in a dose-dependent manner. Inhibitory effect of RHE at 200 μg/ml on NO production was higher than that of NG-monomethyl-L-arginine, a well-known iNOS inhibitor. The MTT assay showed that treatment with RHE for 24 h did not significantly affect cell viability up to 200 μg/ml [Figure 1b]. To determine whether the inhibitory effects of RHE on NO were related to modulation of iNOS, we examined protein expression by Western blot. As shown in Figure 1c, iNOS expression was strongly induced by LPS. However, RHE at 100–200 μg/ml inhibited the LPS-induced iNOS expression.
Figure 1.
Effect of rice hull extract on lipopolysaccharide (LPS)-induced nitric oxide (NO) production and inducible NO synthase (iNOS) expression in macrophage cells
(a) Effect of rice hull extract on LPS-induced NO production by LPS-stimulated RAW 264.7 macrophages. (b) Effect of rice hull extract on viability of LPS-stimulated RAW 264.7 macrophages. (c) Effect of rice hull extract on LPS-induced iNOS expression by LPS-stimulated RAW 264.7 macrophages. Cells (5 × 105) were treated with various rice hull extract concentrations or LPS (1 μg/ml) for 24 h. The cells were then collected and their viability was assessed by MTT assay. The culture medium was collected and subjected to nitrite assay. Nitrate was measured using a Griess reaction. Equal protein loading was confirmed by blotting with anti-β-actin. All data are presented as the mean ± SEM of three independent experiments. * P value less than 0.05, statistically significant differences compared to treatment with LPS alone.
Effect of rice hull extract on lipopolysaccharide-induced Prostaglandin E2 production and cyclooxygenase-2 expression in RAW264.7 cells
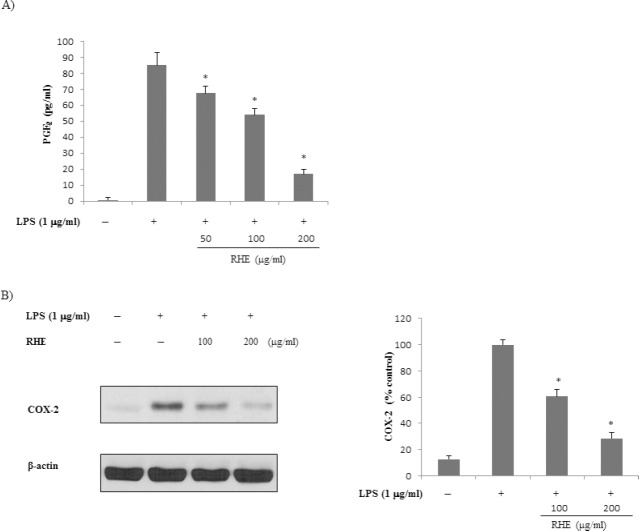
To evaluate the effects of RHE on LPS-induced PGE2 production in RAW 264.7 cells, culture media were harvested and PGE2 levels therein were measured. As shown in [Figure 2a], LPS was associated with significant increases in PGE2 secretion by RAW 264.7 cells. However, RHE significantly inhibited LPS-induced PGE2 production in a dose-dependent manner. We next determined whether inhibition of PGE2 production by RHE was due to a decrease in COX-2 expression. RHE dose-dependently inhibited PGE2 production. Next, Western blot analysis was performed to determine whether the inhibitory effect of RHE on the proinflammatory mediator PGE2 was related to modulation of COX-2 expression. COX-2 protein levels were markedly upregulated in response to LPS; this effect was strongly inhibited by RHE [Figure 2b].
Figure 2.
Effect of rice hull extract on Prostaglandin E2 (PGE2) production by lipopolysaccharide (LPS)-induced macrophage cells
(a) PGE2 level was determined using a competitive enzyme immunoassay kit after treatment with LPS (1 μg/ml) for 24 h in the presence or absence of rice hull extract (50, 100, or 200 μg/ml). (b) Effect of rice hull extract on cyclooxygenase-2 (COX-2) expression by RAW 264.7 cells. COX-2 protein was detected by Western blot 6 h after treatment with LPS (1 μg/ml) in the presence or absence of rice hull extract (100 or 200 μg/ml). Equal protein loading was confirmed by blotting with anti-β-actin. All data are presented as the mean ± SEM of three independent experiments. *P value less than 0.05, statistically significant differences compared to treatment with LPS alone.
Effect of rice hull extract on lipopolysaccharide-induced interleukin-1β and tumor necrosis factor-α expression in RAW264.7 cells
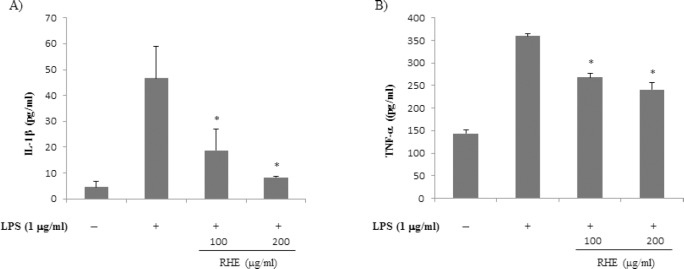
Concentrations of proinflammatory cytokines such as IL-1β and TNF-α are elevated in inflammatory disease, and they play important roles in the immune and inflammatory responses.[6] Therefore, the effect of RHE on the inhibition of IL-1β and TNF-α was investigated. Treatment of RAW 264.7 cells with LPS alone resulted in significant increases in IL-1β and TNF-α production compared with the control group [Figure 3]. However, IL-1β and TNF-α levels in supernatants of cells treated with RHE were significantly decreased compared with the LPS group, in a dose-dependent manner.
Figure 3.
Effect of rice hull extract on interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) expression in lipopolysaccharide (LPS)-induced macrophage cells
Cells were treated with LPS (1 μg/ml) with or without rice hull extract (100 or 200 μg/ml) for 18 h. Culture medium was then collected and IL–1β (a) and TNF-α (b) levels were determined by ELISA. All data are presented as the mean ± SEM of three independent experiments. *P value less than 0.05, statistically significant differences compared to treatment with LPS alone.
Effects of rice hull extract on lipopolysaccharide-induced nuclear factor kappa-B activation
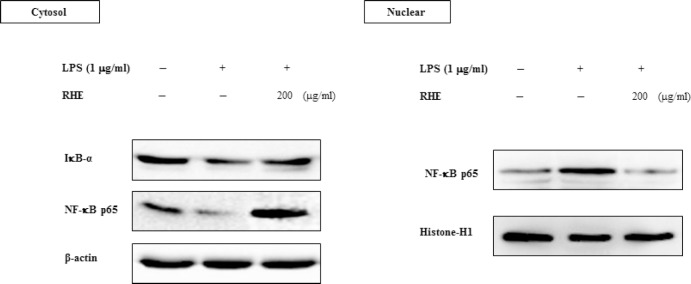
NF-κB is an important transcription factor and is a regulator of inflammation. Therefore, we next determined whether RHE blocked the NF-κB signaling pathway in LPS-stimulated RAW264.7 cells. The NF-κB-p65 subunit was translocated from the cytosol into the nucleus after stimulation by 1 μg/ml LPS for 1 h [Figure 4]. Treatment with RHE caused a significant decrease in cytoplasmic NF-κB-p65 levels and NF-κB-p65 translocation into the nucleus. Because LPS-stimulated activation of NF-κB was correlated with IκBα degradation, the effect of RHE on IκBα expression was examined to clarify the inhibitory action of RHE. LPS stimulation caused a marked reduction in IκBα protein level. RHE reduced LPS-induced IκBα degradation, indicating that IκBα degradation and the subsequent translocation of NF-κB p65 are inhibited by RHE.
Figure 4.
Effect of rice hull extract on lipopolysaccharide (LPS)-induced nuclear factor-κB (NF-κB) activation in macrophage cells
Cells were treated with LPS (1 μg/ml) with or without rice hull extract (200 μg/ml) for 1 h. Cytosolic and nuclear fractions were then prepared using a nuclear extraction kit. IκBα and NF-κB contents of the cytosolic and nuclear fractions were analyzed by Western blot. Equal protein loading was confirmed by blotting for anti-β-actin and histone H1. An immunoblot representative of three independent experiments is shown.
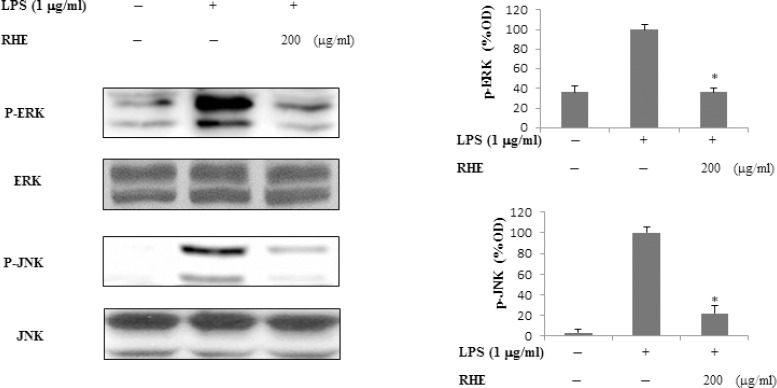
Figure 5.
Effect of rice hull extract on LPS-induced ERK and JNK activation.
Cells were treated with LPS (1 μg/ml) with or without rice hull extract (200 μg/ml) for 30 min, and lysates were prepared to evaluate ERK and JNK levels. Activation of ERK and JNK was evaluated by Western blot analysis using antibodies against the phosphorylated or non-phosphorylated forms of ERK and JNK. All data are presented as the mean ± SEM of three independent experiments. *p < 0.05, statistically significant differences compared to treatment with LPS alone.
Effects of rice hull extract on lipopolysaccharide-induced extracellular signal-regulated kinase and c-Jun N-terminal kinase activation
To investigate whether inhibition of the inflammatory response by RHE was mediated through the MAPKs pathway, we examined its effect on LPS-induced phosphorylation of ERK and JNK in RAW264.7 cells by Western blot. After cells were stimulated with LPS, levels of ERK and JNK phosphorylation were measured. Phosphorylation of ERK and JNK was significantly increased after stimulation with LPS for 30 min. ERK and JNK phosphorylation levels were dramatically lower in RHE-treated cells than in LPS-treated control cells. These data suggest that the anti-inflammatory activity of RHE was due to inhibition of the LPS-induced phosphorylation of ERK and JNK.
DISCUSSION
Rice hull contains large amounts of antioxidants such as flavonoids and their glycosides, hydrocinnamic acid derivatives, isovitexin, γ-Oryzanol, phytic acid, anisole, vanillin, and syringaldehyde. It is reported that γ-Oryzanol, phytic acid, and isovitexin have been shown to exert multiple functions, including antioxidant and antiinflammatory properties.[7,8,9] HPLC (high-performance liquid chromatography) analysis revealed that the RHE used in this study contains 0.62 mg/g of γ-Oryzanol, 0.05 mg/g of phytic acid (Data not shown). The anti-inflammatory action of the RHE may have been because of these constituents from rice hull. We cannot, however, rule out the possible involvement of other chemicals.
Macrophages actively participate in inflammatory responses by releasing proinflammatory cytokines (IL-1β and TNF-α) and inflammatory factors (NO and PGE2) that recruit additional immune cells to sites of infection or tissue injury.[10] NO is involved in modulation of cellular functions and homeostasis. However, excessive production of NO by iNOS in macrophages and in other cells plays a role in the inflammatory response. Large amounts of NO can stimulate production of many proteins and enzymes crucial to inflammatory reactions, such as the NF-κB and MAPKs pathways. PGE2, another proinflammatory mediator involved in inflammatory responses, is generated by the sequential metabolism of arachidonic acid by COX. PGE2 contributes to the development of chronic inflammatory disease.[11,12] Two proinflammatory enzymes that can be induced by LPS or cytokines, iNOS, and COX-2, work in concert in a number of pathophysiological activities and inflammatory diseases.[13,14]
Previous studies have shown that modulation of iNOS-mediated NO release is a major contributor to the inflammatory process.[15] Inhibition of COX-2 activity can reduce the deleterious consequences of sepsis.[16] Therefore, a compound capable of preventing the release of proinflammatory mediators or downregulating iNOS or COX-2 expression may possess anti-inflammatory activities. In this study, RHE significantly inhibited LPS-induced iNOS and COX-2 protein increments in RAW 246.7 cells. These results strongly suggest that RHE could protect against NO and PGE2 cytotoxicity in LPS-induced inflammatory responses and ecto toxin shock.
TNF-α and IL-1β are major proinflammatory cytokines produced by various immune cells such as macrophages, monocytes, and T cells. TNF-α production is crucial for the synergistic induction of NO synthesis in IFN (interferon)-γ and/or LPS-stimulated macrophages. TNF-α elicits a number of physiological effects, such as septic shock, inflammation, cachexia, and cytotoxicity.[17] IL-1β is a prototypic proinflammatory cytokine that has pleiotropic effects on a variety of cells, and plays key roles in acute and chronic inflammatory and autoimmune disorders.[18] In this study, RHE effectively inhibited the production of these cytokines by LPS-stimulated RAW 264.7 cells. This demonstrates that RHE effectively inhibited the generation of proinflammatory cytokines that are paramount in the generation of an inflammatory response in activated macrophages.
LPS stimulation induces inflammation by activating both the NF-κB pathway and MAPK signaling.[19] NF-κB is a key factor regulating the expression of inflammation-associated enzymes and cytokine genes, such as iNOS, COX-2, TNF-α, and IL-1β, which contain NF-κB binding motifs within their respective promoters. In nonstimulated cells, NF-κB is located in the cytosol and is linked to the inhibitory protein, IκB. IκB is phosphorylated, ubiquitinated, and degraded upon stimulation with LPS or proinflammatory cytokines, which allows NF-κB to translocate into the nucleus.[20] Many antiinflammatory agents exert their effects by suppressing NF-κB signaling.[21,22,23]
To investigate the molecular mechanism of RHE-mediated inhibition of inflammatory substances, its effect on NF-κB activity was evaluated by Western blot. In the present study, we found that translocation of activated NF-κB to the nucleus was inhibited by RHE, and that IκBα degradation was also inhibited. These findings indicate that RHE inhibits NF-κB activation by suppressing IκBα degradation and translocation of NF-κB from the cytosol into the nucleus in LPS-induced RAW 264.7 cells.
MAPKs such as p38, ERK, and JNK also play a critical role in the regulation of production of inflammatory mediators by activated macrophages.[24,25] ERK is normally associated with proliferation and growth factors, whereas JNK and p38 are induced by stress responses and cytokines and can mediate differentiation and cell death. Several studies have described the participation of ERK and JNK in inflammation. Activation of ERK is thought to be involved in LPS-induced macrophage responses.[26,27] In addition, JNK is activated by LPS stimulation and has been postulated to play an important role in controlling iNOS gene expression.[28] In this study, treatment with RHE markedly suppressed LPS-stimulated phosphorylation of ERK and JNK. These data suggest that suppression of ERK/JNK phosphorylation by RHE might also be involved in the inhibition of the LPS-induced production of proinflammatory substances by RAW 264.7 cells.
CONCLUSION
The present study demonstrates that RHE inhibits the LPS-induced inflammatory response in RAW264.7 macrophages. RHE may exert its antiinflammatory effect through inhibition of NF-κB and activation of the ERK and JNK signaling pathways. Thus, our findings suggest that rice hulls represent a potential bioactive source derived from an agricultural byproduct that can be considered a potent candidate treatment for inflammatory diseases.
Financial support and sponsorship
This research was supported by Bio-industry Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (112136-4).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Yoonsook Kim
Dr. Yoonsook Kim, is a principal scientist at Korea Food Research Institute, Division of functional food research. Her research interest is in the area of food chemistry and functionality.
REFERENCES
- 1.Kang HS, Lee JY, Kim CJ. Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J Ethnopharmacol. 2008;116:305–12. doi: 10.1016/j.jep.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 2.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 3.Senftleben U, Karin M. The IKK/NF-kappaB pathway. Crit Care Med. 2002;30:S18–S26. [PubMed] [Google Scholar]
- 4.Lee SC, Kim JH, Jeong SM, Kim DR, Ha JU, Nam, et al. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J Agric Food Chem. 2003;51:4400–03. doi: 10.1021/jf0300285. [DOI] [PubMed] [Google Scholar]
- 5.Jeon KI, Park E, Park HR, Jeon YJ, Cha SH, Lee SC, et al. Antioxidant activity of far-infrared radiated rice hull extracts on reactive oxygen species scavenging and oxidative DNA damage in human lymphocytes. J Med Food. 2006;9:42–48. doi: 10.1089/jmf.2006.9.42. [DOI] [PubMed] [Google Scholar]
- 6.Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003;169:203–14. doi: 10.1016/s0021-9150(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 7.Lv Y, Zhang Z, Hou L, Zhang L, Zhang J, Wang Y, et al. Phytic acid attenuates inflammatory responses and the levels of NF-kappaB and p-ERK in MPTP-induced Parkinson's disease model of mice. Neurosci Lett. 2015;597:132–36. doi: 10.1016/j.neulet.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Huang ST, Chen CT, Chieng KT, Huang SH, Chiang BH, Wang LF, et al. Inhibitory effects of a rice hull constituent on tumor necrosis factor alpha, prostaglandin E2, and cyclooxygenase-2 production in lipopolysaccharide-activated mouse macrophages. Ann N Y Acad Sci. 2005;1042:387–95. doi: 10.1196/annals.1338.059. [DOI] [PubMed] [Google Scholar]
- 9.Sakai S, Murata T, Tsubosaka Y, Ushio H, Hori M, Ozaki H, et al. gamma-Oryzanol reduces adhesion molecule expression in vascular endothelial cells via suppression of nuclear factor-kappaB activation. J Agricultural Food Chem. 2012;60:3367–72. doi: 10.1021/jf2043407. [DOI] [PubMed] [Google Scholar]
- 10.Bosca L, Zeini M, Traves PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–58. doi: 10.1016/j.tox.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 12.Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol. 2002;2:603–30. doi: 10.1016/s1567-5769(01)00204-1. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. Faseb J. 1999;13:1121–36. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- 14.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–70. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 15.Kim ND, Kim EM, Kang KW, Cho MK, Choi SY, Kim SG, et al. Ginsenoside Rg3 inhibits phenylephrine-induced vascular contraction through induction of nitric oxide synthase. Br J Pharmacol. 2003;140:661–70. doi: 10.1038/sj.bjp.0705490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoferl MW, Diodato MD, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH, et al. Cyclooxygenase-2-mediated regulation of Kupffer cell interleukin-6 production following trauma-hemorrhage and subsequent sepsis. Shock. 2001;16:479–83. doi: 10.1097/00024382-200116060-00013. [DOI] [PubMed] [Google Scholar]
- 17.Feldmann M. Many cytokines are very useful therapeutic targets in disease. J Clin Invest. 2008;118:3533–36. doi: 10.1172/JCI37346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanghera JS, Weinstein SL, Aluwalia M, Girn J, Pelech SL. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–65. [PubMed] [Google Scholar]
- 20.Sebban H, Courtois G. NF-kappaB and inflammation in genetic disease. Biochem Pharmacol. 2006;72:1153–60. doi: 10.1016/j.bcp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Choi MS, Lee SH, Cho HS, Kim Y, Yun YP, Jung HY, et al. Inhibitory effect of obovatol on nitric oxide production and activation of NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264.7 cells. Eur J Pharmacol. 2007;556:181–89. doi: 10.1016/j.ejphar.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 22.Kim BH, Cho SM, Reddy AM, Kim YS, Min KR, Kim Y, et al. Down-regulatory effect of quercitrin gallate on nuclear factor-kappa B-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264 7. Biochem Pharmacol. 2005;69:1577–83. doi: 10.1016/j.bcp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545:192–99. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97:124–29. doi: 10.1046/j.1365-2567.1999.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YH, Lee SH, Lee JY, Choi SW, Park JW, Kwon TK, et al. Triptolide inhibits murine-inducible nitric oxide synthase expression by down-regulating lipopolysaccharide-induced activity of nuclear factor-kappa B and c-Jun NH2-terminal kinase. Eur J Pharmacol. 2004;494:1–9. doi: 10.1016/j.ejphar.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 26.Chen BC, Lin WW. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134:1055–65. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 28.Uto T, Fujii M, Hou DX. 6-(Methylsulfinyl)hexyl isothiocyanate suppresses inducible nitric oxide synthase expression through the inhibition of Janus kinase 2-mediated JNK pathway in lipopolysaccharide-activated murine macrophages. Biochem Pharmacol. 2005;70:1211–21. doi: 10.1016/j.bcp.2005.07.011. [DOI] [PubMed] [Google Scholar]