Abstract
Background:
Leishmaniasis and African trypanosomiasis are recognized as the leading causes of mortality and morbidity with the greatest prevalence in the developing countries. They affect more than one billion of the poorest people on the globe.
Objective:
To find a cheap, affordable, safe, and efficacious antileshmanial and antitrypanosomal natural drug and to elucidate its probable mode of action.
Materials and Methods:
Phytochemical investigation of the non-polar fraction of the methanol extract of leaves of Ochrosia elliptica Labill. (Apocyanaceae) resulted in the isolation of ursolic acid, which was unambiguously determined based on HR-ESI-FTMS, extensive 1D and 2D NMR spectroscopy. It was further tested for its cytotoxicity, antimicrobial, antimalarial, antileishmanial, and trypanocidal potency. in-silico molecular modeling studies were conducted on six vital parasitic enzymes including farnesyl diphosphate synthase, N-myristoyl transferase, pteridine reductase 1, trypanothione reductase, methionyl-tRNA synthetase, and inosine–adenosine–guanosine nucleoside hydrolase to discover its potential mode of action as antitrypanosomal and antileishmanial agent.
Results:
Ursolic acid displayed considerable antitrypanosomal and antileishmanial activities with IC50 values ranging between 1.53 and 8.79 μg/mL. It showed superior antitrypanosomal activity as compared to the standard drug difluoromethylornithine (DFMO), with higher binding affinities towards trypanothione reductase and pteridine reductase 1. It displayed free binding energy of -30.73 and -50.08 kcal/mole towards the previously mentioned enzymes, respectively. In addition, ursolic acid exhibited considerable affinities to farnesyl diphosphate synthase, N-myristoyl transferase and methionyl-tRNA synthetase with free binding energies ranging from -42.54 to -63.93 kcal/mole.
Conclusion:
Ursolic acid offers a safe, effective and cheap antitrypanosomal and antileishmanial candidate acting on several key parasitic enzymes.
SUMMARY
The fresh leaves of Ochrosia elleptica Labill., family Apocyanaceae are a reliable source of ursolic acid.
Ursolic acid displayed considerable antitrypanosomal and antileishmanial activities. It showed superior antitrypanosomal activity as compared to difluoromethylornithine (DFMO), potent antitrypanosomal reference drug.
In silico molecular modeling studies revealed that the antileishmanial and antitrypanosomal activities of ursolic acid could be partially explained in view of its multiple inhibitory effects on vital parasitic enzymes with the highest potency exerted in the inhibition of pteridine reductase 1 and trypanothione reductase.

Abbreviations used: AHT: African Human Trypanosomiasis, ATCC: American type cell culture, BuOH: n-butanol, DCM: dichloromethane, DFMO: difluoromethylornithine, EtOAc: ethyl acetate, FCS: fetal calf serum, HMBC: Heteronuclear Multiple Bond Correlation, HMQC: Heteronuclear Multiple-Quantum Correlation, HR-ESI-FTMS: High Resolution Electrospray ionozation Mass Spectrometry, MENA: Middle East and North Africa, MeOH: Methanol, MRSA: Methicillin-resistant Staphylococcus aureus, NTDs: Neglected tropical diseases, TLC: Thin layer chromatography, UA: Ursolic acid, UV: Ultra violet, WHO: World Health Organization.
Keywords: Antileishmanial, antitrypanosomal, Ochrosia elliptica, ursolic acid, virtual screening
INTRODUCTION
Neglected tropical diseases (NTDs) represent a massive disease collection mainly caused by parasitic organisms that seriously affect more than one billion of the poor people on the globe. It is booming in impoverished regions, thus perpetuate the cycle of poverty, causing mental retardation in children and forbidding socioeconomic achievements. The Middle East and North Africa (MENA) are highly endemic for several NTDs including malaria, leishmaniasis as well as African trypanosomiasis.[1]
Malaria is a mosquito-borne infectious disease caused mainly by Plasmodium falciparum resulting in unfavorable symptoms as headache, fatigue, fever and vomiting that if left untreated may severely progress to seizures and coma, which ultimately causes death.[2] It is notably responsible for almost one million deaths each year in sub-Saharan Africa[3]
Leishmaniasis is a disease caused by 20 different Leishmania species as Leishmania donovani promastigotes and spread by sand flies bites.[4] The World Health Organization (WHO) reported that there are around 12 million cases of leishmaniasis worldwide with two and half million new cases every year. It embraces three discriminant clinical features, which are generalized visceral infection, dermal ulceration and excessive development of the mucous membranes. Thus, curing is difficult owing to the intramacrophagic location of the infectious form, immune deficiency and malnutrition of patients suffering from the disease.[5,6]
Besides this, African Trypanosomiasis is a protozoan disease of man and livestock. Trypanosoma brucei is the causative agent of African Human Trypanosomiasis (AHT, sleeping sickness) that disseminates by tsetse fly. Anemia, fever accompanied by edema and enlargement of the lymph nodes and spleen are among the common manifestations of trypanosomiasis. Neurological symptoms occur in the late stages of the disease.[7]
The fact that people suffering from NTDs are of low-income, makes the evolution of novel medications unwelcomed by the private pharmaceutical companies. Thus, natural sources remain the favorable mine to find promising, safe and cheap candidates for the attenuation of these terrible health hazards. Additionally, in-silico molecular modeling is recently recognized as a modern and powerful tool to fasten the drug discovery from natural origin and to reduce its cost. Docking is used to predict the affinity and consequently the activity of small drug molecules to various protein targets. Furthermore, by initial prediction of the inactive ones, the total number of compounds, time, resources and efforts for further processing can be markedly reduced accompanied by dramatic elevation in the hit scores.[8]
Based on our recent strategy targeting the discovery of compounds of significant activity combating neglected tropical diseases, the non-polar fraction of methanol extract of leaves of Ochrosia elliptica Labill (family Apocyanaceae) was screened. The extract showed certain activity that encouraged further research on its chemical composition. In this study, we report the isolation and identification of ursolic acid from the non-polar fraction of methanol extract of leaves of Ochrosia elliptica and the assessment of its pharmacological activity on different aspects including cytotoxicity, antimicrobial, antimalarial, antileishmanial, and antitrypanosmal activities. In addition, virtual screening of ursolic acid together with pentamidine and difluoromethylornithine (DFMO), reference antiparasitic agents, was done on six vital parasitic enzymes; namely farnesyl diphosphate synthase, N-myristoyl transferase, pteridine reductase 1, trypanothione reductase, methionyl-tRNA synthetase and inosine–adenosine–guanosine nucleoside hydrolase in order to find out its probable mode of action.
MATERIALS AND METHODS
Plant material
Fresh leaves of Ochrosia elliptica Labill. family Apocyanaceae were collected in April, 2013 in El-Giza Zoo garden, Giza, Egypt. They were authenticated morphologically by Dr. Mohamed El-Gebaly, Department of Botany, National Research Centre (NRC), Giza, Egypt. The voucher specimens of the plant material were deposited at Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt given the identity (PHG-P- OE-171).
Extraction, isolation and identification
Five kilograms of the leaves were extracted with 10 liters of methanol (MeOH). The alcoholic extract was concentrated and the solvent-free residue (110 g) was suspended in distilled water then partitioned using different solvents n-hexane (1 L), dichloromethane (DCM, 8 L), ethyl acetate (EtOAc, 4 L) and n-butanol (BuOH, 4 L) to afford 7, 40, 12 and 38 g, respectively. The dichloromethane (DCM) fraction was subjected to column chromatography using silica gel (200 g) as stationary phase and gradually eluted with petroleum ether and then the polarity was increased by adding ethyl acetate in an increasing polarity in ratios (2:1; then 1:1; then 1:2) followed by ethyl acetate.
Fraction 3 (2.6 g), which was eluted with petroleum ether and ethyl acetate (1:1) was applied on a second silica gel (60 g) column and eluted with n-hexane with gradual addition of ethyl acetate to afford 83 sub-fractions. Sub-fractions (70–79, 941 mg), eluted with n-hexane: ethyl acetate (1:1), gave white crystals having no UV absorbance on TLC plates but giving a dark violet color upon spraying with vanillin/sulphuric reagent. Structural elucidation of these crystals was determined by further spectroscopic, HR-ESI-FTMS and exhaustive 1D and 2D NMR analyses.
Antimicrobial assay
The isolated compound was tested for antimicrobial activity against Staphylococcus aureus ATCC 29213, methicillin-resistant Staphylococcus aureus ATCC 33591 (MRSA), Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Mycobacterium intracellulare ATCC 23068, Candida albicans ATCC 90028, Candida glabrata ATCC 90030, Candida krusei ATCC 6258, Cryptococcus neoformans ATCC 90113, and Aspergillus fumigatus ATCC 204305.[9] Ciprofloxacin and amphotericin B were used as positive controls for bacteria and fungi, respectively.
Antimalarial assay
The isolated compound was tested for antimalarial activity in vitro against chloroquine sensitive (D6, Sierra Leone) and resistant (W2, Indo China) strains of Plasmodium falciparum by measuring plasmodial LDH activity as described earlier.[10] Chloroquine was used as positive control. In order to determine the selectivity index of antimalarial activity, in vitro cytotoxicity to mammalian cells was also determined against Vero cells (monkey kidney fibroblasts)obtained from the American Type Culture Collection CCL-81 (ATCC, Rockville, MD).[9]
Antileishmanial assay
Assays with macrophage-amastigotes models are considered closest to the pathophysiological conditions of leishmaniasis, and are therefore the most appropriate for in vitro screening. Differentiated, non-dividing human acute monocytic leukemia cells (THP1) make an attractive alternative to isolated primary macrophages and can be used for assaying antileishmanial activity of different compounds against intracellular amastigotes. A parasite rescue and transformation assay with differentiated THP1 cells infected in vitro with Leishmania donovani for screening ursolic acid and determining the efficacy against the intracellular Leishmania amastigotes. The assay involves the following steps differentiation of THP1 cells to non-dividing macrophages, followed by infection of macrophages with L. donovani metacyclic promastigotes, then treatment of infected cells with ursolic acid. Finally, controlled lysis of infected macrophages to release/rescue of amastigotes and transformation of live amastigotes to promastigotes was done. The assay was optimized using detergent treatment for controlled lysis of Leishmania-infected THP1 cells to achieve almost complete rescue of the viable intracellular amastigotes with minimal effect on their ability to transform to promastigotes. Different macrophage: promastigotes ratios were tested to achieve maximum infection. Quantification of the infection was performed through transformation of live rescued Leishmania amastigotes to promastigotes and evaluation of their growth by the Alamar Blue fluorometric assay in 96-well microplates. This assay is comparable to the currently used microscopic, transgenic reporter gene and digital image analysis assays. This assay is robust and measures only the live intracellular amastigotes compared to reporter gene and image analysis assays, which may not differentiate between live and dead amastigotes. Also, the assay has been validated with a current panel of antileishmanial drugs and has been successfully applied to large-scale screening of pure compounds and a library of natural products fractions.[11,12] Pentamidine and amphotericin B were used as control drugs.
Trypanocidal assay
Trypomastigotes of Trypanosoma brucei Squib-427 strain (suramin-sensitive) were cultured at 37° C and 5% CO2 in HMI-9 medium[13] (supplemented with 10% fetal calf serum (FCS). Pentamidine and diflouromethylornithine (DFMO) were used as control drugs. The reaction was read at 540 nm and absorbance values were expressed as a percentage of the blank control.[14]
Molecular modeling studies
in-silico molecular modeling of ursolic acid isolated from the non-polar fraction of methanol extract of Ochrosia elliptica leaves together with pentamidine and DFMO, reference drugs, were carried out on six crucial parasitic enzymes using Discovery Studio 2.5 software (Accelrys Inc., San Diego, CA, USA) applying C-Docker protocol. These enzymes are involved in various metabolic processes of Leishmania donovani and Trypanosoma brucei and are required for their survival. The X-ray crystal structure of the enzymes farnesyl diphosphate synthase (PDB ID 4JZB), N-myristoyl transferase (PDB ID 2WUU) and pteridine reductase 1 (PDB ID 2QHX) serving as antileishmanial targets and trypanothione reductase (PDB ID 4NEV), methionyl-tRNA synthetase (PDB ID 4EG4) and inosine–adenosine–guanosine nucleoside hydrolase (PDB ID 4I72) acting as antitrypanosomal targets cocrystallized with their lead compounds were downloaded from protein data bank (www.pdb.org).
The structures of the enzymes were established using the default protein preparation protocol of Accelry's discovery studio 2.5 (Accelrys® Inc., San Diego, CA, USA). First, the proteins were prepared by adding hydrogen atoms and cleansing them from any unwanted interactions. Then, determination of the binding site was achieved via detection of the binding mode of bioactive conformation of the reported lead compounds co-crystallized with the targeted enzymes.[15] Following the binding site determination, the structures of the compounds were docked inside the binding site using C-Docker protocol. CHARM force field was assigned and the binding energies for the selected docking poses were calculated applying the following equation:
ΔGbinding=Ecomplex–(Eenzyme+Eligand)
where; ΔGbinding is the ligand–protein interaction binding energy, Ecomplex is the potential energy for the complex of protein bound with the ligand, Eenzyme is the potential energy of the protein alone, and Eligand is the potential energy for the ligand alone.
RESULTS
Ursolic acid was isolated from the leaves of Ochrosia elliptica cultivated in Egypt as a white crystalline solid. The molecular formula was determined to be C30H48O3 based on the HR-ESI-FTMS revealing a molecular ion peak at m/z 455.35271 [M-H]- (calculated for C30H47O3, 455.35252). Based on the molecular formula, the degrees of unsaturation were found to be seven indicating that the isolated compound is probably a pentacyclic triterpenoidal metabolite. Further confirmation was provided by 13C NMR and DEPT experiment [Table 1] revealing the presence of 30 carbon resonances distinguished into seven methyl resonances (δc 15.3, 16.1, 16.9, 17.0, 21.1, 23.3, and 28.3 ppm), nine methylene moieties (δc 18.0, 22.9, 23.9, 27.0, 27.6, 30.2, 32.7, 36.4, and 38.8 ppm), seven methine carbons (δc 38.5, 38.6, 47.1, 52.4, and 54.8 ppm) in addition to two carbon resonances at δc 76.9 and 124.6 ppm representing one oxygenated aliphatic (C-3) and one olefinic (C-12) carbons, respectively, together with seven quaternary carbons (δc 36.6, 38.4, 40.2, 41.7, 46.9, 138.2, and 178.3 ppm). The appearance of δc 124.6 and 138.2 ppm indicate the presence of a double bond. The most downfield signal at δc 178.3 indicates the carboxylic function.
Table 1.
1H and 13C NMR data for ursolic acid in pyridine-d5
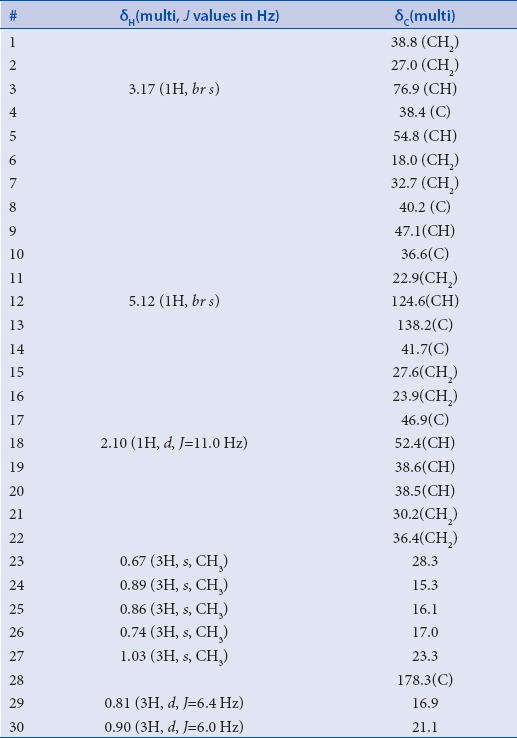
In 1H NMR spectrum, seven methyl resonances can be identified into five singlets at δH 0.67, 0.74, 0.86, 0.89 and 1.03 ppm together with two doublets at δH0.81 (3H, J value = 6.4 Hz) and 0.90 (3H, J value = 6.0 Hz). In addition, 1H NMR spectrum revealed two resonances at δH5.12 and 3.17 ppm which displayed in HMQC spectrum C–H correlations to δc 124.6 (C-12) and 76.9 (C-3), respectively. HMBC spectrum exhibited long range correlations from a singlet methyl resonance (Me-27) at δH 1.03 ppm to four carbon resonances at δc40.2 (C-8), 41.7 (C-14), 27.6 (C-15) and 138.2 (C-13) ppm.
Furthermore, HMBC spectrum revealed long range correlations between another singlet methyl resonance (Me-26) at δH 0.74 ppm and four carbon resonances at δc32.7 (C-7), 40.2 (C-8), 41.7 (C-14) and 47.1 (C-9) ppm. Two singlet methyl resonances at δH 0.67 and 0.89 ppm showed long range correlations to three carbon resonances in common at δc38.4 (C-4), 54.8 (C-5) and 76.9 (C-3) ppm which supports that those two methyls are attached to the same carbon neighboring the oxygenated carbon at C-3. Based on the aforementioned data and by comparison with the reported literature, the major component in the investigated methanol extract of the leaves of Ochrosia elliptica was confirmed to be ursolic acid [Figure 1].[16] Ursolic acid was subjected to antimicrobial activity assay against a panel of Gram positive, Gram negative and fungal strains. Results disclosed that ursolic acid doesn’t possess a potential antimicrobial activity against any of the tested microorganisms proven by the IC50 values, which were found to be more than 44 µg/mL.
Figure 1.
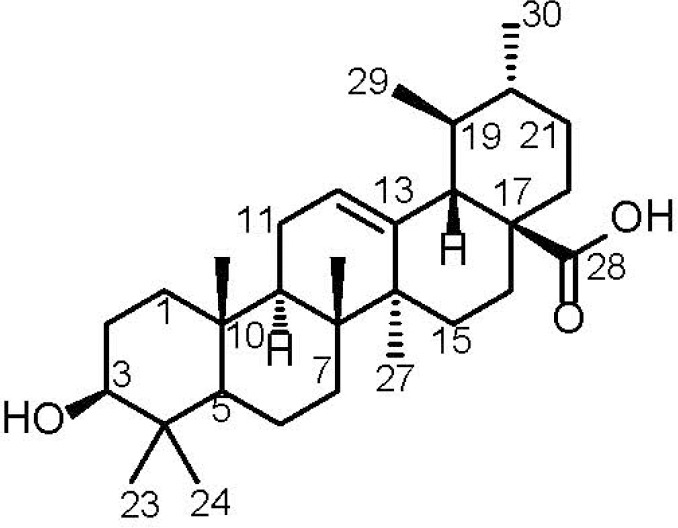
Chemical structure of ursolic acid (UA)
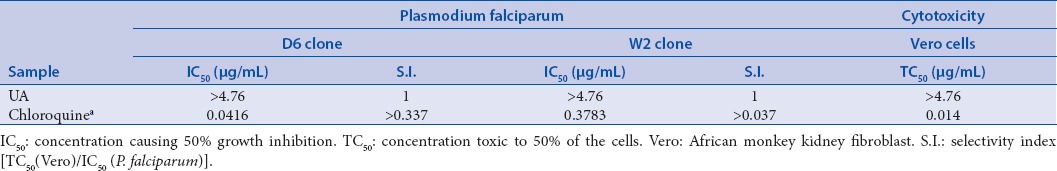
Ursolic acid was also assessed for its cytotoxicity against Vero cells (African monkey kidney fibroblasts); and antimalarial activity against D6 (chloroquine sensitive) and W2 (chloroquine resistant) strains of Plasmodium falciparum. Results [Table 2] revealed that ursolic acid has IC50 in both assays > 4.76 µg/mL with a selectivity index of 1 to both malarial strains in comparison to chloroquine (IC50 ranges from 0.0416 to 0.3783μg/mL). Thus, ursolic acid is considered as a weak antimalarial agent.
Table 2.
Antimalarial and cytotoxic activity of ursolic acid (UA)
Antileishmanial activity of ursolic acid was conducted against Leishmania donovani promastigotes, amastigotes and amastigotes with human acute monocytic leukemia cells (THP1). Ursolic acid [Table 3] exhibited a moderate activity against promastigotes and amastigotes with THP1 cells with IC50 values of 5.80 and 8.79 µg/mL, respectively, compared to pentamidine with IC50 of 1.363 and 2.156 µg/mL and amphotericin B 0.213 and 0.547 μg/mL. In trypanocidal activity assay against Trypanosoma brucei [Table 4], ursolic acid revealed its superior inhibitory activity with IC50 value of 1.53 µg/mL compared to DFMO, the standard antitrypanosomal agent that exhibited IC50 = 3.386 μg/mL and pentamidine (IC50 = 1 ng/mL).
Table 3.
Antileishmanial activity of ursolic acid (UA)
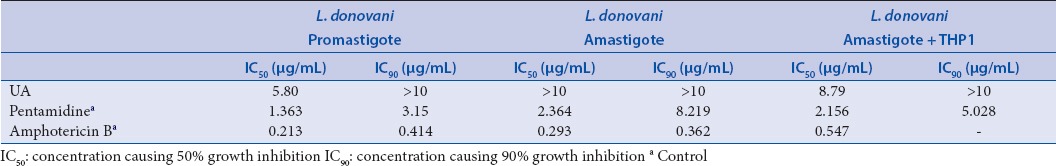
Table 4.
Trypanocidal activity of ursolic acid against T. brucei (UA)

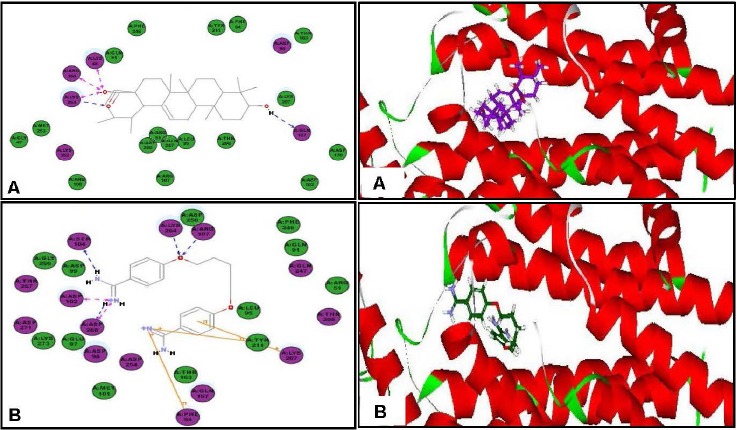
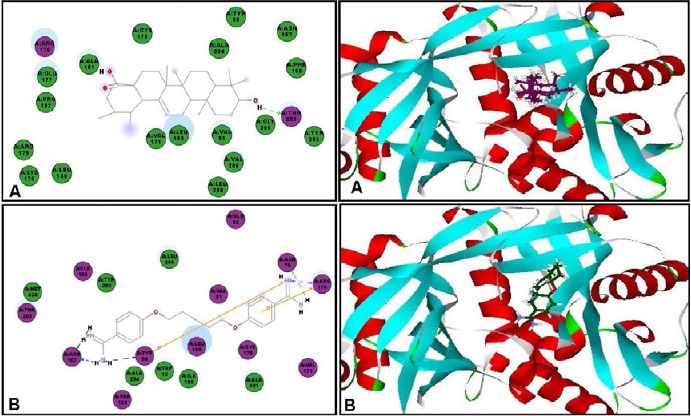
In an effort to find out the probable mode of action of ursolic acid as antileishmanial and antitrypanosomal agent, virtual screening of ursolic acid together with pentamidine and DFMO, reference antiparasitic agents, was done on six vital parasitic enzymes. Regarding the antileishmanial activity, molecular docking was performed within the active sites of farnesyl diphosphate synthase, involved in sterol, isoprenoid biosynthesis as well as protein prenylation in trypanosomatid parasites,[17] and N-myristoyl transferase that is involved in the catalysis of the attachment of the 14-carbon saturated fatty acid, myristate, to the amino-terminal glycine residue of eukaryotic proteins that are important in various cellular processes.[18] Ursolic acid showed moderate inhibition to both enzymes and approaching that of pentamidine as evidenced from the free binding energies [Table 5]. The moderate inhibitory activity of ursolic acid to the enzyme could be attributed to the formation of four strong hydrogen bonds with the amino acid residues Arg360, Gln167, Lys48 and Lys264 present at the active site of the first enzyme. However, for the latter, one hydrogen bond with Thr203 in addition to many hydrophobic interactions with many residues at the active site are formed as shown by the two dimensional binding diagrams [Figure 2 and Figure 3]. Moreover, regarding pteridine reductase 1, an important enzyme reducing pteridines (pterins and folates) required for growth,[19] both ursolic acid and pentamidine exerted an outstanding inhibitory activity with high fitting scores comparable to the original ligand co-crystallized with the enzyme. This prominent activity relied upon the formation of three hydrogen bonds with the Tyr191, Tyr194 and Arg17 residues in addition to many hydrophobic interactions with many amino acid residues at the active site [Figure 4].
Table 5.
Free binding energies (ΔGbinding) of ursolic acid (UA) to several parasitic enzymes using molecular modeling experiment
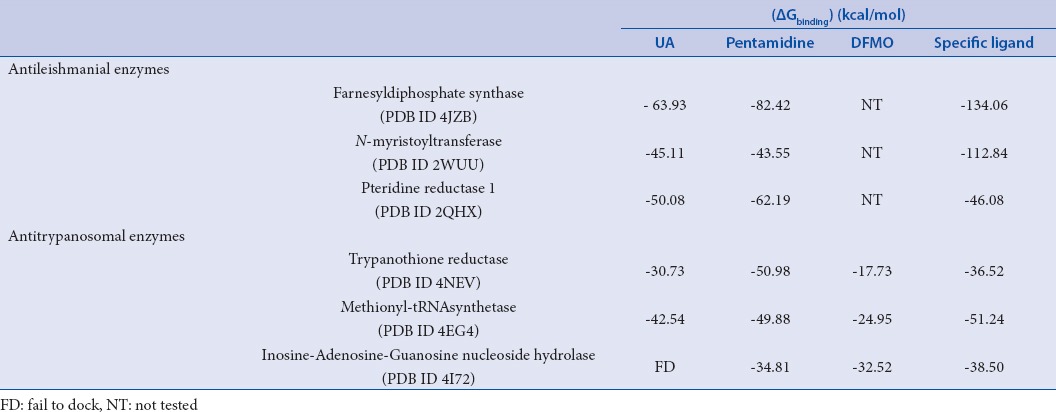
Figure 2.
Two-dimensional and 3-D binding modes of ursolic acid (A) and pentamidine (B) within farnesyl diphosphate synthase active sites
Figure 3.
Two-dimensional and 3-D binding modes of ursolic acid (A) and pentamidine (B) within N-myristoyl transferase active sites
Figure 4.
Two-dimensional and 3D binding modes of ursolic acid (A) and pentamidine (B) within pteridine reductase1active sites
Concerning the antitrypanosomal potency, ursolic acid showed a notable inhibitory activity within the active sites of trypanothione reductase and methionyl-tRNA synthetase exhibiting free binding energies (ΔG) of -30.73 and -42.54 kcal/mole, respectively exceeding the activity of DFMO (a reference antitrypanosomal agent) that showed free binding energies (ΔG) of -17.73 and -24.95 kcal/mole on the previously mentioned enzymes, respectively. Trypanothione reductase is a flavoprotein oxidoreductase present in the parasites and equivalent to glutathione reductase,[20] thus its inhibition is recognized as a potential strategy in the treatment of various parasitic infections, whereas, methionyl-tRNA synthetase plays a significant role in protein synthesis and cell survival.[21]
The formation of two hydrogen bonds with Gly112 and Asp116 in the active site of trypanothione reductase and with Lys292 at the active site of methionyl-tRNA synthetase may be responsible for this significant inhibitory activity [Figure 5 and Figure 6]. On the contrary, ursolic acid failed to dock within the active site of inosine–adenosine–guanosine nucleoside hydrolase, responsible for purine uptake[22] showing no inhibition to the enzyme.
Figure 5.
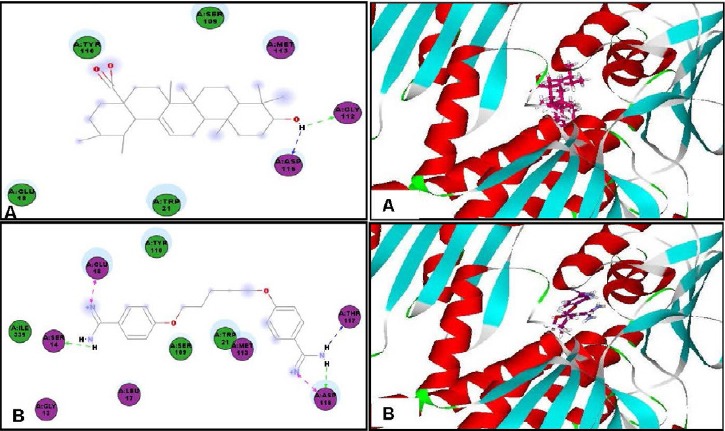
Two-dimensional and 3D binding modes of ursolic acid (A) and pentamidine (B) within Trypanothione reductase active sites
Figure 6.
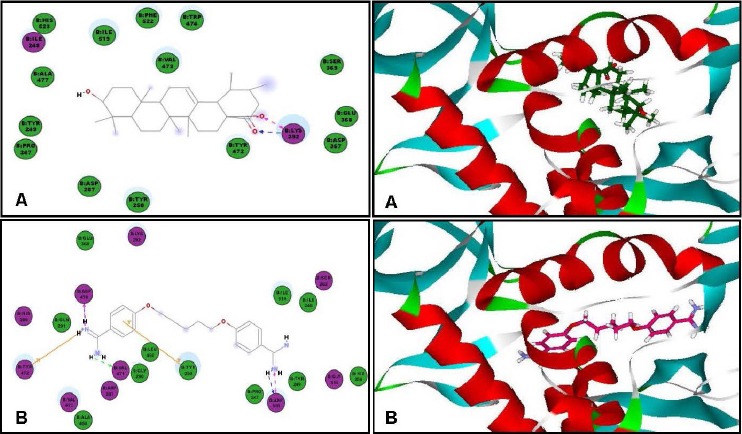
Two-dimensional and 3D binding modes of ursolic acid (A) and pentamidine (B) within methionyl-tRNA synthetase active sites
DISCUSSION
Triterpenoids have been reported in a variety of plants. Ursolic acid was isolated from leaves of Ochrosia elliptica Labill. cultivated in Egypt in a considerable amount and a pure form and its structural elucidation was performed based on HR-ESI-FTMS, extensive 1D and 2D NMR spectroscopy, and comparison of the measured data to the reported literature.
Ursolic acid was previously reported to have analgesic and anti-inflammatory activities.[23] Also it exhibited antitumour,[24,25] antioxidant,[26] antidiabetic,[27] and hepatoprotective activities as well.[28] Previous studies had also showed its antibacterial activity but tested at higher concentration reaching 64mg/L against Gram positive and showed minor or no activity against Gram negative bacteria.[29] Another study was conducted to derivatize ursolic acid to increase its antimicrobial activity.[30] Also ursolic acid is considered a potential therapeutic agent for Alzheimer's disease by its ability to inhibit the β-amyloid protein.[31] In our study, we assessed ursolic acid against a diverse panel of bioactivities such as cytotoxicity, antimicrobial, antimalarial, antileishmanial and trypanocidal activities.
Among the tested activities, ursolic acid had displayed considerable activity in both antitrypanosomal and antileishmanial assays with IC50 values ranging between 1.53 and 8.79 µg/mL. It revealed its superior inhibitory activity compared to DFMO, the standard antitrypanosomal agent that exhibited IC50 = 3.386 μg/mL and pentamidine (IC50 = 1 ng/mL).
Antimonials and pentamidine[32] are the available treatment for leishmaniasis; both drugs have reported many side effects; antimonials is really toxic, expensive and painful and may cause hepatic or cardiac toxicity during therapy, whereas pentamidine causes nausea vomiting, hypoglycemia and hypotension. DFMO toxicity was shown in the form of hearing loss and thrombocytopenia[33] and it is considered antitrypanosomal drug. With the aim of searching for a safe drug that has no or little side effects, our natural source of plants is still the main mine to dig into. In order to avoid these side effects that result from the consumption of the previously mentioned synthetic drugs, ursolic acid might be a drug that helps to overcome these side effects. There is no recorded toxicity for ursolic acid that might urge scientists to carry out further study on its clinical use.[34]
Besides, the results of virtual screening performed on six targeted parasitic enzymes revealed that the antileishmanial and antitrypanosomal activities of ursolic acid could be partially explained in view of its multiple inhibitory effects on various parasitic enzymes with the highest potency exerted in the inhibition of pteridine reductase 1 and trypanothione reductase, respectively. Notable inhibition to farnesyl diphosphate synthase, N-myristoyl transferase and methionyl-tRNA synthetase was recognized as displayed from their two and three dimensional patterns as well as their free binding energies at the active sites approaching the efficacy of pentamidine, the antiparasitic reference drug and exceeding that of DFMO, the standard antitrypanosomal agent that further supports the in vitro assessments.
CONCLUSION
From the current study, it was concluded that the fresh leaves of Ochrosia elliptica Labill., family Apocyanaceae are a reliable source of ursolic acid. Furthermore, ursolic acid exhibited a potent antitrypanosomal and antileishmanial activities approaching that of pentamidine and exceeding that of DFMO (reference drugs). This may be due to its notable inhibition to various vital enzymes that are important in the parasitic survival. Furthermore, in vitro followed by in vivo mechanistic assays should be done to assure the claimed modes of action as initially predicted from the virtual screening.
Financial support and sponsorship
We are grateful to the Egyptian Government and the National Center for Natural Products Research, The University of Mississippi, School of Pharmacy, Mississippi, USA for their financial support.
Conflicts of interest
There is no conflict of interests.
ABOUT AUTHOR

Rola M. Labib
Dr. Rola M. Labib, got B.S. degree in Pharmacy 1994, M.S. in Pharmacognosy 2001 and Ph.D. degree in Pharmacognosy and Phytochemistry 2008 from Faculty of Pharmacy, Ain Shams University, Cairo, Egypt. She got a postdoctoral fellowship 2014 at the National Center for Natural Products Research, University of Mississippi, USA and worked under the supervision of Dr Samir Ross. She was promoted to Associate Professor of Pharmacognosy 2015.
Acknowledgement
We are thankful to Dr. Babu Tekwani, Dr. Shabana Khan and Dr. Melissa Jacob, National Center for Natural Products Research, the University of Mississippi, School of Pharmacy, Mississippi, USA for the antileishmanial, antimalarial and antimicrobial assays. The content is solely the responsibility of the authors and does not necessarily represent the official views of the aforementioned institutions.
REFERENCES
- 1.McDowell MA, Rafati S. Neglected tropical diseases-Middle East and North Africa. New York (NY): Springer-Verlag Wien; 2014. [Google Scholar]
- 2.Caraballo H, King K. Emergency department management of mosquito-borne illness: malaria, dengue, and West Nile virus. Emerg Med Pract. 2014;16:1–23. [PubMed] [Google Scholar]
- 3.Gilbert I. Neglected diseases and drug discovery. 2012. In: Michael J, editor. Palmer and Timothy NC Wells. Darmstadt: Wiley-VCH Verlag GmbH & Co; 2012. [Google Scholar]
- 4.Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175–96. doi: 10.1093/bmb/lds031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha L, Almeida J, Macedo R, Barbosa-Filho J. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–35. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho PB, Arribas MAG, Ferreira EI. Leishmaniasis? Braz J Pharm Sci. 2000;36:69–96. [Google Scholar]
- 7.Mergia E, Shibeshi W, Terefe G, Teklehaymanot T. Evaluation of in vivo antitrypanosomal activity of aqueous and methanol leaf extracts of Clutia abyssinica (Euphorbiaceae) against Trypanosoma congolense. Nat Prod Chem Res. 2014;2:138. doi: 10.4172/2329-6836.1000138. [Google Scholar]
- 8.Rhoades JA, Peterson YK, Zhu H-J, Appel DI, Peloquin CA, Markowitz JS. Prediction and in vitro evaluation of selected protease inhibitor antiviral drugs as inhibitors of carboxylesterase 1: a potential source of drug-drug interactions. Pharm Res. 2012;29:972–82. doi: 10.1007/s11095-011-0637-9. [DOI] [PubMed] [Google Scholar]
- 9.Bharate SB, Khan SI, Yunus NA, Chauthe SK, Jacob MR, Tekwani BL. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg Med Chem. 2007;15:87–96. doi: 10.1016/j.bmc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–10. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 11.Bharate SB, Khan SI, Tekwani BL, Jacob M, Khan IA, Singh IP. S-Euglobals: Biomimetic synthesis, antileishmanial, antimalarial, and antimicrobial activities. Bioorg Med Chem. 2008;16:1328–36. doi: 10.1016/j.bmc.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 12.Jain SK, Sahu R, Walker LA, Tekwani BL. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J Vis Exp. 2012;70:4054. doi: 10.3791/4054. doi: 10.3791/4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75:985–89. [PubMed] [Google Scholar]
- 14.Manda S, Khan SI, Jain SK, Mohammed S, Tekwani BL, Khan IA. Synthesis, antileishmanial and antitrypanosomal activities of N-substituted tetrahydro-β-carbolines. Bioorg Med Chem Lett. 2014;24:3247–50. doi: 10.1016/j.bmcl.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 15.El-Ahmady SH, Ashour ML, Wink M. Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J Essent Oil Res. 2013;25:475–81. [Google Scholar]
- 16.Babalola IT, Shode FO. Ubiquitous ursolic acid: a potential pentacyclic triterpene natural product. J Pharmacogn Phytochem. 2013;2:214–22. [Google Scholar]
- 17.Aripirala S, Gonzalez-Pacanowska D, Oldfield E, Kaiser M, Amzel LM, Gabelli SB. Structural and thermodynamic basis of the inhibition of Leishmania major farnesyl diphosphate synthase by nitrogen-containing bisphosphonates. Acta Crystallogr Sect D-Biol. Crystallogr. 2014;70:802–10. doi: 10.1107/S1399004713033221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brannigan JA, Smith BA, Yu Z, Brzozowski AM, Hodgkinson MR, Maroof A. N-myristoyltransferase from Leishmania donovani: structural and functional characterisation of a potential drug target for visceral leishmaniasis. J Mol Biol. 2010;396:985–99. doi: 10.1016/j.jmb.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavazzuti A, Paglietti G, Hunter WN, Gamarro F, Piras S, Loriga M. Discovery of potent pteridine reductase inhibitors to guide antiparasite drug development. Proc Natl AcadSci. 2008;105:1448–53. doi: 10.1073/pnas.0704384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tovar J, Wilkinson S, Mottram JC, Fairlamb AH. Evidence that trypanothione reductase is an essential enzyme in Leishmania by targeted replacement of the tryA gene locus. Mol Microbiol. 1998;29:653–60. doi: 10.1046/j.1365-2958.1998.00968.x. [DOI] [PubMed] [Google Scholar]
- 21.Deniziak MA, Barciszewski J. Methionyl-tRNA synthetase. Acta Biochim Pol. 2001;48:337–50. [PubMed] [Google Scholar]
- 22.Giannese F, Berg M, Van der Veken P, Castagna V, Tornaghi P, Augustyns K. Structures of purine nucleosidase from Trypanosoma brucei bound to isozyme-specific trypanocidals and a novel metalorganic inhibitor. Acta Crystallogr Sect D-Biol. Crystallogr. 2013;69:1553–66. doi: 10.1107/S0907444913010792. [DOI] [PubMed] [Google Scholar]
- 23.Vasconcelos MAL, Royo VA, Ferreira DS, Crotti AEM, Carvalho JCT, Bastos JK. In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae), Z. Naturforsch. C. 2006;61:477–82. doi: 10.1515/znc-2006-7-803. [DOI] [PubMed] [Google Scholar]
- 24.Saraswati S, Agrawal SS, Alhaider Abdulqader A. Ursolic acid inhibits tumor angiogenesis and induces apoptosis through mitochondrial-dependent pathway in Ehrlich ascites carcinoma tumor. Chem Biol Interact. 2013;206:153–65. doi: 10.1016/j.cbi.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Son H-S, Kwon HY, Sohn EJ, Lee J-H, Woo H-J, Yun M. Activation of AMP-activated protein kinase and phosphorylation of glycogen synthase kinase3b mediate ursolic acid induced apoptosis in HepG2 liver cancer cells. Phytother Res. 2013;27:1714–22. doi: 10.1002/ptr.4925. [DOI] [PubMed] [Google Scholar]
- 26.Ma J-Q, Ding J, Xiao Z-H, Liu C-M. Ursolic acid ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney by inhibiting the STAT3 and NF-kB activities. Int. Immunopharmacol. 2014;21:389–95. doi: 10.1016/j.intimp.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Alqahtani A, Hamid K, Kam A, Wong KH, Abdelhak Z, Razmovski-Naumovski V. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr Med Chem. 2013;20:908–31. [PubMed] [Google Scholar]
- 28.Sultana T, Rashid MA, Ali MA, Mahmood SF. Hepatoprotective and antibacterial activity of ursolic acid extracted from Hedyotis corymbosa L. Bangladesh. J Sci Ind Res. 2010;45:27–34. [Google Scholar]
- 29.Fontanay S, Grare M, Mayer J, Finance C, Duval RE. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J Ethnopharmacol. 2008;120:272–76. doi: 10.1016/j.jep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Zhao L-X, Yang J-W, Zheng C-J, Tang Y, Wang Z-W, Zhao C-H. Synthesis and characterization of ursolic acid derivatives, and the study on the antibacterial activity. J Liaoning Normal University (Natural Science Edition) 2012;35:358–63. [Google Scholar]
- 31.Wilkinson K, Boyd JD, Marcie G, Moore KJ, El Khoury J. A high content drug screen identifies ursolic acid as an inhibitor of amyloid b protein interactions with its receptor CD36. J Biol Chem. 2011;286:34914–22. doi: 10.1074/jbc.M111.232116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fat L A, Esther J, Vrede M A, Soetosenojo R M, Fat LA, Rudy F. Pentamidine, the drug of choice for the treatment of cutaneous leishmaniasis in Surinam. Int J Dermatol. 2002;41:796–800. doi: 10.1046/j.1365-4362.2002.01633.x. [DOI] [PubMed] [Google Scholar]
- 33.MeyskensJr FL, Kingsley EM, Glattke T, Loescher L, Booth A. A phase II study of α-difluoromethylornithine (DFMO) for the treatment of metastatic melanoma. Invest New Drugs. 1986;4:257–62. doi: 10.1007/BF00179593. [DOI] [PubMed] [Google Scholar]
- 34.Kashyap D, Tuli HS, Sharma AK. Ursolic acid (UA): a metabolite with promising therapeutic potential. Life Sci. 2016;146:201–13. doi: 10.1016/j.lfs.2016.01.017. [DOI] [PubMed] [Google Scholar]


