Abstract
Background:
It has been known that oxidative stress induced by alcohol played a crucial role in the formation of alcoholic liver disease. Although the formation mechanisms underlying liver injury induced by alcohol still remained largely unknown, it has been considered that oxidative stress played a core role in the pathogenesis of hepatocyte damage.
Objective:
The aim of this study was to investigate the effects of soyasaponin Bb (Ss-Bb) on oxidative stress in alcohol-induced rat hepatocyte injury.
Results:
It has been shown that the administration of Ss-Bb could significantly restore antioxidant activity in BRL 3A cells. Moreover, the impaired liver function and morphology changes resulting from ethanol exposure were improved by Ss-Bb treatment. Treatment with a pharmacological inhibitor of haem oxygenase-1 (HO-1) indicated a critical role of HO-1 in mediating the protective role. Finally, we found that pretreatment with Ss-Bb to ethanol exposure cells increased the expression level of HO-1.
Conclusion:
It was suggested that Ss-Bb may protect against alcohol-induced hepatocyte injury through ameliorating oxidative stress, and the induction of HO-1 was an important protective mechanism.
SUMMARY
Effects of soyasaponin Bb was investigated on oxidative stress in rat hepatocytes
Cell viability and antioxidant capacities were evaluated to determine the effects
The expression level of HO-1 was measured to reveal the proptective mechanisms
Keywords: Alcohol, heme oxygenase-1, oxidative stress, soyasaponin Bb
INTRODUCTION
It has been known that excessive alcohol consumption results in alcoholic liver disease (ALD), which was the leading cause of advanced liver disease and death among adults in Europe and some other areas.[1] The same thing also happened in developing countries, for instance, the incidence of ALD increased from 2.7 to 4.4% during the first 5 years of the 21st centrury.[2] Fatty liver, alcoholic hepatitis, fibrosis, and cirrhosis were four characterized stages of ALD,[3] and finally the risks of liver cancer increase significantly.[4] Although the formation mechanisms underlying liver injury induced by alcohol still remained largely unknown, it has been considered that oxidative stress played a core role in the pathogenesis of hepatocyte damage.[5,6,7]
Oxidative stress, a principle cause of aging and diseases, was defined as an imbalance between excess production of reactive oxygen species (ROS) relative to the defense of antioxidant.[8] As oxygen-based chemical intermediates with high reactivity, the increase production of ROS was a causal feature in the toxicity of many xenobiotics.[9,10] Excessive intake of alcohol could enhance the production of ROS and restrain the activity of antioxidants in vivo, such as superoxide dismutase (SOD), glutathione (GSH), and NAD(P)H: quinone oxidoreductase.[11,12] Research showed that the induction of cytochrome P450-2E1 (CYP2E1) was the material cause for the generation of ROS and oxidative stress.[13,14,15] The generated ROS could initiate lipid peroxidation, which directly damaged intracellular membranes and plasma. The production of reactive aldehydes negatively influences liver or other vital organs with profibrotic properties and potent proinflammatory.[16] Among various genes encoding for antioxidative proteins, haem oxygenase-1 (HO-1) has attracted particular interest, which could generate metabolites with many important biological activities.[17] Increasing evidence supported that many natural products extracted from food could induce the expression of HO-1, and represent enhanced resistance to various oxidative stresses.[18,19,20,21] These functional extracts have shown good potential on relieving alcohol-induced oxidative stress.
Soyasaponins are one kind of amphiphilic oleanane type triterpenoid glycoside isolated from soybeans (Glycine max) and other legumes, which have been generally classified into four groups: A, B, E, and DDMP (2,3-dihydro-2,5-dihydroxy-6-methyl-4Hpyran-4-one), according to their aglycones.[22] Recent studies reported that soyasaponins presented a wide range of health benefits, including memory impairment prevention, immune adjuvant activities, and cytoprotective and hepatoprotective effects.[23,22,23,24,25,26] Although crude soyasaponins-rich extract has been shown effective on acute alcohol-induced hepatotoxicity in mice,[27] the protective mechanism and monomer compound activities are still unknown, which limited their application in functional foods.
In this study, we chose soyasaponin Bb, the principal monomer compound among soyasaponins extracted from soybeans,[28] and investigated its effects on alcohol-induced oxidative stress in rat hepatocytes. The cell viability and antioxidant capacities were evaluated to determine the effects of soyasaponin Bb on the oxidative stress in BRL-3A cells exposed to alcohol. The expression level of HO-1 was measured to reveal the molecular mechanisms of soyasaponin Bb on alcohol-induced oxidative stress partially.
MATERIALS AND METHODS
Chemicals and reagents
Soyasaponin Bb (Ss-Bb, C48H78O18; molecular weight: 943.134; purity >98%) was provided by Chengdu Biopurify Phytochemicals Ltd. (Chengdu, PR China). The cell culture components and reagent grade chemicals used, streptomycin, penicillin, 3,4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT), Dulbecco's Modified Eagle's Medium, PBS, sodium pyruvate, dimethyl sulfoxide, nonessential amino acids, 2,7-dichlorofluorescin diacetate (DCFH-DA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Bicinchoninic acid protein assay kit and trypsin was purchased from Beyotime Company (Jiangsu, China). Fetal calf serum was purchased from HyClone Company (Logan, UT, USA). Sn(IV) protoporphyrin IX dichloride (SnPP), an inhibitor of HO-1, was obtained from Frontier Scientific (Logan, UT, USA). Deionized water was obtained using a NW10LVF water purification system (Heal Force, Hong Kong, China). Other reagents were of analytical grade. Alanine aminotransferase (ALT), malondialdehyde (MDA), SOD, and GSH kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China).
Cell culture and treatment
BRL 3A-immortalized rat hepatocytes were from Chinese Academy of Science (Shanghai, China), and were grown in Dulbecco's Modified Eagle's Medium culture medium, which is supplemented with 100 μg/mL streptomycin, 100 U/mL penicillin, and 10% fetal calf serum. The cells were incubated at 37°C and pH 7.4 in a 5% CO2 atmosphere. The inoculum density of BRL-3A was 2 × 105 cells/mL in 96-well plates. Ethanol was mixed with deionized water with a concentration of 250 mmol/L before being added to the cell culture.[29] The cells were preincubated with 0, 25, 50, and 100 μg/mL Ss-Bb for 24 h and then incubated with 0 or 250 mmol/L ethanol for 24 h.[26]
Cell viability assay and morphological analysis
The conversion of MTT to a purple color product by cellular mitochondria was used to evaluate cell viability. After a series of treatment, cells of each group were washed by sterile PBS three times and subsequently incubated with at a concentration of 5 mg/mL MTT for 3 h, and finally dissolved in dimethyl sulfoxide. The percent of cell viability was defined as the relative absorbance of Ss-Bb or ethanol-treated cells versus ethanol untreated cells at 570 nm. After the treatment, images of the cells of each group were taken with an AE 31 inverted phase contrast microscope (Motic, Shenzhen, China) equipped with a quick imaging system.
Reactive oxygen species determination
Intracellular production of ROS was measured by DCFH-DA fluorescence probe, which passively diffused into cells and produces a well-retained polar diol. The changes via the production of relative ROS in fluorescence were compared with the fluorescence of the corresponding control. After the treatment, cells of each group were incubated with 10 μmol/L DCFH-DA at 37°C for 30 min in the dark, and then washed three times with PBS. The cells were analyzed in a CytoFLEX flow cytometer (Beckman Coulter, Brea, California, USA) at excitation and emission wavelengths of 488 and 525 nm, respectively.
Measurements of the antioxidant status, intracellular oxidant, and liver function
To measure the level of GSH and MDA, and the activity of SOD, cells of each group were collected and these biochemical indicators were determined by commercially purchased kits for assessing the antioxidant status and intracellular oxidant. The determination of ALT level was used to evaluate liver function, and also detected by purchased assay kits.
Real-time PCR
Total RNA was purified from cells of each group with RNA Prep Pure Tissue Kit (TIANGEN, Beijing, China). Complementary DNA (cDNA) was synthesized through the purified RNA. PCR reaction was carried out in a 20 μL reaction mixture on a 96 real-time quantitative thermal block (Bioneer, Daejeon, Korea). The following sequences of HO-1 with CTGGAATGGAAGGAGATGCC and TCAGAACAGCCGCCTCTACCG, as well as β-actin with GAGATTACTGCCCTGGCTCCTAGC and GGCCGGACTCATCGTACTCCTGCTT, are primers used in this study. Comparative threshold (CT) cycle method was used to analyze the obtained data.
Western blotting assay
Western blot analyses of HO-1 were performed as described.[15] The optical density ratio of the protein product to β-actin was obtained and expressed as percentage or ratio of the control value in the figures.
Statistical analysis
Experiments of Western blotting analysis were repeated three times with similar trends. Data were analyzed using one-way analysis of variance. All results were expressed as mean ± SD. Differences were considered significant for P < 0.05, and all analyses were performed using version 13.0 SPSS for Windows (Chicago, IL, USA).
RESULTS
Cell viability and morphology
Cell viability assay suggested that approximately 60% of BRL 3A cells became inviable after 24 h exposure to ethanol [Figure 1]. After Ss-Bb pretreatment, 50 and 100 μg/mL Ss-Bb groups significantly increased the cell survival rate (P < 0.01), whereas 25 μg/mL Ss-Bb group only increased around 15% and did not obviously alter cell viability compared with the ethanol alone group.
Figure 1.
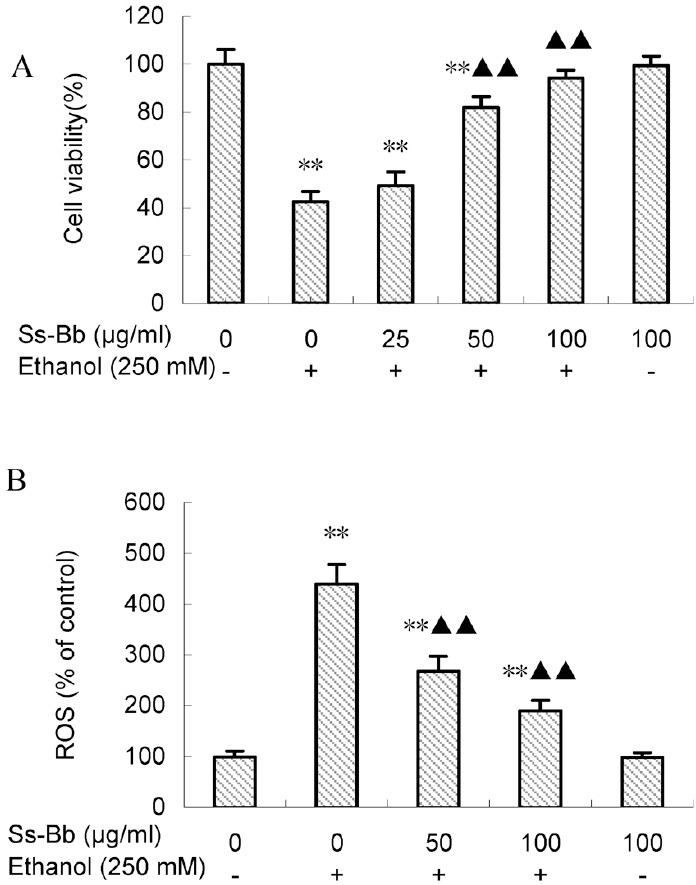
Cell viability (A) and ROS generation (B) of BRL 3A cells treated with ethanol and Ss-Bb. The cells were preincubated with 0, 25, 50, and 100 μg/mL Ss-Bb for 24 h and then incubated with 0 or 250 mmol/L ethanol for 24 h. Data are presented as mean ± SD of three independent experiments performed in triplicate. Significant difference: **P < 0.01, compared with the control. ▶▶P < 0.01, compared with the 250 mmol/L ethanol group
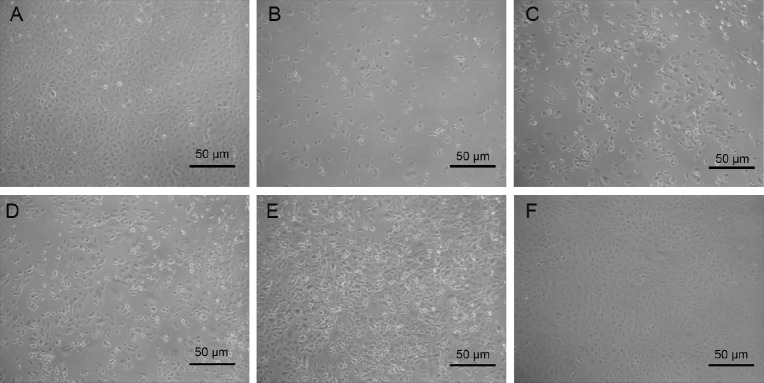
Phase contrast microscopic observations after exposure to 250 mmol/L ethanol revealed morphological changes showing loss of cell integrity, cytoplasmic shrinkage, and typical apoptotic features [Figure 2]. Pre-incubation with 25 μg/mL Ss-Bb had no significant effect on cell morphology compared with the ethanol alone group. Significant improved effects were observed in the cells with incubation concentrations of 50 and 100 μg/mL Ss-Bb before treatment with ethanol (P < 0.01). Most of the cells were restored to normal in both the groups. Synthesizing the results of cell viability and morphology, we selected 50 and 100 μg/mL as the incubation concentration for the following studies.
Figure 2.
Ethanol and Ss-Bb induce morphological changes in BRL 3A cells. The cells were preincubated with 0 μg/mL (A and B), 25 μg/mL (C), 50 μg/mL (D), and 100 μg/mL (E and F) Ss-Bb for 24 h and then incubated with 0 mmol/L (A and F) or 250 mmol/L (B–E) ethanol for 24 h. After the treatment, images were taken with an Motic inverted phase contrast microscope
Effects of soyasaponin bb on reactive oxygen species generation
The generation of ROS was expressed as the measured fluorescence intensity in cells of each group, and representative results are shown in Figure 1. Ethanol (250 mmol/L) significantly increased the level of ROS production compared with the control group (P < 0.01), whereas pre-incubation with 50 and 100 μg/mL Ss-Bb significantly reduced ROS generation induced by ethanol (P < 0.01). The two Ss-Bb pre-incubation groups still showed significant ROS increases compared with the control group (P < 0.01).
Effects of soyasaponin bb on antioxidant status, intracellular oxidant, and liver function
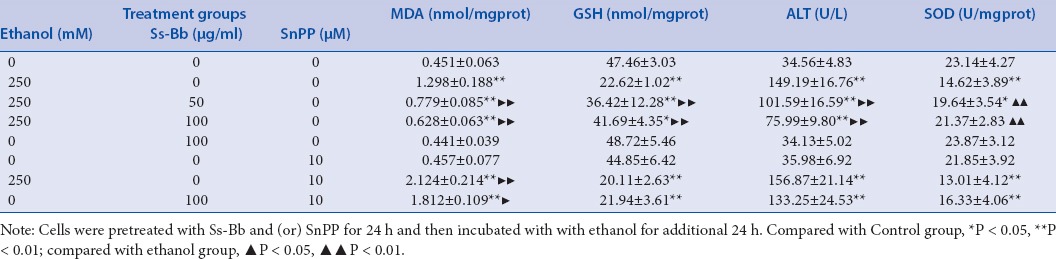
Levels of MDA and GSH and activities of SOD and ALT were evaluated in cells of each group to assess the antioxidant status, intracellular oxidant, and liver function [Table 1]. The MDA levels and ALT activities in the groups treated with 250 mmol/L ethanol were significantly higher than those in the control group (P < 0.01). The indices of MDA and ALT in the cells pre-incubated with 50 and 100 μg/mL Ss-Bb were remarkably lower than those in the cells treated with ethanol alone (P < 0.01). The results also suggested that 250 mmol/L ethanol significantly decreased GSH level and SOD activity (P < 0.01). Compared with the ethanol alone group, GSH levels and SOD activities significantly increased in the group pre-incubated with 50 and 100 μg/mL Ss-Bb (P < 0.01). To investigate whether HO-1, one of the most readily induced enzymatic antioxidants, was involved in the Ss-Bb-induced protection or not, HO-1 inhibitor (SnPP, 10 μmol/L) was used in this study. Results showed that SnPP did not only remarkably abrogate the protective effect of Ss-Bb, but also further promoted ethanol-induced GSH and SOD depletion, lipid peroxidation, and the leakage of ALT.
Table 1.
Effect of ethanol, Ss-Bb, and SnPP on MDA level, GSH level, SOD activity, and ALT activity of BRL 3A cells
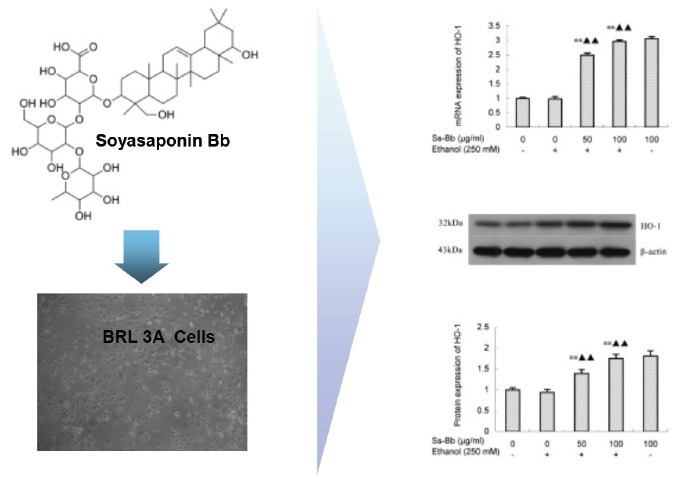
Effects of soyasaponin bb on the mRNA and protein expression levels of haem oxygenase-1
The expression levels of HO-1 in BRL 3A cells were investigated to explore the molecular mechanism of the protective effect of Ss-Bb against ethanol-induced oxidative stress [Figure 3]. There were no significant changes in mRNA and protein expression levels of HO-1 in ethanol exposed group compared with the control via the real-time PCR and Western blot assay. The administration of Ss-Bb significantly activated the expression of HO-1 (P < 0.01), particularly for 100 μg/mL dose of Ss-Bb increased 196.25 and 74.55% of the mRNA and protein expression compared with the ethanol alone group, respectively.
Figure 3.
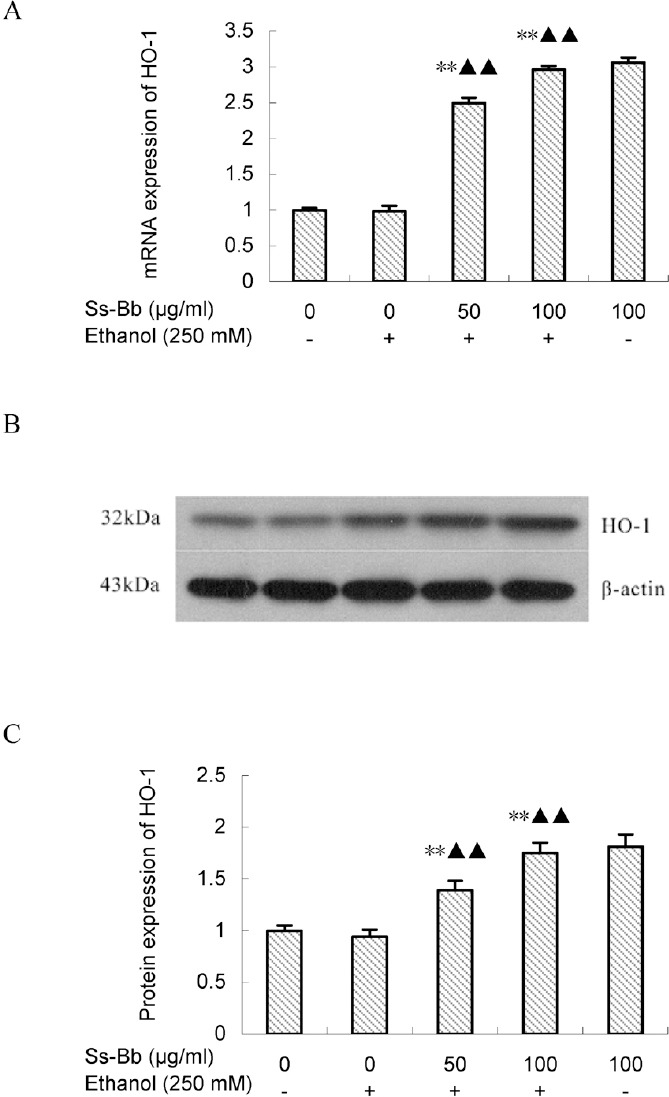
Effect of ethanol and Ss-Bb on mRNA (A, real-time PCR), and protein (B and C, Western blot analysis, with a same ordering) expression levels of HO-1 in BRL 3A cells. The cells were preincubated with 0, 25, 50, and 100 μg/mL Ss-Bb for 24 h and then incubated with 0 or 250 mmol/L ethanol for 24 h. Data are presented as mean ± SD of three independent experiments performed in triplicate. Significant difference: **P < 0.01, compared with the control. ▶▶P < 0.01, compared with the 250 mmol/L ethanol group
DISCUSSION
As a physiological disturbance in redox status, oxidative stress was closely associated with various pathological conditions.[9,10,30] Researchers have found that excessive ROS are produced during ethanol metabolism by catalase, ethanol dehydrogenase, and CYP2E1.[13,31] In this study, we found that Ss-Bb-reduced oxidative stress in BRL 3A rat hepatocytes exposed to ethanol. The apoptosis, morphological changes, impaired antioxidant system, and liver function caused by ethanol exposure were improved after Ss-Bb treatment. Furthermore, the administration of Ss-Bb increased the expression level of HO-1 in these hepatocytes.
As an important group of active ingredients in soybean, soyasaponins have been proved to exhibit various effects.[23,24,25,26] More importantly, other researchers have found that crude soyasaponins-rich extract showed protective effects on acute alcohol-induced hepatotoxicity in mice.[27] Therefore, it was reasonable to hypothesize that monomeric soyasaponin, such as Ss-Bb, protect against ethanol-induced hepatocyte injury through its antioxidant activity. To test this hypothesis, whether the administration of Ss-Bb could attenuate ethanol-induced apoptosis in rat hepatocytes was determined firstly, and the cell viability combined with morphology of three pre-incubated groups showed different improvement effects. Levels of oxidative stress markers and liver function, including the content of ROS, GSH and MDA, and the activities of SOD and ALT, were also tested to evaluate the protective effects. Results showed that Ss-Bb could not completely eliminate the generated ROS caused by ethanol exposure, although the antioxidant activity of the cells was significantly restored. It was suggested that the antioxidant capacity of Ss-Bb was not achieved only by the removal of ROS. HO-1, a stress-responsive protein, was ubiquitous and responsible for catalyzing the cleavage of prooxidative haem into bioactive products such as bilirubin, CO, and free iron.[32] Its activity could be considered as a possible candidate to initiate both anti-inflammatory and hepato-protective mechanisms.[33] In chronic inflammatory diseases, HO-1 played an important anti-inflammatory role, and it also presented antiproliferative properties and antiapoptotic.[34] In this study, the BRL 3A cells exposed to ethanol did not significantly downregulate the expression of HO-1, which showed a different result to the chronic ethanol ingestion in rat or some cell models.[35,36] It may be because of relatively short time of ethanol exposure (24 h) or different ethanol concentrations in cell treatment compared with zoopery. The differences in cell lines and sample treatments may also contribute to the changes on the ethanol sensitivity of the HO-1. However, pretreatment with SnPP, a pharmacological inhibitor of HO-1, markedly decreased antioxidant activity in cells, providing evidence that HO-1 induction is involved in the regulation of Ss-Bb. The mRNA and protein expression of HO-1 also obviously upregulated after in Ss-Bb pretreated groups. Therefore, the induction of HO-1 by Ss-Bb administration may contribute to its beneficial effects on alcohol-induced oxidative stress.
Financial support and sponsorship
This work was supported by the Cultivation Plan for Youth Agricultural Science and Technology Innovative Talents of Liaoning Province (2015001).
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62:S38–46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan J. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28:11–7. doi: 10.1111/jgh.12036. [DOI] [PubMed] [Google Scholar]
- 3.Huang CK, Yu T, Suzanne MM, Wands JR, Derdak Z, Kim M. Restoration of Wnt/β-catenin signaling attenuates alcoholic liver disease progression in a rat model. J Hepatol. 2015;63:191–8. doi: 10.1016/j.jhep.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee R, Mitra A. An overview of effective therapies and recent advances in biomarkers for chronic liver diseases and associated liver cancer. Int Immunopharmacol. 2015;24:335–45. doi: 10.1016/j.intimp.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Cao YW, Jiang Y, Zhang DY, Wang M, Chen WS, Su H. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J Ethnopharmacol. 2015;23:92–8. doi: 10.1016/j.jep.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Yu C, Mei XT, Zheng YP, Xu DH. Zn(II)-curcumin protects against hemorheological alterations, oxidative stress and liver injury in a rat model of acute alcoholism. Environ Toxicol Pharmacol. 2014;37:729–37. doi: 10.1016/j.etap.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Tamura A, Sasaki M, Yamashita H, Matsui-Yuasa I, Saku T, Hikima T. Yerba-mate (Ilex paraguariensis) extract prevents ethanol-induced liver injury in rats. J Funct Foods. 2013;5:1714–23. [Google Scholar]
- 8.Maiwulanjiang M, Chen J, Xin G, Gong AG, Miernisha A, Du CY. The volatile oil of Nardostachyos Radix et Rhizoma inhibits the oxidative stress-induced cell injury via reactive oxygen species scavenging and Akt activation in H9c2 cardiomyocyte. J Ethnopharmacol. 2014;153:491–8. doi: 10.1016/j.jep.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Shankar K, Mehendale HM. Reference module in biomedical sciences, from encyclopedia of toxicology. 3rd ed. Amsterdam Elsevier BV; 2014. Oxidative stress; pp. 735–7. [Google Scholar]
- 10.Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–75. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Lee HI, Yun KW, Seo KI, Kim MJ, Lee MK. Scopoletin prevents alcohol-induced hepatic lipid accumulation by modulating the AMPK–SREBP pathway in diet-induced obese mice. Metabolism. 2014;63:593–601. doi: 10.1016/j.metabol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Yeligar SM, Machida K, Kalra VK. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. J Biol Chem. 2010;285:35359–73. doi: 10.1074/jbc.M110.138636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M, Wang P, Zhu Y, Liu X, Hu X, Chen F. Blueberry anthocyanins extract inhibits acrylamide-induced diverse toxicity in mice by preventing oxidative stress and cytochrome P450 2E1 activation. J Funct Foods. 2015;14:95–101. [Google Scholar]
- 14.Zhang P, Ma D, Wang Y, Zhang M, Qiang X, Liao M. Berberine protects liver from ethanol-induced oxidative stress and steatosis in mice. Food Chem Toxicol. 2014;74:225–32. doi: 10.1016/j.fct.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Zhu L, Wu T, Zhang J, Jiao X, Liu X. Effects of triterpenoid from Schisandra chinensis on oxidative stress in alcohol-induced liver injury in rats. Cell Biochem Biophys. 2015;71:803–11. doi: 10.1007/s12013-014-0266-0. [DOI] [PubMed] [Google Scholar]
- 16.Tilg H, Moschen AR, Kaneider NC. Pathways of liver injury in alcoholic liver disease. J Hepatol. 2011;55:1159–61. doi: 10.1016/j.jhep.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Yao P, Li K, Song F, Zhou S, Sun X, Zhang X. Heme oxygenase-1 upregulated by Ginkgo biloba extract: potential protection against ethanol-induced oxidative liver damage. Food Chem Toxicol. 2007;45:1333–42. doi: 10.1016/j.fct.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Gao C, Shi Y, Zhu L, Hu X, Wang D. Quercetin attenuates ethanol-derived microsomal oxidative stress: implication of haem oxygenase-1 induction. Food Chem. 2012;132:1769–74. [Google Scholar]
- 19.Subramanian P, Anandan R, Jayapalan JJ, Hashim OH. Hesperidin protects gentamicin-induced nephrotoxicity via Nrf2/HO-1 signaling and inhibits inflammation mediated by NF-κB in rats. J Funct Foods. 2015;13:89–9. [Google Scholar]
- 20.Yu M, Wang D, Yang W, Xu M, Liu Y, Xu S. Mechanisms of Nrf2/HO-1 pathway up-regulation induced by pu-erh black tea extract supplementation for quinocetone-treated Sprague-Dawley rats. J Funct Foods. 2015;14:767–78. [Google Scholar]
- 21.Cheng HW, Lee KC, Cheah KP, Chang ML, Lin CW, Li JS. Polygonum viviparum L. inhibits the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through haem oxygenase-1 induction and activation of the Nrf2 pathway. J Sci Food Agric. 2013;93:491–7. doi: 10.1002/jsfa.5795. [DOI] [PubMed] [Google Scholar]
- 22.Guang C, Chen J, Sang S, Cheng S. Biological functionality of soyasaponins and soyasapogenols. J Agric Food Chem. 2014;62:8247–55. doi: 10.1021/jf503047a. [DOI] [PubMed] [Google Scholar]
- 23.Hong SW, Yoo DH, Woo JY, Jeong JJ, Yang JH, Kim DH. Soyasaponins Ab and Bb prevent scopolamine-induced memory impairment in mice without the inhibition of acetylcholinesterase. J Agric Food Chem. 2014;62:2062–8. doi: 10.1021/jf4046528. [DOI] [PubMed] [Google Scholar]
- 24.Sun T, Yan X, Guo W, Zhao D. Evaluation of cytotoxicity and immune modulatory activities of soyasaponin Ab: An in vitro and in vivo study. Phytomedicine. 2014;21:1759–66. doi: 10.1016/j.phymed.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Vila-Donat P, Fernández-Blanco C, Sagratini G, Font G, Ruiz MJ. Effects of soyasaponin I and soyasaponins-rich extract on the Alternariol-induced cytotoxicity on Caco-2 cells. Food Chem Toxicol. 2015;77:44–9. doi: 10.1016/j.fct.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Sun S, Zha D, Wu J, Mao L, Deng H. Soyasaponins prevent H2O2-induced inhibition of gap junctional intercellular communication by scavenging reactive oxygen species in rat liver cells. Nutr Cancer. 2014;66:1342–51. doi: 10.1080/01635581.2014.956245. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Dong C, Ren G. Effect of soyasaponins-rich extract from soybean on acute alcohol-induced hepatotoxicity in mice. J Agric Food Chem. 2011;59:1138–44. doi: 10.1021/jf103749r. [DOI] [PubMed] [Google Scholar]
- 28.Decroos K, Vincken JP, Koningsveld GA, Gruppen H, Verstraete W. Preparative chromatographic purification and surfactant properties of individual soyasaponins from soy hypocotyls. Food Chem. 2007;101:324–33. [Google Scholar]
- 29.Xiao J, Zhu Y, Liu Y, Tipoe GL, Xing F, So KF. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int J Biol Macromol. 2014;69:73–8. doi: 10.1016/j.ijbiomac.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lívero FA, Stolf AM, Dreifuss AA, Bastos-Pereira AL, Chicorski R, de Oliveira LG. The FXR agonist 6ECDCA reduces hepatic steatosis and oxidative stress induced by ethanol and low-protein diet in mice. Chem Biol Interact. 2014;217:19–27. doi: 10.1016/j.cbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Jie Q, Tang Y, Deng Y, Li Y, Shi Y, Gao C. Bilirubin participates in protecting of heme oxygenase-1 induction by quercetin against ethanol hepatotoxicity in cultured rat hepatocytes. Alcohol. 2013;47:141–8. doi: 10.1016/j.alcohol.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Bakhautdin B, Das D, Mandal P, Roychowdhury S, Danner J, Bush K. Protective role of HO-1 and carbon monoxide in ethanol-induced hepatocyte cell death and liver injury in mice. J Hepatol. 2014;61:1029–37. doi: 10.1016/j.jhep.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motterlini R, Haas B, Foresti R. Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs) Med Gas Res. 2012;2:28–40. doi: 10.1186/2045-9912-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng T, Zhang CL, Song FY, Zhao XL, Yu LH, Zhu ZP, Xie KQ. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim Biophys Acta. 2013;1830:4848–59. doi: 10.1016/j.bbagen.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Senthil Kumar KJ, Liao JW, Xiao JH, Gokila Vani M, Wang SY. Hepatoprotective effect of lucidone against alcohol-induced oxidative stress in human hepatic HepG2 cells through the up-regulation of HO-1/Nrf-2 antioxidant genes. Toxicol In Vitro. 2012;26:700–8. doi: 10.1016/j.tiv.2012.03.012. [DOI] [PubMed] [Google Scholar]