Abstract
Background
Psoroptic mange is a chronic, refractory, contagious and infectious disease mainly caused by the mange mite Psoroptes ovis, which can infect horses, sheep, buffaloes, rabbits, other domestic animals, deer, wild camels, foxes, minks, lemurs, alpacas, elks and other wild animals. Features of the disease include intense pruritus and dermatitis, depilation and hyperkeratosis, which ultimately result in emaciation or death caused by secondary bacterial infections. The infestation is usually transmitted by close contact between animals. Psoroptic mange is widespread in the world. In this paper, the transcriptome of P. ovis is described following sequencing and analysis of transcripts from samples of larvae (i.e. the Pso_L group) and nymphs and adults (i.e. the Pso_N_A group). The study describes differentially expressed genes (DEGs) and genes encoding allergens, which help understanding the biology of P. ovis and lay foundations for the development of vaccine antigens and drug target screening.
Methods
The transcriptome of P. ovis was assembled and analyzed using bioinformatic tools. The unigenes of P. ovis from each developmental stage and the unigenes differentially between developmental stages were compared with allergen protein sequences contained in the allergen database website to predict potential allergens.
Results
We identified 38,836 unigenes, whose mean length was 825 bp. On the basis of sequence similarity with seven databases, a total of 17,366 unigenes were annotated. A total of 1,316 DEGs were identified, including 496 upregulated and 820 downregulated in the Pso_L group compared with the Pso_N_A group. We predicted 205 allergens genes in the two developmental stages similar to genes from other mites and ticks, of these, 14 were among the upregulated DEGs and 26 among the downregulated DEGs.
Conclusion
This study provides a reference transcriptome of P. ovis in absence of a reference genome. The analysis of DEGs and putative allergen genes may lay the foundation for studies of functional genomics, immunity and gene expression profiles of this parasitic mite species.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1856-z) contains supplementary material, which is available to authorized users.
Keywords: Psoroptes ovis, Transcriptome, Differentially expressed genes (DEGs), RNA-Seq
Background
Psoroptic mange is a chronic, contagious and infectious disease caused by the mange mite Psoroptes ovis, which infects horses, sheep, buffaloes, rabbits, other domestic animals, deer, wild camels, foxes, minks, lemurs [1, 2], alpacas [3, 4], elks [5, 6] and other wild animals. Psoroptes ovis mites are divided into different subspecies or variants according to their host species, for example, P. ovis var. cuniculi (host: rabbit), P. ovis var. equi (host: horse), P. ovis var. cervinus (host: deer), P. ovis var. natalensis (host: buffalo), etc. [7]. The sophisticated interaction between the host and the mite results into mite infestation [8], which is characterized by skin lesions and formation of characteristic yellowish scabs [9, 10].
Psoroptes ovis is a non-burrowing, surface secretions feeder, capable of consuming a variety of body fluids, lymph and red blood cells [11]; the mites do not appear to penetrate beyond the stratum corneum and survive on the epidermis of mammals [12]. Mites abrade the cuticle and deposit allergens when establishing themselves on the host, that results in severe inflammatory responses [13, 14] and the production of an exudate that, in turn, represents a food source for the mites [15]. Features of psoroptic mange include intense pruritus, extensive dermatitis, depilation and hyperkeratosis, which ultimately result in emaciation or death caused by secondary bacterial infections. The infestation is usually transmitted by close contact between animals [9].
Psoroptic mange is widespread in the world. In agriculture, P. ovis infestations can cause lower feed conversion efficiency, poor weight gain, poor quality of leather and reduced carcass traits [9, 16–18]. Current methods of prevention and control rely heavily on chemotherapy; however, there are concerns with this approach that incluse parasite resistance to chemoterapeutics, biological residues and toxic effects on the environment. Consequently, it is necessary to develop novel strategies for mite prevention and control [19]. One approach is the combination of promoting animal resistance to the infection and exploring new acaricidal drugs, via the discovery of novel drug targets. Alternative control strategies may also rely on vaccine development. However, a lack of available sequence information hinders progress in these areas. This far, only 1,545 unique P. ovis expressed sequence tags (ESTs) have been identified [20]; there is no available reference genome.
Since Velculescu et al. [21] first described the transcriptome of yeast in 1997, transcriptome research has become a hot topic in biology. With second generation massively parallel sequencing platforms, transcriptome sequencing (RNA-Seq) has become widely used. In terms of parasites, the transcriptomes of Plasmodium falciparum [22], Trypanosoma brucei [23], Schistosoma mansoni [24], Trichuris suis [25], Dermanyssus gallinae [26] and others [27–29] have been sequenced. To better understand the intricacy of gene function and the activity of cells, description of the transcriptome is useful [30]. In this paper, RNA-Seq techniques were applied to the study of the transcriptome of P. ovis, to assist current understanding of the biology of this mite and to lay foundations for vaccine antigen development and drug target screening.
Methods
P. ovis var. cuniculi collection
Psoroptes ovis var. cuniculi were reared on rabbits at the Laboratory Animal Center of Sichuan Agricultural University, China. Scrapings in the external auditory canal were harvested using tweezers, placed in microliter plates, and incubated at 37 °C for 2 h [31]. P. ovis that emerged from the scrapings were divided into two groups: larvae (named the Pso_L group), and nymphs and adults (named the Pso_N_A group). The Pso_L group was composed of 100 P. ovis larvae, and the Pso_N_A group was composed of 60 P. ovis nymphs and adults. Care was taken to remove contaminating skin debris by thorough washing. Larvae (160 mg) and nymphs and adults (170 mg) were preserved immediately at −80 °C after harvesting.
Library preparation for transcriptome sequencing
Sequencing was carried out on a second generation sequencing platform (RNA-Seq) [32]. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, USA) from the two groups of P. ovis var. cuniculi in accordance with the manufacturer’s recommendations. A total of 35 μg RNA from the Pso_L group and 41.76 μg RNA from the Pso_N_A group were used as input materials for RNA sample preparation. The RNA integrity number (RIN) values of both samples were > 7.0. Sequencing libraries were obtained using the Illumina TruSeq™ RNA Sample Preparation Kits (Illumina, San Diego, USA) according to the manufacturer’s instructions, and index codes were added to link sequences to each sample. For detailed steps, please refer to Wu et al. [28]. Finally, PCR products were purified using the AMPure XP system (Beckman Coulter, Beverly, USA) and the qualities of the sequencing libraries were assessed on the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). The library preparations were sequenced on an Illumina HiSeq 2000 platform (Illumina,USA). RNA-Seq data were produced by Beijing Novegene Bioinformatics Technology Co., Ltd.
Bioinformatic analysis
Before assembly, clean reads (clean data) were obtained by removing reads including adapters, reads including poly-N, and low quality reads including more than 10 % bases with q-value ≤ 20 from the raw data. Q20, Q30, GC-content and sequence duplication level of the clean data were calculated. All downstream analyses were performed using clean reads. The Trinity program [33] (http://trinityrnaseq.sourceforge.net/) was used for de novo assembly of the sequence data from P. ovis var. cuniculi. Finally, non-redundant unigenes could be identified until they could not be further elongated. For details of the data processing steps, please refer to Wu et al. [28].
Blastx alignment was carried out between unigenes and databases such as NR (NCBI non-redundant protein sequences), NT (NCBI nucleotide sequences), KO (KEGG Orthology), SwissProt (a manually annotated and peer-reviewed protein sequence database), PFAM (Protein Family), GO (Gene Ontology) and KOG (the euKaryotic Ortholog Groups database). Bioinformatics analyses were conducted as previously described [27]. In this analysis, the Blast2GO program [34] and WEGO software [35] were applied. Coding sequences (CDS) were predicted by comparison with sequences from other eukaryotes. The direction and CDS of unigenes in databases were obtained based on the best alignment results. Unigenes that could not be aligned to the above databases were scanned using the ESTScan software [36] to obtain the CDS and the sequence direction.
Analysis of gene expression
The Bowtie software was used for mapping clean reads to the assembled transcriptome. The percentage gene expression coverage was estimated by counting the reads numbers mapped to each gene. Expression levels of individual unigenes were analysed, and differences in gene expression levels were compared between samples from different life-cycle stages of the mite. The standard method of RPKM (reads per kb per million reads) [37] quantify gene expression. The input data for differential gene expression is the read count data obtained in the analysis of gene expression level. Firstly, the read count data was standardized by the edgeR program package through one scaling normalized factor [38]. Secondly, the DEGseq R package (1.12.0) was used for analysis of differential gene expression; the P values were adjusted using the Benjamini and Hochberg method; the screening threshold was Q value < 0.005 and |log2fold-change| > 1 [39]. Blastx alignment was carried out between differential expression genes and KEGG database (Kyoto encyclopedia of genes and genomes) [40]. KOBAS software (2.0) was used to test the statistical enrichment of differential expression genes in KEGG pathways; the statistic method was hypergeometric test; the P-values were adjusted using the Benjamini and Hochberg method; corrected P-value < 0.05 was set as the threshold for significant enrichment pathway [41].
Real-time PCR (qRT-PCR) validation
To validate the P. ovis expression data, qRT-PCR was employed to analyze differentially expressed genes (DEGs). Primer pairs for 9 differentially expressed genes and the housekeeping gene GAPDH-like are available (Additional file 1: Table S1). For qRT- PCR, an ABI7500 FAST real-time PCR System (Applied Biosystems, Forster, USA) and a SYBR®Premix Ex Taq™ II Kit (Takara, Japan) were applied in accordance to the manufacturers’ recommendations. The qRT-PCR conditions were 95 °C for 2 min, followed by 40 cycles of 94 °C for 20 s and 58 °C for 20 s. The final melting curve was analyzed. The relative expression level of each gene was calculated using the 2-△△Ct method [42].
Prediction of putative allergens
To predict putative allergens, the unigenes of P. ovis in each developmental stage and the differentially expressed unigenes of P. ovis were compared by Blast against allergen protein sequences from the allergen database website (http://www.allergome.org).
Results
Illumina sequencing and assembly of P. ovis transcriptome data
We obtained 59.4 million and 55.4 million raw reads from the Pso_L and Pso_N_A groups, respectively, by RNA-Seq. The raw sequence data has been submitted to the SRA division of GenBank (project accession no.: PRJNA317241). After a rigorous screening process, 56.6 million clean reads from the Pso_L group with a Q20 of 96.91 % and a GC of 35.85 % were retained. There were 52.9 million clean reads from the Pso_N_A group with a Q20 of 96.54 % and a GC of 34.90 % (Additional file 2: Table S2). Using the Trinity software, all clean data were assembled into a transcriptome, which was used as a reference sequence for further analyses. We obtained 62,983 transcripts with an average length of 1,480 base pairs (bp) and an N50 of 3,208 bp. Transcripts were clustered using the TGICL program; 38,836 unigenes were generated. The mean length and N50 were 825 bp and 1,607 bp, respectively. Of the 38,836 unigenes, 15,108 (38.90 %) were ≥ 500 bp, and 8,029 (20.67 %) were ≥ 1,000 bp (Additional file 3: Table S3 and Additional file 4: Table S4). The length distribution of the transcripts and unigenes is shown in Fig. 1.
Fig. 1.
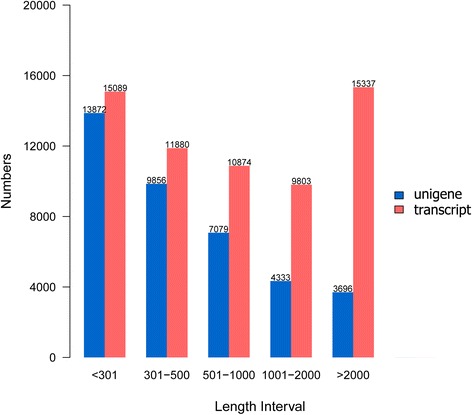
Length distribution of P. ovis transcripts and unigenes
Functional annotation of unigenes in P. ovis
The unigenes of the P. ovis transcriptome were compared with sequences in the NR, NT, KO, SwissProt, PFAM, GO and KOG databases using the Blastx program. Many of the unigenes showed homology when mapped against the databases (Table 1). When aligned through the KOG database, 7,798 unigenes were categorized into 25 molecular families (Fig. 2). Only 16.07 % (1,253/7,798) of the unigenes were contained in the cluster ‘general function prediction only’. ‘Signal transduction mechanisms’, ‘post-translational modification, ‘protein turnover, chaperones’, ‘translation, ribosomal structure and biogenesis’ and ‘intracellular trafficking, secretion, and vesicular transport’ included 15.72 % (1,226), 10.52 % (820), 8.45 % (659) and 5.81 % (453) of the unigenes, respectively. ‘Function unknown’ contained 4.90 % (382) of the unigenes.
Table 1.
Annotations of the P. ovis transcriptome. The percentage, in a database, equals the number of unigenes that were annotated successfully accounted for the proportion of the total number of unigenes
| Number of unigenes | Percentage (%) | |
|---|---|---|
| Annotated in NR | 12,121 | 31.21 |
| Annotated in NT | 7,681 | 19.77 |
| Annotated in KO | 6,624 | 17.05 |
| Annotated in SwissProt | 10,805 | 27.82 |
| Annotated in PFAM | 12,157 | 31.3 |
| Annotated in GO | 13,213 | 34.02 |
| Annotated in KOG | 7,798 | 20.07 |
| Annotated in all databases | 2,330 | 5.99 |
| Annotated in at least one database | 17,366 | 44.71 |
| Total unigenes | 38,836 |
Abbreviations: NR NCBI non-redundant protein sequences, NT NCBI nucleotide sequences, KO KEGG orthology, SwissProt a manually annotated and reviewed protein sequence database, PFAM protein family, GO gene ontology, KOG the euKaryotic Ortholog Groups database
Fig. 2.
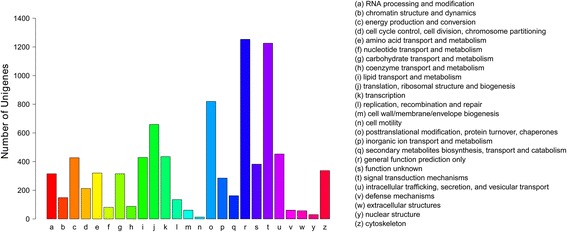
Histogram presenting clusters of orthologous groups (KOG) classifications. Of 38,836 unigenes, 7,798 sequences were assigned to 25 KOG classifications
GO functional cluster analysis was also undertaken. Of the most significant Blastx hits against the NR known species dataset, 13,213 unigenes were mapped to GO terms using the Blast2GO program (Table 1). These GO terms were categorized into the 3 main GO categories and 55 subcategories. The 3 main GO categories (i.e. molecular function, cellular components and biological process) contain 14, 18 and 23 subcategories, respectively (Fig. 3). The predominant GO annotation was ‘biological processes’ (46,840, 48.26 % of the total), followed by ‘cellular component’ (32,627, 33.61 %) and ‘molecular function’ (17,594, 18.13 %).
Fig. 3.
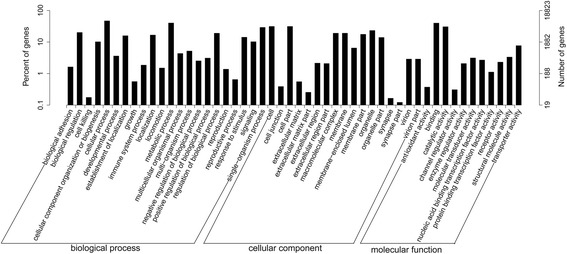
GO annotations of unigenes in the P. ovis transcriptome
KEGG pathway analysis of unigene consensus sequences
We were able to assign 2,201 different KO terms and 265 KEGG pathways to the P. ovis transciptome to 6,624 unigenes (Additional file 5: Table S5). The KEGG pathways included six categories (‘Metabolism’, ‘Genetic Information Processing (GIP)’, ‘Environmental Information Processing (EIP)’, ‘Cellular Processes’, ‘Organismal Systems’, and ‘Human Diseases’). We removed all the pathways belonging to Human Diseases and showed the remaining classification results in a histogram (Fig. 4). The most abundant subcategory in the ‘EIP’ category was ‘signal transduction’ (943 unigenes, 14.24 %); the most abundant subcategory in the ‘GIP’ category was ‘translation’ (678, 10.24 %); and the most abundant subcategory in the ‘Organismal Systems’ was ‘endocrine system’ (567, 8.56 %). Among the 265 KEGG pathways, the predominant terms were: ‘ribosome’ (ko03010, 393), ‘carbon metabolism’ (ko01200, 287), ‘protein processing in endoplasmic reticulum’ (ko04141, 209), ‘oxidative phosphorylation’ (ko00190, 202), ‘PI3K-Akt signaling pathway’ (ko04151, 181) and ‘MAPK signaling pathway’ (ko04010, 180).
Fig. 4.
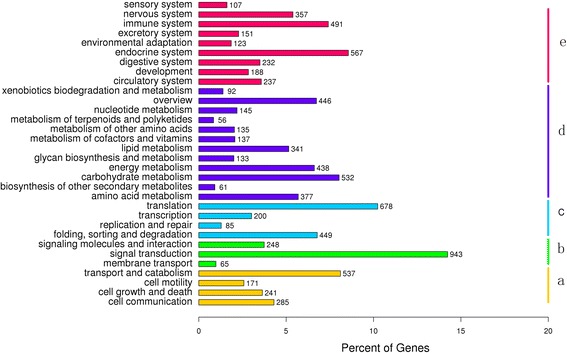
KEGG categories of P. ovis unigenes. Overall, 6,624 unigenes were annotated against the KEGG database. a Cellular processes; b Environmental information processing; c Genetic information processing; d Metabolism; e Organismal systems
Alignment of CDS
The CDS of unigenes were determined using the Blastx program, then by translating the corresponding P. ovis gene sequence into amino acids in accordance with the standard code table. The unigenes that did not match known genes were predicted using the ESTScan program. We identified 17,731 (45.66 %) CDS from the 38,836 unigenes in the P. ovis transcriptome. Of these, 12,950 were identified using the Blastx algorithmic program; their length distribution is shown in Additional file 6: Figure S1; the ESTScan software identified 4,781 CDS and their length distribution is shown in Additional file 7: Figure S2.
Analysis of differentially expressed genes from P. ovis in different developmental stages
To detect gene expression differences in different developmental life-cycle stages of P. ovis, we analyzed differentially expressed genes in the Pso_L (larval) and Pso_N_A (nymph/adult) groups. A total of 1,316 DEGs (Additional file 8: Table S6) were identified, including 496 upregulated unigenes and 820 downregulated unigenes in the Pso_L group compared with the Pso_N_A group. DEGs were assigned to 151 KEGG pathways. Amongst them, ‘lysosome’ (ko04142, 31 unigenes) and ‘phagosome’ (ko04145, 29) showed significant enrichment (Additional file 9: Table S7). The upregulated DEGs in the Pso_L group were assigned to 101 KEGG pathways (Additional file 10: Table S8). Amongst them, 5 showed significant enrichment, including ‘NF-kappa B signaling pathway’ (ko04064, 11), ‘cytokine-cytokine receptor interaction’ (ko04060, 12), ‘TNF signaling pathway’ (ko04668, 11), ‘phagosome’ (ko04145, 16)’, and ‘regulation of actin cytoskeleton’ (ko04810, 15). Downregulated DEGs in the Pso_L group relative to Pso_N_A were assigned to 138 KEGG pathways (Additional file 11: Table S9). Amongst them, 3 showed significant enrichment, including ‘lysosome’ (ko04142, 19), ‘protein processing in endoplasmic reticulum’ (ko04141, 24), and ‘cell cycle’ (ko04110, 14).
qRT-PCR was employed to validate the P. ovis transcriptome data. Specifically, nine DEGs were selected for experimental validation and statistical analysis. Pdis (comp20116_c0), bcap31 (comp24383_c0), sec61a (comp9210_c0), hsp90 (comp16267_c1, comp16267_c0, comp22146_c0), p97 (comp20363_c0), and rad32 (comp22004_c2) were found to be upregulated in the Pso_N_A group relative to the Pso_L group by qRT-PCR. hsp70 (comp15363_c1) was downregulated (Fig. 5). These results were consistent with the transcriptome sequencing data.
Fig. 5.
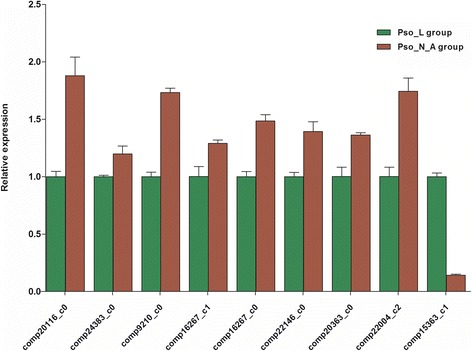
qRT-PCR validation of nine selected genes that were differentially expressed between the Pso_L group (samples from P. ovis larvae) and the Pso_N_A group (samples from adults and nymphs) in the transcriptomic data. Error bars indicate SDs
Protein processing in the endoplasmic reticulum
To help obtain a better understanding of the biochemistry and physiology of P. ovis, we chose the ‘protein processing in the endoplasmic reticulum’ pathway of ‘GIP’ (Additional file 12: Figure S3) as a case-study for further analysis. Across the transcriptome, this pathway was mapped to 209 unigenes grouped into three categories (‘endoplasmic reticulum protein processing’, ‘endoplasmic reticulum stress response’ and ‘endoplasmic reticulum associated degradation-ERAD’). This pathway was one of the most enriched in upregulated DEGs in the Pso_N_A group relative to the Pso_L group; there were 24 upregulated DEGs related to this pathway. ERAD, coupled with the ubiquitin-proteasome degradation pathway [43], is the main pathway of intracellular protein degradation and is involved in > 80 % of protein degradation in the cell. Five key genes in ERAD, heat-shock protein hsp90, heat-shock protein hsp70, transitional endoplasmic reticulum ATPase p97, ERAD enhancers mannosidase edem, and transporters sec61, were mapped by 14, 19, 8, 3 and 3 transcripts, respectively.
Prediction of allergen genes
The unigenes of P. ovis in the two developmental stages were compared by Blastx against allergen protein sequences, and 1,294 putative allergen genes were identified (Additional file 13: Table S10). Amongst them, there were 205 which resulted in Blast hits to genes from other mites (such as Dermatophagoides pteronyssinus, Dermatophagoides farina, etc.) and ticks (Additional file 14: Table S11), and 1,089 which did not result in Blast hits to genes from other mites or ticks (Additional file 15: Table S12). The upregulated DEGs were similarly analyzed in the Pso_L group relative to the Pso_N_A group, and 72 putative allergen genes were predicted (Additional file 16: Table S13). Of these, 14 were shared with other mites and ticks (Table 2), and 58 were not (Additional file 17: Table S14). A similar analysis of the downregulated DEGs predicted 116 putative allergen genes (Additional file 18: Table S15), of which 26 produced Blast hits to sequences from mites and ticks (Table 3), while 90 did not (Additional file 19: Table S16).
Table 2.
Allergen genes which Blast hits with genes from mites and ticks among the upregulated DEGs
| Unigene ID | Allergen protein ID | Species | Description | Blast e-value |
|---|---|---|---|---|
| comp21772_c1 | A0A088SAG5 | Dermatophagoides farinae | A0A088SAG5_DERFA Der f 30 allergen | 1.12e-102 |
| comp16294_c0 | L7UZ91 | Dermatophagoides farinae | L7UZ91_DERFA Ferritin | 1.48e-66 |
| comp22600_c16 | C6ZDB5 | Tyrophagus putrescentiae | C6ZDB5_TYRPU Tyr p 3 | 1.02e-11 |
| comp21796_c0 | Q8WQ47 | Lepidoglyphus destructor | TBA_LEPDS Tubulin alpha chain | 0 |
| comp20560_c0 | A0A088SAY1 | Dermatophagoides farinae | A0A088SAY1_DERFA Der f 31 allergen | 4.63e-06 |
| comp7410_c0 | A0A088SAY1 | Dermatophagoides farinae | A0A088SAY1_DERFA Der f 31 allergen | 1.70e-11 |
| comp22325_c14 | L7N6F8 | Dermatophagoides pteronyssinus | L7N6F8_DERPT Dust mite allergen | 5.95e-06 |
| comp21866_c0 | Q4JK69 | Dermatophagoides pteronyssinus | Q4JK69_DERPT Group 15 allergen protein short isoform | 8.53e-76 |
| comp22602_c8 | Q6Y2F9 | Dermatophagoides pteronyssinus | Q6Y2F9_DERPT HDM allergen | 9.29e-18 |
| comp15615_c0 | A0A088SAX2 | Dermatophagoides farinae | A0A088SAX2_DERFA Triosephosphate isomerase | 2.91e-120 |
| comp15363_c1 | A0A088SAS1 | Dermatophagoides farinae | A0A088SAS1_DERFA Der f 28 allergen | 4.99e-61 |
| comp22460_c6 | Q6Y2F9 | Dermatophagoides pteronyssinus | Q6Y2F9_DERPT HDM allergen | 9.80e-27 |
| comp35289_c0 | A0A088SAG5 | Dermatophagoides farinae | A0A088SAG5_DERFA Der f 30 allergen | 1.16e-31 |
| comp22588_c0 | L7UZ85 | Dermatophagoides farinae | L7UZ85_DERFA Alpha–actinin | 4.46e-07 |
Table 3.
Allergen genes which Blast hits with genes from mites and ticks among the downregulated DEGs
| Unigene ID | Allergen protein ID | Species | Description | Blast e-value |
|---|---|---|---|---|
| comp22562_c0 | A0A088SCP3 | Dermatophagoides farinae | A0A088SCP3_DERFA Der f 32 allergen | 5.57e-179 |
| comp22544_c0 | Q8N0N0 | Dermatophagoides pteronyssinus | Q8N0N0_DERPT Group 14 allergen protein (Fragment) | 1.33e-20 |
| comp16295_c1 | A1KXH2 | Dermatophagoides farinae | A1KXH2_DERFA Der f 1 allergen | 2.58e-31 |
| comp19324_c1 | Q9U6R7 | Dermatophagoides farinae | Q9U6R7_DERFA 98 kDa HDM allergen | 7.44e-94 |
| comp16185_c0 | Q8WQ47 | Lepidoglyphus destructor | TBA_LEPDS Tubulin alpha chain | 2.44e-91 |
| comp20889_c0 | L7N6F8 | Dermatophagoides pteronyssinus | L7N6F8_DERPT Dust mite allergen | 2.81e-07 |
| comp7240_c0 | Q9Y197 | Dermatophagoides pteronyssinus | Q9Y197_DERPT Alpha–amylase (Fragment) | 0 |
| comp19263_c0 | L7N6F8 | Dermatophagoides pteronyssinus | L7N6F8_DERPT Dust mite allergen | 2.67e-07 |
| comp22789_c0 | Q1EIQ3 | Psoroptes ovis | PEPT1_PSOOV Peptidase 1 | 0 |
| comp22115_c5 | A1KXG7 | Dermatophagoides farinae | A1KXG7_DERFA Der f 7 allergen | 1.87e-59 |
| comp21147_c0 | Q9U1G2 | Lepidoglyphus destructor | ALL7_LEPDS Mite allergen Lep d 7 | 7.02e-21 |
| comp22817_c0 | E0A8N8 | Dermatophagoides pteronyssinus | E0A8N8_DERPT Der p 13 allergen | 9.01e-80 |
| comp21324_c0 | P49273 | Dermatophagoides pteronyssinus | ALL7_DERPT Mite allergen Der p 7 | 1.19e-93 |
| comp22866_c0 | A8B8G7 | Dermatophagoides farinae | A8B8G7_DERFA Group 21 allergen | 3.91e-48 |
| comp16295_c0 | Q1EIQ3 | Psoroptes ovis | PEPT1_PSOOV Peptidase 1 | 6.01e-80 |
| comp16271_c0 | L7UZ91 | Dermatophagoides farinae | L7UZ91_DERFA Ferritin | 7.49e-56 |
| comp7938_c0 | Q965E2 | Psoroptes ovis | ALL2_PSOOV Mite group 2 allergen Pso o 2 | 2.18e-73 |
| comp16261_c0 | Q52PV9 | Tyrophagus putrescentiae | Q52PV9_TYRPU Alpha–tubulin | 1.88e-17 |
| comp22807_c0 | Q8N0N0 | Dermatophagoides pteronyssinus | Q8N0N0_DERPT Group 14 allergen protein (Fragment) | 0 |
| comp22811_c0 | L7N6F8 | Dermatophagoides pteronyssinus | L7N6F8_DERPT Dust mite allergen | 6.09e-13 |
| comp23114_c0 | A1KXH1 | Dermatophagoides farinae | A1KXH1_DERFA Der f 13 allergen | 2.73e-37 |
| comp22853_c0 | A8B8I1 | Dermatophagoides farinae | A8B8I1_DERFA Der f 5 allergen | 2.16e-45 |
| comp7750_c0 | L7UW58 | Dermatophagoides farinae | L7UW58_DERFA Translation elongation factor 2 (Fragment) | 0 |
| comp22673_c2 | Q86R84 | Dermatophagoides farinae | Q86R84_DERFA 60 kDa allergen Der f 18p | 0 |
| comp16261_c1 | Q8WQ47 | Lepidoglyphus destructor | TBA_LEPDS Tubulin alpha chain | 3.93e-87 |
| comp22539_c0 | A3F5F1 | Dermatophagoides farinae | A3F5F1_DERFA Der f 2 allergen | 2.97e-14 |
Discussion
Coverage and quality of consensus sequences
Analysis of transcriptome data is useful to the understanding of genomic function and cellular activities in organisms [30]. Paired-end transcriptome sequencing technology is an effective approach for the analysis of transcriptomic data [44], and is also efficient in elucidating the complexity of the transcriptome. In the present study, the transcriptome of P. ovis was determined using a HiSeq 2000 paired-end sequencing platform and Trinity assembling software. This analysis provided extensive coverage of the transcriptome in long fragments, which provided us with the opportunity to: (i) analyze gene expression; (ii) predict new genes; and (iii) explore metabolic pathways in the absence of a reference genome.
Kenyon et al. sequenced 484 P. ovis ESTs in 2003 [45]. To further expand knowledge of the P. ovis transcriptome, a cDNA library of P. ovis was constructed by Burgess et al. [20]; 1,574 ESTs were sequenced and, combined with 484 previously obtained P. ovis sequences such that, resulted in 1,545 mite sequences. Li et al. sequenced 33 P. ovis var. cuniculi ESTs by constructing a cDNA library [46]. Thus, overall, relatively few P. ovis sequences were obtained in previous investigations. In the present study, reads were assembled into 38,836 unigenes. On the basis of sequence similarity sequences stored in seven databases (NR, NT, KO, SwissProt, PFAM, GO and KOG), 17,366 unigenes were annotated, which greatly expands the P. ovis transcript database. The average length of P. ovis unigenes was 825 bp, which is longer than some species in earlier studies, for example, Taenia pisiformis (398 bp) [27]. The unigene annotations included a description of the gene or protein name, prediction of conserved regions and analysis of GO terms and metabolic/signaling pathways (only in the KEGG database), which provides biological function information, metabolic/signaling pathways of nominee genes at a specific time. Such data enables a more in-depth understanding of gene expression in P. ovis. In this study, 17,260 CDS were obtained. Amongst them, we identified many novel genes, which provides a platform for research into P. ovis functional genes. For example, heat-shock proteins [47] can be screened as candidate diagnostic antigens.
Gene functional clusters of the P. ovis transcriptome
Identified unigenes could be annotated with a variety of KOG functional clusters, which showed that transcriptomic data on P. ovis contained extensive transcript diversity. KOG, short for euKaryotic Ortholog Groups, is an NCBI database based on relationships between orthologous genes. Based on KOG and evolutionary relationships, homologous genes from different species can be divided into different ortholog clusters. To date, there are 4,852 KOG classifications. Orthologous genes are annotated with the same function, so functional annotation is inherited directly by other members of the same KOG cluster. In 25 molecular families, the clusters for ‘coenzyme transport and metabolism’, ‘replication, recombination and repair’, ‘intracellular trafficking secretion, and vesicular transport’ and ‘defense mechanisms’ had a meaningful impact, because they were closely related to the immune and physiological functions of P. ovis. Analysis of gene expression levels, GO functional clusters and prediction of protein metabolic pathways, respectively, indicated the sifting of high abundance expression genes, GO functional classification of different genes and positioning of relevant metabolic pathways. The P. ovis transcriptome data in this work add to the available data on this parasite and will facilitate research into gene functions and antigen mechanism.
Analysis of putative allergen genes
Proinflammatory cytokines cause skin damage; the source of these factors may be excretions/secretions containing enzymes and allergens from mites, such as cysteine proteinases, gst, pso2 et al.[15]. In particular, we characterized putative P. ovis allergen genes and analyzed differences in in gene expression between larval and adult/nymph stages, which lays the foundations for research into the pathogenic properties of these two mite stages. At present, only eight allergen genes have been studied in-depth in P. ovis: pso2 [48], actin [49], pso1 [50], pso10 [51], pso11 [51], vitellogenin [52], gst [53], and troponin C [31]. In this study, comparisons of P. ovis DEGs against allergen protein sequences resulted in 188 predicted allergen genes. Amongst them, 40 matched sequences in other mites and ticks, including some important allergen genes such as derf15 98 kDa chitinase [54], derp7 allergen peptides [55], and derf18 chitinase-like chitin-binding protein [54]. Further study of these genes will help in understanding the interactions between P. ovis and its hosts.
Collection of experimental materials and analysis of DEGs
The collection of suitable experimental materials lays the foundation for subsequent experiments. cDNA libraries of P. ovis were constructed by Kenyon et al. [45] and Burgess et al. [20], and a cDNA library of P. ovis var. cuniculi was constructed by Hu et al.[46]. The experimental materials used in these studies were all mixed life-cycle stages of P. ovis, including larvae, nymphs and adults. In the present work, specimens of P. ovis var. cuniculi were assigned to two groups, larvae (the Pso_L group) and nymphs and adults (the Pso_N_A group). Larvae are characterized by distinct features, including a smaller body, a lighter color than nymphs and adults and three pairs of legs (rather than four in nymphs and adults) [56]. Adult males (with an average length of 396 μm), male tritonymphs (414 μm) and adult females (402 μm) have very similar body lengths [57]. The mean body widths of adult males and male tritonymphs are 380 μm and 370 μm, respectively [57]. In addition, female tritonymphs and adult males are attached during mating and cannot be separated easily. Moreover, adult females is similar morphologically to female tritonymphs except for very small differences in reproductive organs [56]. Taken together, there is difficulty in identifying and separating nymphs and adults.
We analyzed DEGs in different life-cycle stages of P. ovis (i.e. in larvae and nymphs/adults). A total of 1,316 DEGs were analyzed. The analysis of differential gene expression may lay the foundation for research into the development of the reproductive system. Besides, as described above, morphologically, the difference is obvious between the samples in the Pso_L and Pso_N_A groups, especially the differences of legs. It is necessary to further study if there are genes that control the fourth pair of legs in the upregulated DEGs in the Pso_N_A group relative to the Pso_L group. Therefore, the analysis of differential gene expression may also lay the foundation for research into the morphological differences between the stages of P. ovis.
Analysis of ER-associated degradation pathway
The function of many unigenes is closely related to signaling and metabolic pathways. In this study, we specifically characterized the endoplasmic reticulum associated degradation pathway (ERAD) of the ‘protein processing in endoplasmic reticulum’ KEGG pathway. The ERAD pathway guides ubiquitin-mediated degradation of a range of ER-associated misfolded and normal proteins and functions in protein quality control [58]. That selective degradation of normal proteins is the base for cellular regulation of many processes [59]. For example, in order to control the cell cycle, cyclins or their inhibitors are degradated. By comparison, the degradation of quality control requires recognition of damaged or misfolded proteins. hsp90, hsp70 and edem are key genes in the ERAD pathway. When cells are stressed (such as as a consequence of infection, heat-shock or oxidative damage), heat-shock proteins are expressed; their role is to identify misfolded proteins and mark them for ubiquitin-mediated degradation. As a molecular chaperone, lumenal heat-shock protein hsp70 plays an important role in recognition and degradation of lumenal ERAD substrates [60]. Four cytosolic hsp70 proteins play a key role in identifying and degrading integral membrane substrates [61].
An earlier study showed that a calnexin cycle mechanism was closely related to ERAD in the endoplasmic reticulum [62]. The calnexin cycle refered to that the newly synthesized glycoproteins began to fold after combing with transmembrane protein calnexin and soluble protein calreticulin in the endoplasmic reticulum through the glucose residues. Correctly folded glycoproteins were separated from the two proteins, while the misfolded ones would recirculate and repeat the folding process. Since only those proteins that were out of the calnexin cycle could pass into the ERAD pathway, the calnexin cycle limited the rate of misfolded proteins going into the ERAD which effected the degradation of these proteins [63]. Molinari et al. [64] found that a mannosidase-1-like protein (edem) could adjust a misfolded glycoprotein released from the calnexin cycle and passed into the ERAD pathway. edem overexpression could cause a faster release of misfolded proteins from the calnexin cycle and an earlier start of ERAD. The upregulated edem during endoplasmic reticulum stress may promote cell recovery by clearing the calnexin cycle and by facilitating ERAD of terminally folding-incompetent polypeptides.
In our data, 14, 19 and 3 transcripts could be mapped to heat-shock protein hsp90, heat-shock protein hsp70 and ERAD enhancer mannosidase edem of the ERAD pathway, respectively. These important genes provide opportunities to investigate vaccine antigens against P. ovis and drug targets in this mite.
Conclusion
Here we have identified 38,836 unigenes from P. ovis by RNA-Seq and bioinformatic analyses of sequence data. A total of 17,366 unigenes were annotated and 17,260 CDS were identified. We also analyzed 1,316 DEGs in different life-cycle stages of P. ovis. This work will lay the foundation for studies of functional genomics, immunoregulation mechanism, vaccine antigens and gene expression profiles of this parasitic mite species.
Acknowledgements
We are grateful to Yu Wang, Ning Wang, Yu Zheng, Jia-Hai Wang and Xing Huang for their help and suggestions.
Funding
This study was supported by a grant from the Key Technology R&D Program of Sichuan Province, China (no. 2015NZ0041). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The transcriptome raw sequence data has been submitted to the SRA division of GenBank (project accession no.: PRJNA317241). The other data supporting our findings and conclusions are available in the article and its Additional files.
Authors’ contributions
GYY conceived and designed the experiments. MLH, XRP contributed reagents/materials/analysis tools. MLH, RH performed the experiments. JX, NXS performed bioinformatics analyses. MLH, JX, NXS, XBG analyzed the data. MLH wrote the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
All animals were raised in strict accordance with the animal protection laws of the People's Republic of China (a draft of an animal protection law released on September 18, 2009). All procedures were conducted strictly according to the Guide for the Care and Use of Laboratory Animals of the Animal Ethics Committee of Sichuan Agricultural University (Ya'an, China) (Approval No. 2013–028).
Abbreviations
- DEG
Differentially expressed gene
- GO
Gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- KO
KEGG Orthology
- KOG
The euKaryotic Ortholog Groups database
- NR
NCBI non-redundant protein sequences
- NT
NCBI nucleotide sequences
- PFAM
Protein family
- RIN
RNA integrity number.
- RPKM
Reads per kb per million reads
- RT-qPCR
Reverse-transcription quantitative PCR
- SwissProt
A manually annotated and reviewed protein sequence database
Additional files
Primer sequences used for qRT-PCR. (DOCX 14 kb)
The quality of transcriptome data. (XLSX 10 kb)
The frequency distribution of splice length. (XLSX 9 kb)
The distribution of splice length. (XLSX 9 kb)
Detailed KEGG pathway categories of the P. ovis unigenes. (XLSX 10 kb)
Length distribution of CDS determined using the Blastx algorithm. (TIF 1334 kb)
Length distribution of CDS determined by ESTScan software. (TIF 1342 kb)
DEGseq analysis results for DEGs. (XLSX 110 kb)
The enrichment results of DEGs in KEGG pathways. (XLS 72 kb)
The enrichment results of upregulated DEGs in KEGG pathways. (XLSX 20 kb)
The enrichment results of downregulated DEGs in KEGG pathways. (XLS 64 kb)
The ‘Protein processing in endoplasmic reticulum’ pathway. (TIF 46 kb)
Allergen genes among the unigenes in the two developmental stages. (XLSX 83 kb)
Allergen genes which resulted in Blast hits with genes from other mites and ticks among the unigenes of P. ovis in the two developmental stages. (XLSX 20 kb)
Allergen genes which did not result in Blast hits with genes from other mites or ticks among the unigenes of P. ovis in the two developmental stages. (XLSX 64 kb)
Allergen genes among the upregulated DEGs. (XLSX 15 kb)
Allergen genes which did not result in Blast hits with genes from other mites or ticks among the upregulated DEGs. (XLSX 13 kb)
Allergen genes among the downregulated DEGs. (XLSX 16 kb)
Allergen genes which did not result in Blast hits with genes from other mites or ticks among the downregulated DEGs. (XLSX 16 kb)
Contributor Information
Man-Li He, Email: aliciaheml@hotmail.com.
Jing Xu, Email: 635851095@qq.com.
Ran He, Email: ran.susan@qq.com.
Neng-Xing Shen, Email: 935952544@qq.com.
Xiao-Bin Gu, Email: 448070309@qq.com.
Xue-Rong Peng, Email: 445081670@qq.com.
Guang-You Yang, Email: guangyou1963@aliyun.com.
References
- 1.Junge RE, Louis EE. Biomedical evaluation of two sympatric lemur species (Propithecus verreauxi deckeni and Eulemur fulvus rufus) in Tsiombokibo Classified Forest, Madagascar. J Zoo Wildl Med. 2005;36(4):581–9. doi: 10.1638/05-025.1. [DOI] [PubMed] [Google Scholar]
- 2.Junge RE, Louis EE. Biomedical evaluation of black lemurs (Eulemur macaco macaco) in Lokobe Reserve, Madagascar. J Zoo Wildl Med. 2007;38(1):67–76. doi: 10.1638/06-006.1. [DOI] [PubMed] [Google Scholar]
- 3.D'Alterio GL, Batty A, Laxon K, Duffus P, Wall R. Psoroptes species in alpacas. Vet Rec. 2001;149(3):96. [PubMed] [Google Scholar]
- 4.Frame NW, Frame RK. Psoroptes species in alpacas. Vet Rec. 2001;149(4):128. [PubMed] [Google Scholar]
- 5.Garris GI, Prullage JB, Prullage JL, Wright FC, Miller JA. Control of Psoroptes cuniculi in captive white-tailed deer with ivermectin-treated corn. J Wildl Dis. 1991;27(2):254–7. doi: 10.7589/0090-3558-27.2.254. [DOI] [PubMed] [Google Scholar]
- 6.Ziccardi MH, Boyce WM, Gardner IA. Seroprevalence of Psoroptes sp. mites in free-ranging elk (Cervus elaphus) as determined by kinetic ELISA. J Wildl Dis. 1996;32(1):51–6. doi: 10.7589/0090-3558-32.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Wall R, Kolbe K. Taxonomic priority in Psoroptes mange mites: P. ovis or P. equi? Exp Appl Acarol. 2006;39(2):159–62. doi: 10.1007/s10493-006-9000-y. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair AN, Filan SJ. Confirmation of degenerative effects on psoroptic mites from scab lesions. Vet Rec. 1991;129(22):492. doi: 10.1136/vr.129.22.492. [DOI] [PubMed] [Google Scholar]
- 9.Mullen GR, Durden LA. Medical and veterinary entomology. Boston: Academic Press; 2009.
- 10.Stromberg PC, Guillot FS. Pathogenesis of psoroptic scabies in Hereford heifer calves. Am J Vet Res. 1989;50(4):594–601. [PubMed] [Google Scholar]
- 11.DeLoach JR, Wright FC. Ingestion of rabbit erythrocytes containing 51Cr-labeled hemoglobin by Psoroptes spp. (Acari: Psoroptidae) that originated on cattle, mountain sheep, or rabbits. J Med Entomol. 1981;18(4):345–8. doi: 10.1093/jmedent/18.4.345. [DOI] [PubMed] [Google Scholar]
- 12.Mathieson BR, Lehane MJ. Ultrastructure of the alimentary canal of the sheep scab mite, Psoroptes ovis (Acari: Psoroptidae) Vet Parasitol. 2002;104(2):151–66. doi: 10.1016/S0304-4017(01)00620-3. [DOI] [PubMed] [Google Scholar]
- 13.Burgess ST, Frew D, Nunn F, Watkins CA, McNeilly TN, Nisbet AJ, et al. Transcriptomic analysis of the temporal host response to skin infestation with the ectoparasitic mite Psoroptes ovis. BMC Genomics. 2010;11:624. doi: 10.1186/1471-2164-11-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Broek AH, Huntley JF. Sheep scab: the disease, pathogenesis and control. J Comp Pathol. 2003;128(2–3):79–91. doi: 10.1053/jcpa.2002.0627. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton KA, Nisbet AJ, Lehane MJ, Taylor MA, Billingsley PF. A physiological and biochemical model for digestion in the ectoparasitic mite, Psoroptes ovis (Acari: Psoroptidae) Int J Parasitol. 2003;33(8):773–85. doi: 10.1016/S0020-7519(03)00089-4. [DOI] [PubMed] [Google Scholar]
- 16.Cole NA, Guillot FS. Influence of Psoroptes ovis on the energy metabolism of heifer calves. Vet Parasitol. 1987;23(3–4):285–95. doi: 10.1016/0304-4017(87)90014-8. [DOI] [PubMed] [Google Scholar]
- 17.Lonneux J-F, Nguyen T, Detry J, Farnir F, Losson B. The relationship between parasite counts, lesions, antibody titres and daily weight gains in Psoroptes ovis infested cattle. Vet Parasitol. 1998;76(1):137–48. doi: 10.1016/S0304-4017(97)00220-3. [DOI] [PubMed] [Google Scholar]
- 18.Rehbein S, Visser M, Winter R, Trommer B, Matthes HF, Maciel AE, et al. Productivity effects of bovine mange and control with ivermectin. Vet Parasitol. 2003;114(4):267–84. doi: 10.1016/S0304-4017(03)00140-7. [DOI] [PubMed] [Google Scholar]
- 19.Nisbet AJ, Huntley JF. Progress and opportunities in the development of vaccines against mites, fleas and myiasis-causing flies of veterinary importance. Parasite Immunol. 2006;28(4):165–72. doi: 10.1111/j.1365-3024.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 20.Burgess ST, Nisbet AJ, Kenyon F, Huntley JF. Generation, analysis and functional annotation of expressed sequence tags from the ectoparasitic mite Psoroptes ovis. Parasit Vectors. 2011;4:145. doi: 10.1186/1756-3305-4-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velculescu VE, Zhang L, Zhou W, Vogelstein J, Basrai MA, Bassett DE, Jr, et al. Characterization of the yeast transcriptome. Cell. 1997;88(2):243–51. doi: 10.1016/S0092-8674(00)81845-0. [DOI] [PubMed] [Google Scholar]
- 22.Sorber K, Dimon MT, DeRisi JL. RNA-Seq analysis of splicing in Plasmodium falciparum uncovers new splice junctions, alternative splicing and splicing of antisense transcripts. Nucleic Acids Res. 2011;39(9):3820–35. doi: 10.1093/nar/gkq1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolev NG, Franklin JB, Carmi S, Shi H, Michaeli S, Tschudi C. The transcriptome of the human pathogen Trypanosoma brucei at single-nucleotide resolution. PLoS Pathog. 2010;6(9):e1001090. doi: 10.1371/journal.ppat.1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida GT, Amaral MS, Beckedorff FC, Kitajima JP, DeMarco R, Verjovski-Almeida S. Exploring the Schistosoma mansoni adult male transcriptome using RNA-seq. Exp Parasitol. 2012;132(1):22–31. doi: 10.1016/j.exppara.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Cantacessi C, Young ND, Nejsum P, Jex AR, Campbell BE, Hall RS, et al. The transcriptome of Trichuris suis-first molecular insights into a parasite with curative properties for key immune diseases of humans. PLoS One. 2011;6(8):e23590. doi: 10.1371/journal.pone.0023590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schicht S, Qi W, Poveda L, Strube C. Whole transcriptome analysis of the poultry red mite Dermanyssus gallinae (De Geer, 1778) Parasitology. 2014;141(3):336–46. doi: 10.1017/S0031182013001467. [DOI] [PubMed] [Google Scholar]
- 27.Yang D, Fu Y, Wu X, Xie Y, Nie H, Chen L, et al. Annotation of the transcriptome from Taenia pisiformis and its comparative analysis with three Taeniidae species. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0032283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Fu Y, Yang D, Zhang R, Zheng W, Nie H, et al. Detailed transcriptome description of the neglected cestode Taenia multiceps. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0045830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Y, Lan J, Zhang Z, Hou R, Wu X, Yang D, et al. Novel insightsinto the transcriptome of Dirofilaria immitis. Plos One. 2012;7(7) doi: 10.1371/journal.pone.0041639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang LX, He D, Dong WR, Zhang YW, Shao JZ. Deep sequencing-based transcriptome profiling analysis of bacteria-challenged Lateolabrax japonicus reveals insight into the immune-relevant genes in marine fish. BMC Genomics. 2010;11:472. doi: 10.1186/1471-2164-11-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng W, Zhang R, Wu X, Ren Y, Nong X, Gu X, et al. Evaluating troponin C from Psoroptes cuniculi as a diagnostic antigen for a dot-ELISA assay to diagnose mite infestations in rabbits. Parasite Immunol. 2014;36(2):53–9. doi: 10.1111/pim.12076. [DOI] [PubMed] [Google Scholar]
- 32.Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456(7218):53–9. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 35.Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, et al. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:W293–7. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iseli C, Jongeneel CV, Bucher P. ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc Int Conf Intell Syst Mol Biol. 1999;99:138–48. [PubMed]
- 37.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 38.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):1. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–4. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21(19):3787–93. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Hiller MM, Finger A, Schweiger M, Wolf DH. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273(5282):1725–8. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- 44.Lu T, Lu G, Fan D, Zhu C, Li W, Zhao Q, et al. Function annotation of the rice transcriptome at single-nucleotide resolution by RNA-seq. Genome Res. 2010;20(9):1238–49. doi: 10.1101/gr.106120.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenyon F, Welsh M, Parkinson J, Whitton C, Blaxter ML, Knox DP. Expressed sequence tag survey of gene expression in the scab mite Psoroptes ovis-allergens, proteases and free-radical scavengers. Parasitology. 2003;126(05):451–60. doi: 10.1017/S0031182003003044. [DOI] [PubMed] [Google Scholar]
- 46.Hu L, Zhao Y, Cheng J, Yang Y, Li C, Lu Z. Constructing and detecting a cDNA library for mites. Parasitol Res. 2015;114(10):3893–901. doi: 10.1007/s00436-015-4621-x. [DOI] [PubMed] [Google Scholar]
- 47.Ferrer E, Gonzalez LM, Foster-Cuevas M, Cortez MM, Davila I, Rodriguez M, et al. Taenia solium: characterization of a small heat shock protein (Tsol-sHSP35.6) and its possible relevance to the diagnosis and pathogenesis of neurocysticercosis. Exp Parasitol. 2005;110(1):1–11. doi: 10.1016/j.exppara.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Temeyer KB, Soileau LC, Pruett JH. Cloning and sequence analysis of a cDNA encoding Pso o II, a mite group II allergen of the sheep scab mite (Acari: Psoroptidae) J Med Entomol. 2002;39(2):384–91. doi: 10.1603/0022-2585-39.2.384. [DOI] [PubMed] [Google Scholar]
- 49.Zheng W, Tang Q, Zhang R, Jise Q, Ren Y, Nong X, et al. Vaccination with recombinant actin from scab mites and evaluation of its protective efficacy against Psoroptes cuniculi infection. Parasite Immunol. 2013;35(2):91–8. doi: 10.1111/pim.12015. [DOI] [PubMed] [Google Scholar]
- 50.Nisbet AJ, MacKellar A, McLean K, Brennan GP, Huntley JF. Eukaryotic expression of recombinant Pso o 1, an allergen from Psoroptes ovis, and its localization in the mite. Parasitology. 2007;134(01):83–9. doi: 10.1017/S0031182006001235. [DOI] [PubMed] [Google Scholar]
- 51.Nisbet AJ, MacKellar A, Wright HW, Brennan GP, Chua KY, Cheong N, et al. Molecular characterization, expression and localization of tropomyosin and paramyosin immunodominant allergens from sheep scab mites (Psoroptes ovis) Parasitology. 2006;133(04):515–23. doi: 10.1017/S0031182006000631. [DOI] [PubMed] [Google Scholar]
- 52.Huntley JF, Machell J, Nisbet AJ, Van den Broek A, Chua KY, Cheong N, et al. Identification of tropomyosin, paramyosin and apolipophorin/vitellogenin as three major allergens of the sheep scab mite, Psoroptes ovis. Parasite Immunol. 2004;26(8–9):335–42. doi: 10.1111/j.0141-9838.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee AJ, Huntley J, Van den Broek A, Coates D, Isaac RE. Expression and characterisation of a Psoroptes ovis glutathione S-transferase. Vet Parasitol. 2002;105(1):49–63. doi: 10.1016/S0304-4017(01)00622-7. [DOI] [PubMed] [Google Scholar]
- 54.Thomas WR. Hierarchy and molecular properties of house dust mite allergens. Allergol Int. 2015;64(4):304–11. doi: 10.1016/j.alit.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Shen HD, Chua KY, Lin WL, Hsieh KH, Thomas WR. Molecular cloning and immunological characterization of the house dust mite allergen Der f 7. Clin Exp Allergy. 1995;25(10):1000–6. doi: 10.1111/j.1365-2222.1995.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 56.Sweatman GK. On the life history and validity of the species in Psoroptes, a genus of mange mites. Can J Zool. 1958;36(6):905–29.
- 57.Sanders A, Froggatt P, Wall R, Smith KE. Life-cycle stage morphology of Psoroptes mange mites. Med Vet Entomol. 2000;14(2):131–41. doi: 10.1046/j.1365-2915.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 58.Hampton RY. ER-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 2002;14(4):476–82. doi: 10.1016/S0955-0674(02)00358-7. [DOI] [PubMed] [Google Scholar]
- 59.Ciechanover A, Schwartz AL. Ubiquitin-mediated degradation of cellular proteins in health and disease. Hepatology. 2002;35(1):3–6. doi: 10.1053/jhep.2002.30316. [DOI] [PubMed] [Google Scholar]
- 60.Plemper RK, Bohmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388(6645):891–5. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Nijbroek G, Sullivan ML, McCracken AA, Watkins SC, Michaelis S, et al. Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol Biol Cell. 2001;12(5):1303–14. doi: 10.1091/mbc.12.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedlander R, Jarosch E, Urban J, Volkwein C, Sommer T. A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol. 2000;2(7):379–84. doi: 10.1038/35017001. [DOI] [PubMed] [Google Scholar]
- 63.Qing J, Peng J. Endoplasmic reticulum associated protein degradation and its mechanism. Chin J Cell Biol. 2004;26(2):103–7. [Google Scholar]
- 64.Molinari M, Calanca V, Galli C, Lucca P, Paganetti P. Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science. 2003;299(5611):1397–400. doi: 10.1126/science.1079474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The transcriptome raw sequence data has been submitted to the SRA division of GenBank (project accession no.: PRJNA317241). The other data supporting our findings and conclusions are available in the article and its Additional files.


