Abstract
Major depressive disorder (MDD) is a leading cause of disability worldwide characterized by altered neuronal activity in brain regions involved in the control of stress and emotion. Although multiple lines of evidence suggest that altered stress-coping mechanisms underlie the etiology of MDD, the homeostatic control of neuronal excitability in MDD at the molecular level is not well established. In this review, we examine past and current evidence implicating dysregulation of the polyamine system as a central factor in the homeostatic response to stress and the etiology of MDD. We discuss the cellular effects of abnormal metabolism of polyamines in the context of their role in sensing and modulation of neuronal, electrical, and synaptic activity. Finally, we discuss evidence supporting an allostatic model of depression based on a chronic elevation in polyamine levels resulting in self-sustained stress response mechanisms maintained by maladaptive homeostatic mechanisms.
Keywords: Polyamine system, Major depression, suicide, excitability, homeostasis, ion channels, neurotransmitter receptors
Graphical Abstract
Major depressive disorder (MDD) is a leading cause of disability worldwide (WHO, 2011). Multiple lines of evidence suggest that altered stress-coping mechanisms underlie the etiology of MDD by affecting neuronal communication in brain areas involved in the control of emotions and in stress response. Genetic and environmental factors, such as childhood trauma and chronic stress, can also increase the risk of developing MDD and may determine individual differences in response to diverse forms and durations of stress (Hammen, 2005; Hankin et al., 2007; Kendler et al., 1998; Tennant, 2002). Little is known about whether homeostatic mechanisms are affected in MDD stress response at the molecular level. In this review, we examine past and current evidence implicating a dysregulation of the polyamine system in MDD as a central factor in the etiology of MDD and in the homeostatic response to stress.
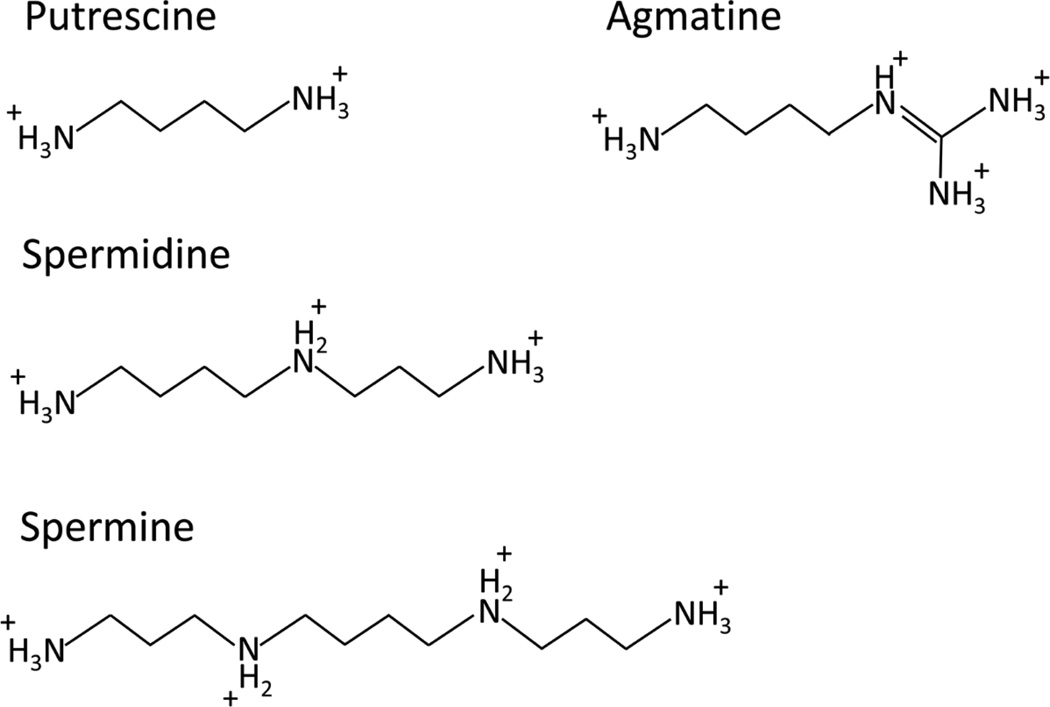
Polyamines (putrescine, spermidine, spermine, and agmatine) are ubiquitous, short, positively charged aliphatic amines that have fundamental roles in homeostatic mechanisms (Fig. 1). Their cellular roles include the regulation of gene transcription and post-transcriptional modifications, the modulation of synaptic activity, and the modulation of ion channels that participate in the excitability of neuronal networks (Table 1) and as reviewed by Pegg (Pegg, 2009)). Polyamine levels are tightly regulated by two key rate-limiting enzymes - Ornithine decarboxylase (ODC1) and Spermidine/Spermine N1-Acetyltransferase 1 (SAT1) (Fig. 2). ODC1 is the first enzyme in the synthesis of polyamines and produces putrescine from ornithine (Pegg, 2009); alternatively, putrescine can also be produced from agmatine by the enzyme agmatinase (AGMAT) (Sastre et al., 1996). Agmatine is also thought to be a neurotransmitter, with antagonistic effects at NMDA receptors and interactions with β2-adrenoreceptors (Reis and Regunathan, 2000), and has been shown to have antidepressant properties in animal models (Zomkowski et al., 2002), and in one clinical trial (Shopsin, 2013). Putrescine is the substrate to produce spermidine and spermine in sequential enzymatic reactions, while SAT1 is the rate limiting enzyme in the catabolism of polyamines (Pegg, 2009). Polyamine levels, particularly putrescine and agmatine, have been demonstrated to increase during stress, a phenomenon called the polyamine stress response (PSR) (Gilad and Gilad, 2003; Turecki, 2014). PSR magnitude is correlated with the intensity of the stressor and with the intensity of the behavioral response to stress, suggesting that a dysfunction of the polyamine system may be involved in the development of abnormal stress responses in conditions like MDD (Gilad and Gilad, 2003).
Figure 1. Chemical structure of positively charged polyamines in solution.
Table 1.
Membrane targets of polyamine modulation involved in control of electrical activity
| Category | Gene | Protein | Action | Function of protein |
|---|---|---|---|---|
| NMDA receptors | GRIN1 GRIN2A |
NR1A NR2A |
↓ Voltage dependent inhibition (NR1A-NR2A) (Gallagher et al., 1997; Traynelis et al., 1995; Williams et al., 1995; Williams et al., 1994; Zhang et al., 1994) ↑ Glycine-dependent stimulation (NR1A-NR2B) |
Glutamate receptor, synaptic component, Ca2+ permeability, Involved in LTD, LTP and excitotoxicity |
| GRIN2B | NR2B | ↑ Glycine independent- stimulation (NR1A-NR2B) ↑ Glycine-dependent stimulation (NR1A-NR2B) ↓ Decrease affinity for agonists (NR1A-NR2B) ↓ Voltage dependent inhibition (NR1A-NR2B) |
||
| AMPA receptors | GRIA1 GRIA2 GRIA3 GRIA4 |
GluR1 GLuR2(Q) GLuR3 GLuR4 |
↓ Voltage dependent blocking (inward rectification) (Hume et al., 1991; Verdoorn et al., 1991) |
Glutamate receptor, synaptic component, involved in induction of LTP, Ca2+ permeability |
| Kainate receptors | GRIK2 | GluR6(Q) | ↓ Inhibition by spermine and spermidine (Mott et al., 2003) |
Glutamate receptor, Synaptic component. |
| GLuR6(R) | ↑ Potentiation by relieving proton inhibition of the receptor |
|||
|
Ach nicotinic receptors |
CHRNA7 | α7 | ↓ Voltage dependent blocking (inward rectification)(Charnet et al., 1992; Gerzanich et al., 1994) |
Presynaptic and postsynaptic receptor. Modulates release of 5-HT, Dopamine and noradrenaline |
| CHRNA4 CHRNB2 |
α4β2 α4β2 |
↓ open pore blocker(Haghighi and Cooper, 1998) | ||
| K+ channels | KCNJ1 KCNJ2 |
Kir1.1 Kir2.1 |
↓ Voltage dependent blocking (mild inward rectification) (Ishihara et al., 1996; Kucheryavykh et al., 2007; Lopatin et al., 1994; Murrough et al., 2013; Yang et al., 1995) |
Voltage dependent K+ channels, modeling of action potentials, membrane potential. |
| KCNJ3 KCNJ4 |
Kir3.1 Kir2.3 |
↓ Voltage dependent blocking (strong inward rectification) |
||
| KCNJ5 | Kir3.4 | ↓ Voltage dependent blocking. Intermediate sensitivity |
||
| KCNJ10 | Kir1.2 | ↓ Fast blocking and posterior permeation |
||
| KCNQ2 KCNQ3 |
Kv7.2 Kv7.3 |
↓ Inhibition, indirectly by binding to intracellular PIP2 (Suh and Hille, 2007) (Chemin et al., 2005) |
Voltage dependent K+ channel. Inhibited by muscarine |
|
| KCNK2 | TREK-1 | ↓ Inhibition by intracellular spermine |
Control of resting membrane potential |
|
| KCNK9 | TASK-3 | ↓ Blocking by extracellular spermine (Musset et al., 2006) |
||
| H+ receptors | ASIC1 | ASIC1 | ↑ Spermine-mediated sensitization of ASIC1a (Babini et al., 2002; Li et al., 2010) |
Cationic permeability, |
| Na+ channels | ? | Nav ? | ↓ Activity dependent inhibition (Chen et al., 2007; Fleidervish et al., 2008) |
Initiation and maintenance of action potentials |
| Ca2+ channels | CACNA1B | Cav 2.2 (N-type) |
↓ Inhibition, shift in activation and decreased permeability (Chen et al., 2007; Cino and Formenti, 2008; Herman et al., 1993; Lasater and Solessio, 2002; Weiger and Hermann, 1994) |
Synaptic vesicular release, Ca2+ permeability |
| CACNA1C | Cav 1.2 (L-type) |
↑ PKC-mediated potentiation (Putrescine)(Herman et al., 1993) |
Ca2+ permeability, NMDA-independent LTP |
|
|
Ca2+ activated K+ channels |
KCNMA1 | BK, KCa1.1 |
↓ Blocking and reduction of open probability(Weiger and Hermann, 1994; Weiger et al., 1998) |
K+ permeability dependent on voltage and Ca2+ convergence |
|
Ca2+ activated cationic channel |
TRPM4 TRPM5 |
TRPM4 TRPM5 |
↓ Channel blocker, (Antagonist) (Nilius et al., 2004; Ullrich et al., 2005) |
Ca2+ permeability, Ca2+ sensor, intracellular ATP binding |
| TRPM7 | TRPM7 | ↓ Channel blocker, (inhibition) (Kerschbaum et al., 2003) |
||
| Capsaicin receptor | TRPV1 | TRPV1 | ↑ Agonist (Ahern et al., 2006) | Cationic permeability, involved in LTD |
|
Extracellular Ca2+ sensor |
CASR | CaS receptor |
↑ Agonist (Hofer and Brown, 2003; Quinn et al., 1997) |
Membrane Ca2+- sensing GPCR |
| Na+ leak channel | NALCN | NALCN | ↓ when coupled to Cas Receptor spermine inhibits the channel (Lu et al., 2010) |
Resting membrane potential and excitability |
|
Hyperpolarization- activated cyclic nucleotide-gated (HCN) channels |
HCN1 HCN2 HCN3 HCN4 |
HCN1 HCN2 HCN3 HCN4 |
↓ Voltage dependent blocking (inward rectification)(Vemana et al., 2008) |
Resting membrane, excitability. |
|
Intracellular enzymes |
PLCD1 | PLCδ1 | ↑ Endogenous activator (spermine) (Haber et al., 1991) |
Membrane Phospholipase |
| Transporter | SLC12A8 | CCC9 | Spermine and spermidine are substrates (Daigle et al., 2009) |
Na+/K+/Cl− membrane transporter |
| GABA receptor | ? | ? | Modulation of diazepam and flunitrazepam binding(Gilad et al., 1992a) |
Inhibitory receptor |
Abbreviations: LTP, long term potentiation; LTD, long term depression; GPCR, G-protein coupled receptor.
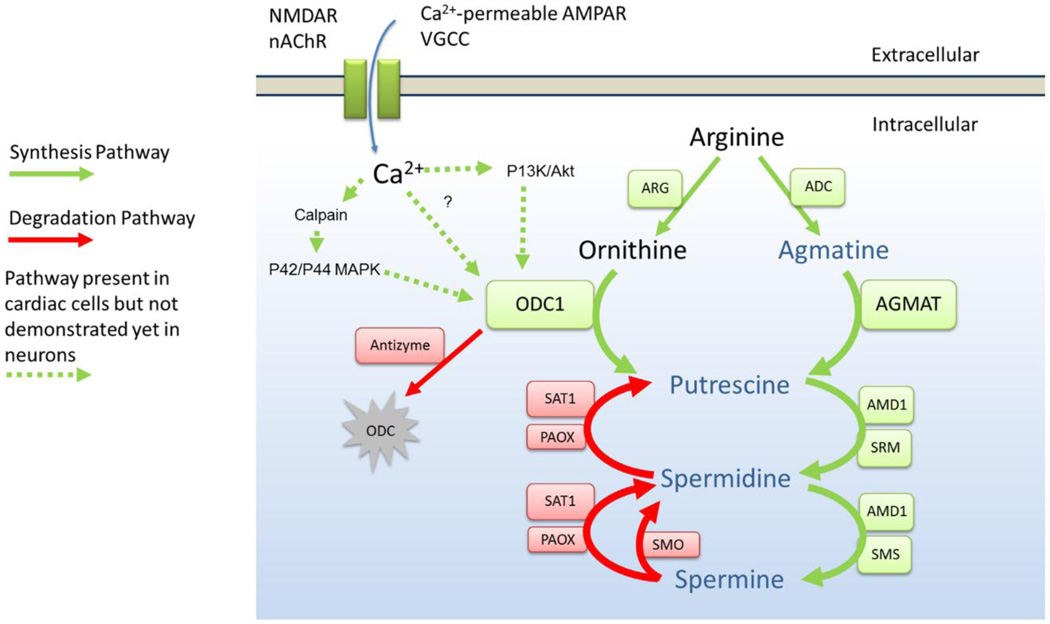
Figure 2. Polyamine metabolism and potential induction pathways in neurons.
Membrane proteins that permeate Ca2+ during cellular activity induce ODC activity and increases cytosolic polyamine concentration. Although It is not known if Ca2+ can directly induce ODC activity there are two alternative pathways that are known to induce ODC activity in cardiac cells. The activity of ODC is negatively regulated by antizyme and the concentration of polyamines are controlled by anabolic (green boxes) and catabolic (red boxes) enzymes. NMDAR, NMDA-type glutamate receptor; nAChR, nicotinic acetylcholine receptor; AMPAR, AMPA-type glutamate receptor; VGCC, voltage-gated Ca2+ channels; P42/P44 MAPK, P42/P44 mitogen-activated protein kinase signaling; P13K/AKt, phosphoinositide 3-kinase/Protein kinase B signaling; ODC, L-ornithine decarboxylase; AMD1, S-adenosylmethionine decarboxylase; SRM, Spermidine synthase; SMS spermine synthase; SMO, spermine oxidase; SAT1, spermidine/spermine-N1-acetyltransferase; PAOX, acetylpolyamine oxidase.
The effects of lithium in animal models and humans also evidence PSR alterations in MDD and suicide. Lithium is a commonly used mood stabilizer with significant anti-suicidal properties (Ahrens and Muller-Oerlinghausen, 2001; Bocchetta et al., 1998). Lithium has been shown to block PSR in the rodent brain when chronically delivered following dexamethasone treatment (Gilad and Gilad, 2003; Gilad et al., 1992b). A similar blockage of brain PSR is observed when lithium is administered pre or post the application of a stressful stimuli (Gilad and Gilad, 1996). Recently, Squassina and colleagues (Squassina et al., 2013) examined the effect of lithium on SAT1 gene expression in a cohort of lymphoblastoid cell lines which included cell lines from controls and subjects with bipolar disorder. The bipolar subjects were grouped into three suicide categories; suicide completers, high suicide risk and low suicide risk. Seven-day lithium treatment increased SAT1 gene expression for all groups except the suicide completers, suggesting differential regulation of SAT1 in suicide completers.
Gene expression studies in post-mortem human brains have consistently supported an alteration of the polyamine system in MDD and suicide. The first study suggesting a dysregulation of the polyamine system in MDD-suicides showed significant decreases in gene and protein expression of SAT1 in the precentral gyrus (BA4) and cortical frontal lobe areas (BA11 and BA8,9) in both non-depressed suicides and MDD-suicides compared to controls (Sequeira et al., 2006). This finding has been confirmed by our group and several others across brain regions and several population groups (Fiori et al., 2011; Guipponi et al., 2009b; Klempan et al., 2009a; Klempan et al., 2009b; Pantazatos et al., 2015; Sequeira et al., 2007). It was also demonstrated that the rs6526342 SNP, located in the promoter regulatory region of SAT1, had a significant effect on gene expression and that this SNP was associated with suicide in a French-Canadian sample (Sequeira et al., 2006), known to have a founder population effect (Scriver, 2001). The genetic effect of the rs6526342 SNP on brain SAT1 gene expression was confirmed in French-Canadian (Fiori et al., 2009) and Mexican (Tovilla-Zárate et al., 2015) cohorts but, not in more genetically heterogeneous European cohorts (Guipponi et al., 2009a), suggesting the presence of other genetic variants involved in the control of SAT1 gene expression. Other genetic association studies also showed an association between ODC1 SNPs (rs1049500 and rs2302614) and suicide attempts (Sokolowski et al., 2013). Ornithine decarboxylase antizyme 1, and 2 (OAZ1 and OAZ2), enzymes that control ODC1 activity by degradation (Fig. 2), have also been found to be upregulated in suicide completers across multiple psychiatric diagnoses including MDD (Gross et al., 2013). Moreover, gene expression of AMD1, which participates in the synthesis of spermidine and spermine from putrescine is altered in middle temporal cortex (BA21) of MDD individuals (Aston et al., 2005). Overall, these gene expression changes suggest abnormal concentrations of polyamines in MDD. Concordantly, putrescine and spermidine levels were significantly elevated in cortical areas (BA4, BA8/9, and BA11) of male MDD-suicide victims when compared to controls (Chen et al., 2010) and suggest that abnormal metabolism of polyamines might be involved in the pathophysiology of suicide and MDD by modulating stress response. Henceforth, we will discuss the polyamine system in the context of its role in sensing and controlling of neuronal electrical activity and its involvement in MDD and suicide.
Signatures of brain activity in MDD
Neuroimaging studies have shown that the strength of functional, reciprocal connectivity between the amygdala, a region critical in the generation and processing of negative emotions, and frontal cortical regions, which are involved in the modulation of amygdala reactivity and cognitive interpretation of negative stimuli, predicts successful emotional regulation (Banks et al., 2007; Comte et al., 2016; Hariri et al., 2002). Similar studies in people with MDD have shown amygdala hyperactivity at rest and during emotional and cognitive processing (Fales et al., 2008; Sheline et al., 2001; Siegle et al., 2002). In parallel, prefrontal cortex (PFC) areas like the left dorsolateral prefrontal cortex (DLPFC), dorsal anterior cingulate cortex (ACC) and rostral cingulate show decreased activity at rest and fail to regulate amygdala hyper-reactivity during processing of negative stimuli (Davidson et al., 2002; Mayberg et al., 1999; Pizzagalli et al., 2006). Hyperactivity during resting state in ventral anterior midline regions, like perigenual ACC, ventromedial prefrontal cortex (VMPFC), ventral striatum, putamen and the dorsomedial thalamus in MDD is also observed in homologous regions of animal models of depression (Alcaro et al., 2010; Krishnan and Nestler, 2008; Shumake and Gonzalez-Lima, 2003), suggesting shared mechanisms across biological species (Alcaro et al., 2010).
Evidence of regional hyperactivity in MDD patients comes principally from imaging studies measuring increased metabolism via blood-oxygen-level dependent (BOLD) signal using functional Magnetic Resonance Imaging (fMRI) (Hasler and Northoff, 2011). Simultaneous measurements of the BOLD signal and electrophysiological data in non-human primates have shown that the BOLD signal mostly represents: 1) excitatory and inhibitory synaptic activity, 2) voltage-dependent membrane oscillations, and 3) spike after-potentials, generated by Ca2+-activated K+ currents, that participate in the modulation of spiking frequency (Logothetis, 2003). In MDDs, the reported regional hyperactivity therefore most likely reflects enhanced firing rate and hyperexcitability of regions involved in stress response. Interestingly, MDD patients have 3 to 7-fold higher risk of developing epilepsy, and epilepsy itself increases the risk of developing MDD (Danzer, 2012; Forsgren and Nystrom, 1990; Hesdorffer et al., 2000; Kanner, 2011), supporting the idea that hyperexcitability is one of the key features of MDD.
In animal models, stress at early prenatal developmental stages increases the risk of seizures later in life due to hyperexcitability of the amygdala and the hypothalamic-pituitary-adrenal (HPA) axis (Dube et al., 2015). Similarly, chronic stress produces depression-like behavioral changes in rodents (Rosenkranz et al., 2010) that correlate with unambiguous evidence of hyperexcitablity in lateral amygdala (LAT) neurons. Interestingly, this hyperexcitablity is better explained by reduction in the expression of Ca2+-activated K+ channels than by the reduction of synaptic inhibitory control (Rosenkranz et al., 2010). In summary, neuronal excitability alterations suggest a dysregulation in activity-feedback mechanisms involved in the development of MDD.
Polyamines in activity-feedback mechanisms
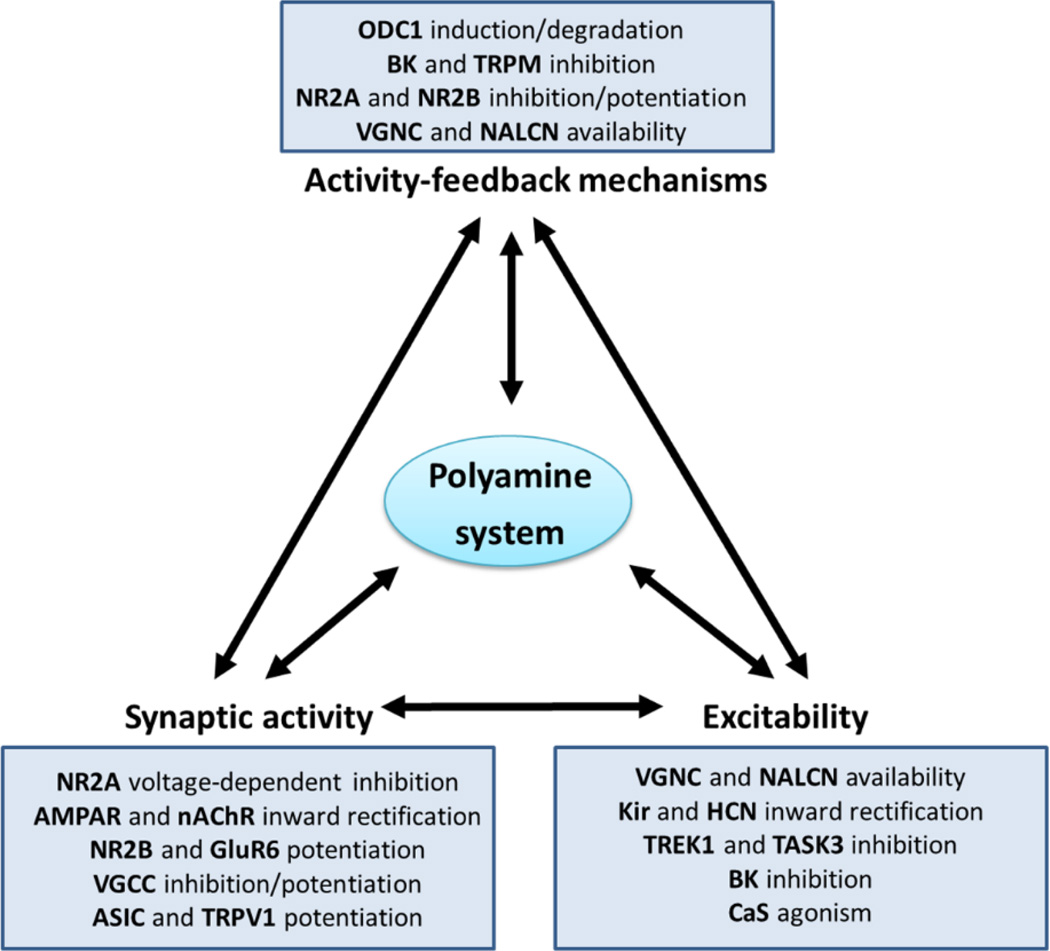
While the role of polyamines on synaptic and ion channel modulation is well known, their role in activity-feedback mechanisms that control neuronal firing rate is somewhat less recognized. Evidence from the past three decades shows that firing rate and ODC1 activity are tightly correlated across a broad range of excitability levels, from small changes of ODC1 occurring during physiological stimulation of visual pathways, to large increments (≥ 50-fold) during epileptiform activity (Baudry et al., 1986) or electrical stimulation (Bondy et al., 1987; Pajunen et al., 1978). Notably, even small changes in the concentration of polyamines during physiological stimulation can have important modulatory effects on excitatory synaptic inputs that modulate sensory experience (Aizenman et al., 2002), indicating that the polyamine system participates in activity-feedback mechanism that sense and regulate neuronal activity (Fig. 3).
Figure 3. Polyamine system interdependence with fundamental properties of neuronal electrical activity.
Each box indicates the membrane protein targets (in bold) that are modulated by cytosolic or extracellular polyamines. More information about the specific type of modulation is found in table 1.
How neuronal activity induces ODC1 is not clearly understood but in peripheral tissue there is evidence of a physiological coupling between polyamine levels and intracellular calcium (Ca2+) concentrations (Chang, 1991; Koenig et al., 1987; Langdon et al., 1984). In a pioneer study of a phenomenon called the calcium paradox, Koening et al. linked Ca2+ and polyamines with excitability and pathological consequences of maladaptive homeostatic mechanisms in the heart (Koenig et al., 1987). Koening et al. (1987) demonstrated that changes in ODC1 activity and polyamine concentrations followed changes in intracellular Ca2+ concentration ([Ca2+]) with a time delay of approximately 15 seconds. These series of experiments showed that ODC1 and polyamines are part of an activity feedback mechanisms involving intracellular [Ca2+], and that abnormal polyamine-mediated compensation may trigger pathological excitability, intracellular [Ca2+] overload, and cell death (Koenig et al., 1987). Similarly, in neurons, a reduction in extracellular [Ca2+] from 2 to 1 mM produces hyperexcitability and seizure-like activity (Isaev et al., 2012). Interestingly, a Ca2+ sensor receptor (CaSR) that is activated by both Ca2+ and spermine, inhibits the Na+ leak channel (NALCN), so when Ca2+ concentrations decrease, the CaSR can no longer inhibit the NALCN channel producing a continuous depolarizing Na+ current that increases excitability (Lu et al., 2010).
Induction of ODC1 by inflow of extracellular [Ca2+] has also been observed in lymphocytes (Otani et al., 1985), osteoblasts (Van Leeuwen et al., 1989), and pancreatic (Chang, 1991) and epithelial cells (Langdon et al., 1984). In cardiac cells, this ODC1 induction is caused by the activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2; also known as P42/P44 MAPK), and P13K/Akt pathways (Pernet et al., 2007; Zhang et al., 2014b). ODC1 activation by ERK1/2 is also dependent on Ca2+-mediated activation of calpain (Moldoveanu et al., 2002). Interestingly, downregulation of mRNA, protein expression of ERK1/2 and activity of the P42/P44 MAPK complex was reported in the prefrontal cortex (PFC) and hippocampus of MDD-suicide subjects (Dwivedi et al., 2001). Moreover, dual-specificity phosphatase-1 (DUSP1), which is a negative modulator of ERK1/2, was significantly upregulated in CA1 and dentate gyrus (DG) of postmortem MDD subjects (Duric et al., 2010). In animal models acute stress leads to ERK1/2 activation in ventral regions of CA1 and DG, and in the basolateral amygdala (Ritov et al., 2014). In agreement with a role of stress in the activation of ERK1/2, intracerebroventricular injections of the stress hormone corticotropin-releasing hormone (CRH) activates ERK1/2 in principal neurons in CA1 and CA3 of the hippocampus and the lateral and basolateral amygdala (Refojo et al., 2005). Notably, chronic stress leads to hyperphosphorylation of ERK1/2 in distal dendrites of layers II and III of the medialPFC (mPFC) (Trentani et al., 2002), suggesting that activation of ERK1/2 may have localized effects on dendrites and synaptic inputs that alter polyamine biosynthesis metabolism and levels of polyamines locally. Future studies aiming to determine how electrical activity, intracellular [Ca2+] and polyamines are linked through ERK1/2 pathways in neurons is a research priority.
Modulation of neuronal excitability by cytosolic polyamines
Polyamines modulate a broad range of neurotransmitter receptors and ion channels that permeate cations (Table 1). Some polyamine targets drive excitability (e.g. Voltage gated Na+ and Ca2+ channels, ionotropic glutamate and nicotinic receptors) and others maintain resting membrane potential and modulate neuronal firing rate (e.g. inward rectifiers K+ channels). Therefore, the net effect of polyamine levels on neuronal excitability depends on the particular set, and proportions, of membrane receptors and ion channels in any given region of the brain.
The most well known modulatory effect of cytosolic polyamines on excitability is to limit membrane permeability to cations at depolarized voltages (e.g. voltage-dependent block). For example, polyamines are responsible for the ion current rectification of Ca2+-permeable AMPA receptors (Hume et al., 1991; Pellegrini-Giampietro, 2003; Verdoorn et al., 1991), inward rectifying K+ channels (Ishihara et al., 1996; Kucheryavykh et al., 2007; Lopatin et al., 1994; Yang et al., 1995) and neuronal nicotinic receptors (Haghighi and Cooper, 2000). Polyamines also contribute, along with Mg+, to the rectification of hyperpolarization-activated cyclic nucleotide-gated channels (HCN) (Vemana et al., 2008).
Polyamines normally block AMPA and nicotinic receptors limiting the flow of Na+ and Ca2+ into the cell during large depolarizations, thereby preventing hyperexcitability and Ca2+ overloading. Conversely, a voltage-dependent block of inward rectifying K+ currents by polyamines can limit the efflux of K+ during the initiation phase of the action potential, thus promoting excitability. In both situations, polyamines determine a temporal- and voltage-dependent window of cation permeability that is optimal for coherent electrical activity and neuronal communication. Polyamines also block Ca2+-permeable AMPA receptors in resting synapses and this block is only removed by fast synaptic activity, whereby high frequency stimulation results in reduction of the polyamine block and potentiation of excitatory synapses. This state-dependent polyamine block of Ca2+-permeable AMPA receptors highlights a role of polyamines in the determination of the timing of synaptic activity (Traynelis et al., 2010).
In general, polyamines have “excitability buffer” properties as they can also negatively modulate the open probability of voltage gated Na+ channels (Chen et al., 2007; Fleidervish et al., 2008). Depletion of cytosolic polyamines in layer 5 cortical pyramidal neurons results in increased amounts of openings of Na+ channels producing a persistent Na+ current and enhanced excitability, leading to spontaneous activity and paroxysmal discharges in these cells (Fleidervish et al., 2008). In contrast, the presence of polyamines reduces the firing of neurons by decreasing the openings of Na+ channels (Fleidervish et al., 2008). Thus, when ODC1 is induced by electrical activity, the increased concentration of cytosolic polyamines decreases the amplitude of Na+ currents, therefore acting as an important intracellular feedback mechanism to maintain firing rate near a physiological set point.
Modulation of synaptic activity by extracellular polyamines
Polyamines reach the extracellular space by Ca2+-independent and Ca2+-dependent efflux processes (Fage et al., 1993; Fage et al., 1992; Harman and Shaw, 1981). Ca2+-dependent processes can be triggered by depolarization of the membrane via activation of NMDA receptors or blockade of Na+/K+ transporters (Fage et al., 1993; Fage et al., 1992; Masuko et al., 2003). Additionally, at highly depolarized voltages internal polyamines can permeate through Ca2+-permeable AMPA receptors resulting in the efflux of polyamines to the extracellular space (Traynelis et al., 2010). Thus, overactivation of NMDA receptors or reduced activity of ion pumps by energetic deficiencies may enhance the release of polyamines into the extracellular space where they can positively modulate synaptic receptors like the transient receptor potential cation channel subfamily V member 1 (TRPV1), acid-sensing ion channel 1a (ASIC1a) and some subtypes of NMDA receptors (Table 1).
TRPV1, also known as the capsaicin or vanilloid 1 receptor, is a receptor gated by heat, acidic pH, and endogenous lipids termed “endovanilloids” like unsaturated N-acyldopamines, lipoxygenase products of arachidonic acid and the endocannabinoid anandamide (Van Der Stelt and Di Marzo, 2004). In humans TRPV1 is expressed in hippocampus (Cristino et al., 2006), amygdala (Zschenderlein et al., 2011) and hypothalamus (Cavanaugh et al., 2011), brain regions that participate in the modulation of the stress response. Animal model studies have shown that synaptic TRPV1 activation is critical for long term depression (LTD) in interneurons and participate in long term potentiation (LTP) mechanisms in hippocampal neurons (Brown et al., 2013; Chavez et al., 2010). TRPV1 is sensitized by physiological concentrations of polyamines which lead to a potentiation of responses gated by endogenous agonists (Ahern et al., 2006). At high concentrations, polyamines directly activate TRPV1, with spermine producing the largest response followed by spermidine and putrescine. Because TRPV1 is permeable to polyamines, TRPV1 has also been proposed to be involved in polyamine transport (Ahern et al., 2006). Interestingly, a TRPV1-KO (knockout) mouse model shows less anxiety-related behavior and impaired hippocampus dependent fear memory (Marsch et al., 2007). Because of the TRPV1-KO resilient phenotype, TRPV1 polyamine activation might have a protective effect against anxiety and depression.
Overexpression of the acid-sensing ion channel 1a (ASIC1a) increases amygdala activity and conditioned fear (Wemmie et al., 2004). ASIC1a is involved in the induction of synaptic long term potentiation (Du et al., 2014), is robustly expressed in the amygdala, cingulate and nucleus accumbens (Wemmie et al., 2004) and is potentiated by extracellular polyamines (Babini et al., 2002). Conversely, ASIC1a-KO mice show attenuated amygdala activity and, similarly to TRPV1-KO mice, these mice display depressive-resistant behaviors (Coryell et al., 2009; Coryell et al., 2007; Wemmie et al., 2003). Restoration of ASIC1a in the amygdala eliminates the depressive-resistant phenotype of the knockout mice, suggesting a link between ASIC1a expression in the amygdala and depressive behaviors.
NMDA receptors are central in brain function because they participate in synaptic plasticity regulating neuronal communication and modulating behavior (Traynelis et al., 2010). Interestingly, among NMDA receptors, NR2B-containing receptors are the most sensitive to polyamine modulation (reviewed by (Igarashi and Kashiwagi, 2010; Mony et al., 2009; Rock and Macdonald, 1995)). Polyamines enhance ion currents trough NR2B-containing NMDA receptors by glycine-dependent and glycine independent potentiation of ion currents. Polyamines increase the affinity for glycine, an obligated co-agonist of NMDA receptors and reduce proton-mediated tonic inhibition of NMDA receptors at physiological pH (reviewed by (Johnson, 1996)). Interestingly, the binding site for polyamines in NR2B receptors is adjacent to the binding site for NR2B antagonists with antidepressant effects (e.g. ifenprodil).
Role of polyamines in stress response and depression
It is well known in animal models that stress activates both the amygdala and the HPA axis, and triggers the PSR, it is not very clear however how regional increases in activity and the PSR are mechanistically linked. Based on the available evidence, activation of the amygdala-HPA axis during stressful events increases neuronal firing and cytosolic [Ca2+], which, consequently, will produce a fast, neuroprotective increase in ODC1 activity and synthesis of polyamines that would reduce the open probability of Na+ channels (Fleidervish et al., 2008). The resulting reduction in Na+ currents will lower the electrical firing rate and prevent neuronal hyperexcitability. Thus, the PSR may be part of redundant homeostatic mechanisms aimed to regulate the firing rate and amygdala-HPA axis activity during stressful events.
Because polyamines have important roles in the regulation of gene transcription and post-transcriptional modifications, chronic elevation in polyamine levels, by either ODC1 overactivity or deficits in catabolic enzymes like SAT1, can lead to compensatory regulation of membrane proteins involved in excitability, including Na+ channels. Indeed, the SCN10A gene, which codes for Nav1.8 sodium channels, was differentially expressed in MDD and MDD-suicide postmortem brains (Sequeira et al., 2009). Also, the subunit SCN8A, coding for Nav1.6 and found to be highly expressed in the CNS and the hippocampus (Hawrylycz et al., 2012), has been associated with suicide attempts (Wasserman et al., 2005).
Many gene targets of cytosolic polyamines are also affected in MDD and suicide. GRIA3 downregulation in BA46 of suicide victims without depression as well as in MDD-suicides, and GRIA4 and GRIA2 increased expression in the amygdala and the frontal pole (BA10) in MDD-suicides have been reported using microarrays (Sequeira et al., 2009). Meanwhile, two SNPs (rs4825476 and rs2518224) located within GRIA3 and GRIK2, the latter encoding for a kainate receptor modulated by polyamines, have been significantly associated with treatment-emergent suicidal ideation in MDD (Laje et al., 2007). Similarly, epigenetic studies have found that GRIK2 in MDD and suicide cases is hypomethylated in intron13 suggesting a prevalence of specific splice variants of kainate receptors in MDD and suicide (Nagy et al., 2014).
The CACNA1C gene, coding for the α1c subunit of the Cav1.2 voltage dependent L-type Ca2+ channel, has also been associated with mood disorders and MDD (Bhat et al., 2012; Casamassima et al., 2010; Dao et al., 2012; Liu et al., 2011; Shi et al., 2011). L-type Ca2+ currents in hippocampus and amygdala are enhanced by corticosteroids through increased synthesis of voltage gated Ca2+ channels (Joels and Karst, 2011), and participate in non-NMDA synaptic plasticity events and the formation of fear memory (Bauer et al., 2002). L-type Ca2+ channels expressed in neuroblastoma cells are enhanced by cytosolic putrescine, but not spermidine or spermine, via the protein kinase C (PKC) pathway (Herman et al., 1993). During episodes of amygdala excitability, increased activity of ODC1 and higher levels of polyamines will likely increase the consolidation of non-NMDA-dependent fear memory.
Several population based genetic studies have also shown an association between K+ channels modulated by polyamines and depression. In patients with bipolar disorder for instance, two SNPs (rs2190547 and rs41368245) located near the KCNJ2 gene, were associated with the number of depressive episodes (Fabbri and Serretti, 2016). The KCNJ2 gene is of interest because it codes for a K+ channel with strong polyamine-dependent inward rectification (Table 1), and is a gene in which mutations have been implicated in the pathogenesis of Andersen–Tawil syndrome which includes major depression among its clinical symptoms (Chan et al., 2010).
Similarly, a strong association between the rs6686529 SNP, within the KCNK2 gene (potassium channel, subfamily K, member 2; also known as TREK-1), and both MDD and antidepressant response was observed in a clinical cohort. Individuals with homozygous genotypes (CC or GG) for rs6686529 showed greater susceptibility to MDD than those heterozygous for the SNP. Concerning antidepressant response, C-allele carriers demonstrated better remission rates following treatment (Liou et al., 2009). In humans and rodents TREK-1 is highly expressed in the putamen, caudate, amygdala and hippocampus supporting a role in the control of emotion and stress (Hawrylycz et al., 2012; Meadows et al., 2000). The modulation of TREK-1 by polyamines is dependent on the presence of phosphatidylinositol 4,5-bisphosphate (PIP2) in the membrane. At rest, cytosolic spermine keeps TREK-1 in a closed and inactive state, however, incorporation of PIP2 into the inner leaflet of the membrane counteracts the polyamine inhibition and renders the channel ready to be activated by stretch, membrane depolarization or H+ (Chemin et al., 2005). Interestingly, deletion of TREK-1 in mice produces a phenotype that is resistant to induction of depressive behavior, shows enhanced 5-HT transmission in neurons from the hippocampus and dorsal raphe nucleus, and is insensitive to antidepressants typically enhancing 5-HT neurotransmission (e.g. fluoxetine) (Heurteaux et al., 2006). These results strongly suggest that TREK-1 participates in the abnormal hyperexcitability, which characterizes MDD, and might be a potential pharmacological antidepressant target (Heurteaux et al., 2006).
Potassium channels can be modulated by polyamines. The large conductance Ca2+-activated K+ channels (BK), encoded by the KCNMA1 gene, are also activated by voltage and can regulate neuronal excitability by modifying K+ permeability depending on the cytosolic Ca2+ concentration and voltage depolarizations (Hille, 2001). BK currents participate in the repolarization and after-hyperpolarization of the action potential and their blockade by toxins leads to increases in excitability (Gribkoff et al., 2001; Limon et al., 2005). Because BK channels are blocked by cytosolic polyamines (Weiger and Hermann, 1994), an increase in polyamine levels in cells with large expression of BK channels may lead to hyperexcitability. In support of this process, acute and chronic stress has been shown to reduce BK expression in the lateral amygdala of rodents leading to hyperexcitability (Guo et al., 2012; Rosenkranz et al., 2010). Moreover, hyperexcitability in lateral amygdala increased NMDA-mediated excitability of thalamo-amygdalar connections (Rosenkranz et al., 2010), a potential step in the transition to a depressive state. Notably, the rs10740467 SNP near KCNMA1 is one of the most significant markers of comorbidity between MDD and anxiety (Schosser et al., 2013).
Neuronal nicotinic acetylcholine receptors (nAChRs) show polyamine-mediated inward rectification and are blocked by extracellular polyamines (Cooper, 2001). Interestingly, nAChRs have a long and complex history of involvement in stress and MDD. In humans and animal models, blocking the degradation of ACh triggers anxiety and depression (Janowsky et al., 1972; Mineur et al., 2013) and pharmacological antagonism of nAChRs has an antidepressant effect in mice by reducing neuronal activity in the basolateral amygdala. Downregulation of homomeric α7 receptors or heteromeric nAChRs containing the β2 subunit in the amygdala mimics the effects of antidepressants (Mineur et al., 2015). Moreover, depressive symptoms and nicotine dependence are strongly associated across age and duration of lifetime smoking exposure (Dierker et al., 2015). It is still not clear however whether the association between MDD and nicotine-dependence results from one causing the other, or from shared neurobiological circuitry. Association studies found that the rs2072660 SNP in the 3’ UTR of the CHRNB2 gene, encoding the β2 nAChR subunit, is associated with suicidal ideation but, has no direct association with MDD unless maternal bonding has been altered (Csala et al., 2015). CHRNB2 has also been shown to have higher levels of methylation in neurons of suicide completers (Labonte et al., 2013).
In addition to nAChRs’ role in depression, the antidepressant action of scopolamine, a non-specific muscarinic acetylcholine receptor (mAChR) antagonist, indicates that global alterations of cholinergic neurotransmission are present in MDD (Drevets and Furey, 2010; Furey and Drevets, 2006), and because nAChRs and mAChRs have synergic activity on several cognitive functions (Ellis et al., 2006), it is likely that maladaptive interactions between these classes of receptors participate in the maintenance of the depressive status.
Maybe the strongest evidence for the participation of NMDA receptors in MDD came from the fast antidepressant effects of sub-anesthetic doses of ketamine, an antagonist of NMDA receptors with consistent antidepressant and anti-suicidal effects (reviewed by (Reinstatler and Youssef, 2015)). A temporal strengthening of synaptic connectivity in PFC areas seems to be responsible for ketamine effects in MDD, and two principal hypotheses have been proposed to explain the mechanism (Duman et al., 2012; Miller et al., 2016). The first proposes that the direct inhibition of NR2B receptors in cortical, excitatory pyramidal neurons produces a homeostatic upregulation of excitatory drive into these neurons, which leads to enhanced connectivity in PFC areas (Li et al., 2010; Maeng et al., 2008). The other hypothesis proposes that the main target of ketamine are NMDA receptors located at the soma of cortical interneurons which provide inhibitory tone to pyramidal neurons; thus, antagonism of NR2B receptors in interneurons reduces inhibitory drive to principal neurons leading to disinhibition and enhanced connectivity in PFC areas (Miller et al., 2016). Interestingly, both hypotheses require the participation of NR2B receptors, these receptors are highly sensitive to polyamine potentiation. Consistent with a specific role of enhanced NR2B in the pathophysiology of MDD, antagonists of NR2B should produce antidepressant actions similar to ketamine (Maeng et al., 2008; Preskorn et al., 2008).
Although the effects of ketamine and other NR2B-specific antagonists develop fast (sometimes within 20 min (Reinstatler and Youssef, 2015)), they only last for about a week. This temporary effect suggests an increased activity of NR2B in MDD, a possible consequence of a self-sustained maladaptive PSR. In a polyamine-mediated allostatic equilibrium, the pharmacological reduction of NR2B will produce a deviation from the excitability set-point that will be gradually compensated for by other polyamine targets. Therefore, determining whether the expression of NR2B receptors is altered in MDD or if their function is enhanced by abnormal concentrations of polyamines is a research priority.
Association studies have found SNPs in GRIN2B, the NR2B subunit coding gene, to be associated with MDD. Thus, the rs220549 SNP was found to be associated with neuroticism, a risk factor for MDD in an European cohort (Aragam et al., 2013). Zhang et al., found that the GRIN2B A-G haplotype of the rs1805502 and rs890 SNPs might have a protective role in MDD in a sample of Han Chinese, whereas the G-T haplotype may be a risk factor for the development of treatment-resistant depression (Zhang et al., 2014a). Recent data have found that females with MDD have a higher expression of NMDA receptors in the DLPFC with the expression of GRIN2B also being higher in overall MDD-suicides than in MDD (Gray et al., 2015).
Further studies have highlighted a more specific role of NR2B as a central factor in the pathophysiology of MDD. In animal models NR2B expression is upregulated after stress and its inhibition is sufficient to prevent behavioral deficits induced by stress (Li et al., 2010; Wong et al., 2007). Notably, a mouse model that overexpresses reelin, an extracellular protein involved in neuronal development and glutamatergic neurotransmission (Herz and Chen, 2006), showed less anxiety and was less susceptible to depressive-like behaviors after chronic corticosterone treatment compared to WT mice (Teixeira et al., 2011). This depressive-resistant effect was correlated with a reduction in the expression of NR2B receptors, despite NMDA currents being larger in the reelin-overexpressing mice (Teixeira et al., 2011).
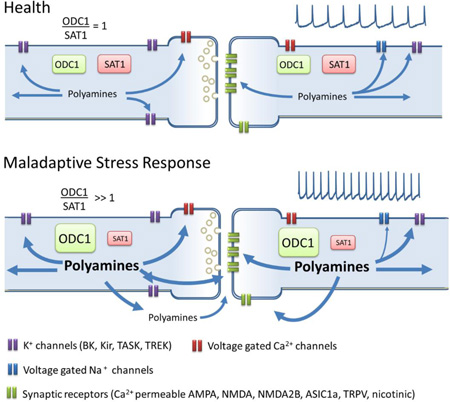
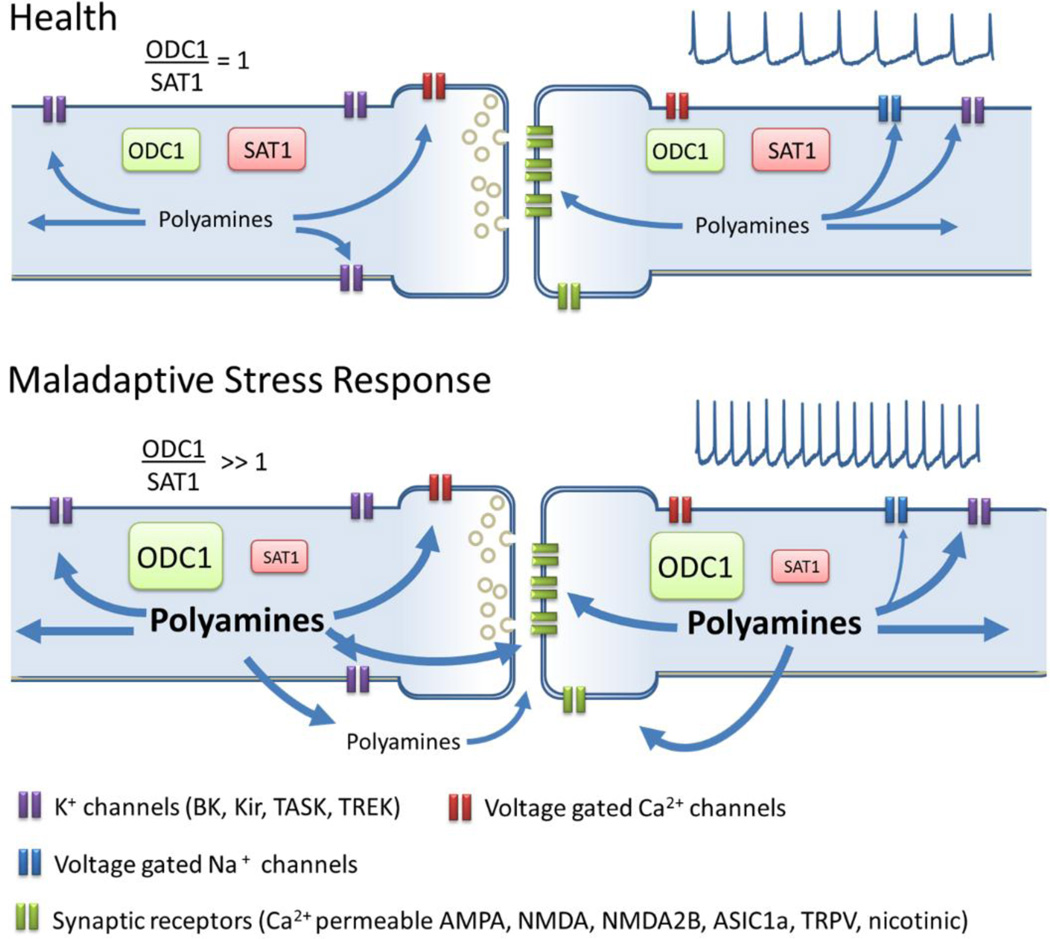
Overall, stress mediated hyperactivity in brain areas involved in depression can result in changes in polyamine metabolism and altered polyamine levels, which can have detrimental effects through modulation of synaptic transmission. A Maladaptive stress response in at risk patients can lead to abnormal polyamine metabolic ratio, due to ODC1 overactivity or SAT1 deficits, this metabolic imbalance can lead to long term increases in polyamines concentrations that saturates their “excitability buffer” capacity and lead to hyperexcitation (Fig. 4).
Figure 4. Polyamine dysregulation in MDD.
In health a balanced polyamine metabolism (represented by the ratio ODC1/SAT1 = 1) will provide a concentration of polyamines required for the normal modulation of cationic receptors and channels. Abnormal polyamine metabolic ratio, due to ODC1 overactivity or SAT1 deficits, may produce a long term increase in polyamines concentrations that saturates its “excitability buffer” capacity and lead to hyperexcitation. Excess of polyamines may increase the efflux of polyamine and potentiate excitatory synapses through sensitization of NMDAB, ASIC1a, nicotinic and TRPV receptors that are highly expressed in brain regions involved in emotion and stress control, and are positively modulated by polyamines. Long term stress-driven and polyamine-enabled excitation may lead to remodeling of neuronal properties that underlies the hyperexcitability and hyper-reactivity found in MDD.
Conclusions
In this review, we summarize evidence in support of an allostatic model of depression. According to this model, chronic elevations of polyamine levels result in a self-sustained stress response which is maintained by maladaptive homeostatic mechanisms (Fig. 4). Specifically, in vulnerable individuals exposure to chronic or acute stress can lead to hyperexcitabity of the amygdala, which will induce a persistent elevation of ODC1 gene expression leading to subsequent increases in polyamine levels.
Under normal conditions a transient induction of cytosolic polyamines occurs and excitability is buffered through modulation of Na+, K+ and Ca2+ channels, as discussed above. However, in depression this increase in polyamine levels does not return to baseline, due to an excess of polyamine anabolism, deficits in polyamine catabolism, and alterations of polyamine targets, and a feed-positive mechanism of excitability is set in motion. Chronic stress and high amygdala activity will maintain high levels of cytosolic polyamines potentiating L-type Ca2+ channels and blocking K+ and Ca2+-activated K+ channels, promoting excitability. The resultant high levels of electrical and neuronal activity may lead to energetic deficits that, together with high concentrations of cytosolic polyamines, would incrementally increase polyamine efflux to the extracellular space where they positively modulate ASIC1, TRPV1, and NR2B and further promote excitability. Under these conditions PFC circuits and compensatory mechanisms cannot modulate amygdala hyperexcitability. Therefore, changes in intrinsic properties and remodeling of neuronal connectivity are put in place until a novel allostatic equilibrium within frontocortical-limbic structures, with higher amygdala activity is established. A better understanding of the role of polyamines during the transition from health to MDD and the compensatory changes occurring in neuronal activity, within the context of excitability, may identify novel nodes of homeostatic control that could be susceptible to therapeutic manipulation.
Highlights.
Evidence of polyamine dysregulation in neuronal hyperexcitability in Major Depressive Disorder
Table of extracellular and intracellular polyamine membrane targets involved in the control of neuronal excitability
Examination of the polyamine system in activity-feedback mechanisms.
Discussion of polyamines as mediators of allostasis in Major Depressive Disorder.
Acknowledgments
The research was supported by The Brain & Behavior Research Foundation (former NARSAD) Young Investigator Award (AS) and by NIMH research grant R01MH097082 (AS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern GP, Wang X, Miyares RL. Polyamines are potent ligands for the capsaicin receptor TRPV1. J Biol Chem. 2006;281:8991–8995. doi: 10.1074/jbc.M513429200. [DOI] [PubMed] [Google Scholar]
- Ahrens B, Muller-Oerlinghausen B. Does lithium exert an independent antisuicidal effect? Pharmacopsychiatry. 2001;34:132–136. doi: 10.1055/s-2001-15878. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Munoz-Elias G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron. 2002;34:623–634. doi: 10.1016/s0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci Biobehav Rev. 2010;34:592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Aragam N, Wang KS, Anderson JL, Liu X. TMPRSS9 and GRIN2B are associated with neuroticism: a genome-wide association study in a European sample. J Mol Neurosci. 2013;50:250–256. doi: 10.1007/s12031-012-9931-1. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Lynch G, Gall C. Induction of ornithine decarboxylase as a possible mediator of seizure-elicited changes in genomic expression in rat hippocampus. J Neurosci. 1986;6:3430–3435. doi: 10.1523/JNEUROSCI.06-12-03430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, Gould TD. CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol. 2012;99:1–14. doi: 10.1016/j.pneurobio.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchetta A, Ardau R, Burrai C, Chillotti C, Quesada G, Del Zompo M. Suicidal behavior on and off lithium prophylaxis in a group of patients with prior suicide attempts. J Clin Psychopharmacol. 1998;18:384–389. doi: 10.1097/00004714-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Mitchell CL, Rahmaan S, Mason G. Regional variation in the response of cerebral ornithine decarboxylase to electroconvulsive shock. Neurochem Pathol. 1987;7:129–141. doi: 10.1007/BF02834213. [DOI] [PubMed] [Google Scholar]
- Brown TE, Chirila AM, Schrank BR, Kauer JA. Loss of interneuron LTD and attenuated pyramidal cell LTP in Trpv1 and Trpv3 KO mice. Hippocampus. 2013;23:662–671. doi: 10.1002/hipo.22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassima F, Huang J, Fava M, Sachs GS, Smoller JW, Cassano GB, Lattanzi L, Fagerness J, Stange JP, Perlis RH. Phenotypic effects of a bipolar liability gene among individuals with major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:303–309. doi: 10.1002/ajmg.b.30962. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HF, Chen ML, Su JJ, Ko LC, Lin CH, Wu RM. A novel neuropsychiatric phenotype of KCNJ2 mutation in one Taiwanese family with Andersen-Tawil syndrome. J Hum Genet. 2010;55:186–188. doi: 10.1038/jhg.2010.2. [DOI] [PubMed] [Google Scholar]
- Chang BK. Inhibitory effects of a calcium antagonist on ornithine decarboxylase induction in pancreatic cancer cell lines. Pancreas. 1991;6:631–636. doi: 10.1097/00006676-199111000-00003. [DOI] [PubMed] [Google Scholar]
- Charnet P, Labarca C, Cohen BN, Davidson N, Lester HA, Pilar G. Pharmacological and kinetic properties of alpha 4 beta 2 neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Physiol. 1992;450:375–394. doi: 10.1113/jphysiol.1992.sp019132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GG, Fiori LM, Moquin L, Gratton A, Mamer O, Mechawar N, Turecki G. Evidence of altered polyamine concentrations in cerebral cortex of suicide completers. Neuropsychopharmacology. 2010;35:1477–1484. doi: 10.1038/npp.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Harnett MT, Smith SM. Modulation of neuronal voltage-activated calcium and sodium channels by polyamines and pH. Channels (Austin) 2007;1:281–290. doi: 10.4161/chan.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cino I, Formenti A. Spermine biphasically affects N-type calcium channel currents in adult dorsal root ganglion neurons of the rat. Biochim Biophys Acta. 2008;1778:2437–2443. doi: 10.1016/j.bbamem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Comte M, Schon D, Coull JT, Reynaud E, Khalfa S, Belzeaux R, Ibrahim el C, Guedj E, Blin O, Weinberger DR, Fakra E. Dissociating Bottom-Up and Top-Down Mechanisms in the Cortico-Limbic System during Emotion Processing. Cereb Cortex. 2016;26:144–155. doi: 10.1093/cercor/bhu185. [DOI] [PubMed] [Google Scholar]
- Cooper E. Nicotinic acetylcholine receptors on vagal afferent neurons. Ann N Y Acad Sci. 2001;940:110–118. doi: 10.1111/j.1749-6632.2001.tb03670.x. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Csala I, Egervari L, Dome P, Faludi G, Dome B, Lazary J. The possible role of maternal bonding style and CHRNB2 gene polymorphisms in nicotine dependence and related depressive phenotype. Prog Neuropsychopharmacol Biol Psychiatry. 2015;59:84–90. doi: 10.1016/j.pnpbp.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Daigle ND, Carpentier GA, Frenette-Cotton R, Simard MG, Lefoll MH, Noel M, Caron L, Noel J, Isenring P. Molecular characterization of a human cation-Cl− cotransporter (SLC12A8A, CCC9A) that promotes polyamine and amino acid transport. J Cell Physiol. 2009;220:680–689. doi: 10.1002/jcp.21814. [DOI] [PubMed] [Google Scholar]
- Danzer SC. Depression, stress, epilepsy and adult neurogenesis. Exp Neurol. 2012;233:22–32. doi: 10.1016/j.expneurol.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O'Donnell P, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, Levinson DF, Thompson SM, Potash JB, Gould TD. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 2012;68:801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Dierker L, Rose J, Selya A, Piasecki TM, Hedeker D, Mermelstein R. Depression and nicotine dependence from adolescence to young adulthood. Addict Behav. 2015;41:124–128. doi: 10.1016/j.addbeh.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Reznikov LR, Price MP, Zha XM, Lu Y, Moninger TO, Wemmie JA, Welsh MJ. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc Natl Acad Sci U S A. 2014;111:8961–8966. doi: 10.1073/pnas.1407018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube CM, Molet J, Singh-Taylor A, Ivy A, Maras PM, Baram TZ. Hyper-excitability and epilepsy generated by chronic early-life stress. Neurobiol Stress. 2015;2:10–19. doi: 10.1016/j.ynstr.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–189. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- Fabbri C, Serretti A. Genetics of long-term treatment outcome in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:17–24. doi: 10.1016/j.pnpbp.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Fage D, Voltz C, Carter C. Ouabain releases striatal polyamines in vivo independently of N-methyl-D-aspartate receptor activation. J Neurochem. 1993;61:261–265. doi: 10.1111/j.1471-4159.1993.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Fage D, Voltz C, Scatton B, Carter C. Selective release of spermine and spermidine from the rat striatum by N-methyl-D-aspartate receptor activation in vivo. J Neurochem. 1992;58:2170–2175. doi: 10.1111/j.1471-4159.1992.tb10960.x. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori LM, Bureau A, Labbe A, Croteau J, Noel S, Merette C, Turecki G. Global gene expression profiling of the polyamine system in suicide completers. Int. J. Neuropsychopharmacol. 2011;14:595–605. doi: 10.1017/S1461145710001574. [DOI] [PubMed] [Google Scholar]
- Fiori LM, Mechawar N, Turecki G. Identification and characterization of spermidine/spermine N1-acetyltransferase promoter variants in suicide completers. Biol Psychiatry. 2009;66:460–467. doi: 10.1016/j.biopsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Libman L, Katz E, Gutnick MJ. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 2008;105:18994–18999. doi: 10.1073/pnas.0803464105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren L, Nystrom L. An incident case-referent study of epileptic seizures in adults. Epilepsy Res. 1990;6:66–81. doi: 10.1016/0920-1211(90)90010-s. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MJ, Huang H, Grant ER, Lynch DR. The NR2B-specific interactions of polyamines and protons with the N-methyl-D-aspartate receptor. J Biol Chem. 1997;272:24971–24979. doi: 10.1074/jbc.272.40.24971. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Anand R, Lindstrom J. Homomers of alpha 8 and alpha 7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol Pharmacol. 1994;45:212–220. [PubMed] [Google Scholar]
- Gilad GM, Gilad VH. Brain polyamine stress response: recurrence after repetitive stressor and inhibition by lithium. J Neurochem. 1996;67:1992–1996. doi: 10.1046/j.1471-4159.1996.67051992.x. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH. Overview of the brain polyamine-stress-response: regulation, development, and modulation by lithium and role in cell survival. Cell Mol Neurobiol. 2003;23:637–649. doi: 10.1023/A:1025036532672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH, Wyatt RJ. Polyamines modulate the binding of GABAA-benzodiazepine receptor ligands in membranes from the rat forebrain. Neuropharmacology. 1992a;31:895–898. doi: 10.1016/0028-3908(92)90127-b. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH, Wyatt RJ, Casero RA., Jr Chronic lithium treatment prevents the dexamethasone-induced increase of brain polyamine metabolizing enzymes. Life Sci. 1992b;50:PL149–PL154. doi: 10.1016/0024-3205(92)90289-2. [DOI] [PubMed] [Google Scholar]
- Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry. 2015;20:1057–1068. doi: 10.1038/mp.2015.91. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Starrett JE, Jr, Dworetzky SI. Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist. 2001;7:166–177. doi: 10.1177/107385840100700211. [DOI] [PubMed] [Google Scholar]
- Gross JA, Fiori LM, Labonte B, Lopez JP, Turecki G. Effects of promoter methylation on increased expression of polyamine biosynthetic genes in suicide. J Psychiatr Res. 2013;47:513–519. doi: 10.1016/j.jpsychires.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guipponi M, Deutsch S, Kohler K, Perroud N, Le Gal F, Vessaz M, Laforge T, Petit B, Jollant F, Guillaume S, Baud P, Courtet P, La Harpe R, Malafosse A. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. 2009a;150B:799–807. doi: 10.1002/ajmg.b.30901. [DOI] [PubMed] [Google Scholar]
- Guipponi M, Deutsch S, Kohler K, Perroud N, Le GF, Vessaz M, Laforge T, Petit B, Jollant F, Guillaume S, Baud P, Courtet P, La HR, Malafosse A. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009b;150B:799–807. doi: 10.1002/ajmg.b.30901. [DOI] [PubMed] [Google Scholar]
- Guo YY, Liu SB, Cui GB, Ma L, Feng B, Xing JH, Yang Q, Li XQ, Wu YM, Xiong LZ, Zhang W, Zhao MG. Acute stress induces down-regulation of large-conductance Ca2+-activated potassium channels in the lateral amygdala. J Physiol. 2012;590:875–886. doi: 10.1113/jphysiol.2011.223784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber MT, Fukui T, Lebowitz MS, Lowenstein JM. Activation of phosphoinositide-specific phospholipase C delta from rat liver by polyamines and basic proteins. Arch Biochem Biophys. 1991;288:243–249. doi: 10.1016/0003-9861(91)90191-k. [DOI] [PubMed] [Google Scholar]
- Haghighi AP, Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J Neurosci. 1998;18:4050–4062. doi: 10.1523/JNEUROSCI.18-11-04050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi AP, Cooper E. A molecular link between inward rectification and calcium permeability of neuronal nicotinic acetylcholine alpha3beta4 and alpha4beta2 receptors. J Neurosci. 2000;20:529–541. doi: 10.1523/JNEUROSCI.20-02-00529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: stress exposure and reactivity models. Child Dev. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harman RJ, Shaw GG. The spontaneous and evoked release of spermine from rat brain in vitro. Br J Pharmacol. 1981;73:165–174. doi: 10.1111/j.1476-5381.1981.tb16786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, Daly BD, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SG, Jones AR. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MD, Reuveny E, Narahashi T. The effect of polyamines on voltage-activated calcium channels in mouse neuroblastoma cells. J Physiol. 1993;462:645–660. doi: 10.1113/jphysiol.1993.sp019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47:246–249. [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Third. Sinauer Associates Inc; 2001. [Google Scholar]
- Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Isaev D, Ivanchick G, Khmyz V, Isaeva E, Savrasova A, Krishtal O, Holmes GL, Maximyuk O. Surface charge impact in low-magnesium model of seizure in rat hippocampus. J Neurophysiol. 2012;107:417–423. doi: 10.1152/jn.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Hiraoka M, Ochi R. The tetravalent organic cation spermine causes the gating of the IRK1 channel expressed in murine fibroblast cells. J Physiol. 1996;491(Pt 2):367–381. doi: 10.1113/jphysiol.1996.sp021222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H. Corticosteroid effects on calcium signaling in limbic neurons. Cell Calcium. 2011;51:277–283. doi: 10.1016/j.ceca.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Johnson TD. Modulation of channel function by polyamines. Trends Pharmacol Sci. 1996;17:22–27. doi: 10.1016/0165-6147(96)81566-5. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Depression and epilepsy: A bidirectional relation? Epilepsia. 2011;52(Suppl 1):21–27. doi: 10.1111/j.1528-1167.2010.02907.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment Dis. 1998;186:661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Kerschbaum HH, Kozak JA, Cahalan MD. Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J. 2003;84:2293–2305. doi: 10.1016/S0006-3495(03)75035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempan TA, Rujescu D, Merette C, Himmelman C, Sequeira A, Canetti L, Fiori LM, Schneider B, Bureau A, Turecki G. Profiling brain expression of the spermidine/spermine N1-acetyltransferase 1 (SAT1) gene in suicide. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009a;150B:934–943. doi: 10.1002/ajmg.b.30920. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, Ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Molecular Psychiatry. 2009b;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- Koenig H, Goldstone AD, Trout JJ, Lu CY. Polyamines mediate uncontrolled calcium entry and cell damage in rat heart in the calcium paradox. J Clin Invest. 1987;80:1322–1331. doi: 10.1172/JCI113209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucheryavykh YV, Pearson WL, Kurata HT, Eaton MJ, Skatchkov SN, Nichols CG. Polyamine permeation and rectification of Kir4.1 channels. Channels (Austin) 2007;1:172–178. doi: 10.4161/chan.4389. [DOI] [PubMed] [Google Scholar]
- Labonte B, Suderman M, Maussion G, Lopez JP, Navarro-Sanchez L, Yerko V, Mechawar N, Szyf M, Meaney MJ, Turecki G. Genome-wide methylation changes in the brains of suicide completers. Am J Psychiatry. 2013;170:511–520. doi: 10.1176/appi.ajp.2012.12050627. [DOI] [PubMed] [Google Scholar]
- Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, McMahon FJ. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- Langdon RC, Fleckman P, McGuire J. Calcium stimulates ornithine decarboxylase activity in cultured mammalian epithelial cells. J Cell Physiol. 1984;118:39–44. doi: 10.1002/jcp.1041180109. [DOI] [PubMed] [Google Scholar]
- Lasater EM, Solessio E. Regulation of voltage-sensitive Ca2+ channels in bipolar cells by divalent cations and polyamines. Adv Exp Med Biol. 2002;514:275–289. doi: 10.1007/978-1-4615-0121-3_16. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon A, Perez C, Vega R, Soto E. Ca2+-activated K+-current density is correlated with soma size in rat vestibular-afferent neurons in culture. J Neurophysiol. 2005;94:3751–3761. doi: 10.1152/jn.00177.2005. [DOI] [PubMed] [Google Scholar]
- Liou YJ, Chen TJ, Tsai SJ, Yu YW, Cheng CY, Hong CJ. Support for the involvement of the KCNK2 gene in major depressive disorder and response to antidepressant treatment. Pharmacogenet Genomics. 2009;19:735–741. doi: 10.1097/FPC.0b013e32832cbe61. [DOI] [PubMed] [Google Scholar]
- Liu Y, Blackwood DH, Caesar S, de Geus EJ, Farmer A, Ferreira MA, Ferrier IN, Fraser C, Gordon-Smith K, Green EK, Grozeva D, Gurling HM, Hamshere ML, Heutink P, Holmans PA, Hoogendijk WJ, Hottenga JJ, Jones L, Jones IR, Kirov G, Lin D, McGuffin P, Moskvina V, Nolen WA, Perlis RH, Posthuma D, Scolnick EM, Smit AB, Smit JH, Smoller JW, St Clair D, van Dyck R, Verhage M, Willemsen G, Young AH, Zandbelt T, Boomsma DI, Craddock N, O'Donovan MC, Owen MJ, Penninx BW, Purcell S, Sklar P, Sullivan PF. Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry. 2011;16:2–4. doi: 10.1038/mp.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Lu B, Zhang Q, Wang H, Wang Y, Nakayama M, Ren D. Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex. Neuron. 2010;68:488–499. doi: 10.1016/j.neuron.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Marsch R, Foeller E, Rammes G, Bunck M, Kossl M, Holsboer F, Zieglgansberger W, Landgraf R, Lutz B, Wotjak CT. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci. 2007;27:832–839. doi: 10.1523/JNEUROSCI.3303-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko T, Kusama-Eguchi K, Sakata K, Kusama T, Chaki S, Okuyama S, Williams K, Kashiwagi K, Igarashi K. Polyamine transport, accumulation, and release in brain. J Neurochem. 2003;84:610–617. doi: 10.1046/j.1471-4159.2003.01558.x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Meadows HJ, Benham CD, Cairns W, Gloger I, Jennings C, Medhurst AD, Murdock P, Chapman CG. Cloning, localisation and functional expression of the human orthologue of the TREK-1 potassium channel. Pflugers Arch. 2000;439:714–722. doi: 10.1007/s004249900235. [DOI] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Fote GM, Blakeman S, Cahuzac EL, Newbold SA, Picciotto MR. Multiple Nicotinic Acetylcholine Receptor Subtypes in the Mouse Amygdala Regulate Affective Behaviors and Response to Social Stress. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu T, Hosfield CM, Lim D, Elce JS, Jia Z, Davies PL. A Ca(2+) switch aligns the active site of calpain. Cell. 2002;108:649–660. doi: 10.1016/s0092-8674(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Washburn MS, Zhang S, Dingledine RJ. Subunit-dependent modulation of kainate receptors by extracellular protons and polyamines. J Neurosci. 2003;23:1179–1188. doi: 10.1523/JNEUROSCI.23-04-01179.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B, Meuth SG, Liu GX, Derst C, Wegner S, Pape HC, Budde T, Preisig-Muller R, Daut J. Effects of divalent cations and spermine on the K+ channel TASK-3 and on the outward current in thalamic neurons. J Physiol. 2006;572:639–657. doi: 10.1113/jphysiol.2006.106898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, Turecki G. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2014;20:320–328. doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Voets T, Droogmans G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflugers Arch. 2004;448:70–75. doi: 10.1007/s00424-003-1221-x. [DOI] [PubMed] [Google Scholar]
- Otani S, Matsui I, Kuramoto A, Morisawa S. Induction of ornithine decarboxylase in guinea-pig lymphocytes. Synergistic effect of diacylglycerol and calcium. Eur J Biochem. 1985;147:27–31. doi: 10.1111/j.1432-1033.1985.tb08713.x. [DOI] [PubMed] [Google Scholar]
- Pajunen AE, Hietala OA, Virransalo EL, Piha RS. Ornithine decarboxylase and adenosylmethionine decarboxylase in mouse brain--effect of electrical stimulation. J Neurochem. 1978;30:281–283. doi: 10.1111/j.1471-4159.1978.tb07066.x. [DOI] [PubMed] [Google Scholar]
- Pantazatos SP, Andrews SJ, Dunning-Broadbent J, Pang J, Huang YY, Arango V, Nagy PL, John MJ. Isoform-level brain expression profiling of the spermidine/spermine N1-Acetyltransferase1 (SAT1) gene in major depression and suicide. Neurobiol. Dis. 2015;79:123–134. doi: 10.1016/j.nbd.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE. An activity-dependent spermine-mediated mechanism that modulates glutamate transmission. Trends Neurosci. 2003;26:9–11. doi: 10.1016/s0166-2236(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Pernet V, Bourgeois P, Di Polo A. A role for polyamines in retinal ganglion cell excitotoxic death. J Neurochem. 2007;103:1481–1490. doi: 10.1111/j.1471-4159.2007.04843.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: a 128-channel EEG study. Hum Brain Mapp. 2006;27:185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Quinn SJ, Ye CP, Diaz R, Kifor O, Bai M, Vassilev P, Brown E. The Ca2+-sensing receptor: a target for polyamines. Am J Physiol. 1997;273:C1315–C1323. doi: 10.1152/ajpcell.1997.273.4.C1315. [DOI] [PubMed] [Google Scholar]
- Refojo D, Echenique C, Muller MB, Reul JM, Deussing JM, Wurst W, Sillaber I, Paez-Pereda M, Holsboer F, Arzt E. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc Natl Acad Sci U S A. 2005;102:6183–6188. doi: 10.1073/pnas.0502070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinstatler L, Youssef NA. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D. 2015;15:37–43. doi: 10.1007/s40268-015-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol Sci. 2000;21:187–193. doi: 10.1016/s0165-6147(00)01460-7. [DOI] [PubMed] [Google Scholar]
- Ritov G, Ardi Z, Richter-Levin G. Differential activation of amygdala, dorsal and ventral hippocampus following an exposure to a reminder of underwater trauma. Front Behav Neurosci. 2014;8:18. doi: 10.3389/fnbeh.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock DM, Macdonald RL. Polyamine regulation of N-methyl-D-aspartate receptor channels. Annu Rev Pharmacol Toxicol. 1995;35:463–482. doi: 10.1146/annurev.pa.35.040195.002335. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Regunathan S, Galea E, Reis DJ. Agmatinase activity in rat brain: a metabolic pathway for the degradation of agmatine. J Neurochem. 1996;67:1761–1765. doi: 10.1046/j.1471-4159.1996.67041761.x. [DOI] [PubMed] [Google Scholar]
- Schosser A, Butler AW, Uher R, Ng MY, Cohen-Woods S, Craddock N, Owen MJ, Korszun A, Gill M, Rice J, Hauser J, Henigsberg N, Maier W, Mors O, Placentino A, Rietschel M, Souery D, Preisig M, Craig IW, Farmer AE, Lewis CM, McGuffin P. Genome-wide association study of co-occurring anxiety in major depression. World J Biol Psychiatry. 2013;14:611–621. doi: 10.3109/15622975.2013.782107. [DOI] [PubMed] [Google Scholar]
- Scriver CR. Human genetics: lessons from Quebec populations. Annu Rev Genomics Hum Genet. 2001;2:69–101. doi: 10.1146/annurev.genom.2.1.69. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, Casero RA, Jr, Rouleau G, Benkelfat C, Turecki G. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Klempan T, Canetti L, Ffrench-Mullen J, Benkelfat C, Rouleau GA, Turecki G. Patterns of gene expression in the limbic system of suicides with and without major depression. Molecular Psychiatry. 2007;12:640–655. doi: 10.1038/sj.mp.4001969. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shi J, Potash JB, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, DePaulo JR, Jr, Gejman PV, Sanders AR, Johnson JK, Adams P, Chaudhury S, Jancic D, Evgrafov O, Zvinyatskovskiy A, Ertman N, Gladis M, Neimanas K, Goodell M, Hale N, Ney N, Verma R, Mirel D, Holmans P, Levinson DF. Genome-wide association study of recurrent early-onset major depressive disorder. Mol Psychiatry. 2011;16:193–201. doi: 10.1038/mp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsin B. The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: a pilot study. Acta Neuropsychiatr. 2013;25:113–118. doi: 10.1111/j.1601-5215.2012.00675.x. [DOI] [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Sokolowski M, Ben-Efraim YJ, Wasserman J, Wasserman D. Glutamatergic GRIN2B and polyaminergic ODC1 genes in suicide attempts: associations and gene-environment interactions with childhood/adolescent physical assault. Mol Psychiatry. 2013;18:985–992. doi: 10.1038/mp.2012.112. [DOI] [PubMed] [Google Scholar]
- Squassina A, Manchia M, Chillotti C, Deiana V, Congiu D, Paribello F, Roncada P, Soggiu A, Piras C, Urbani A, Robertson GS, Keddy P, Turecki G, Rouleau GA, Alda M, Del Zompo M. Differential effect of lithium on spermidine/spermine N1-acetyltransferase expression in suicidal behaviour. Int J Neuropsychopharmacol. 2013;16:2209–2218. doi: 10.1017/S1461145713000655. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Electrostatic interaction of internal Mg2+ with membrane PIP2 Seen with KCNQ K+ channels. J Gen Physiol. 2007;130:241–256. doi: 10.1085/jgp.200709821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CM, Martin ED, Sahun I, Masachs N, Pujadas L, Corvelo A, Bosch C, Rossi D, Martinez A, Maldonado R, Dierssen M, Soriano E. Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology. 2011;36:2395–2405. doi: 10.1038/npp.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant C. Life events, stress and depression: a review of recent findings. Aust N Z J Psychiatry. 2002;36:173–182. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- Tovilla-Zárate CA, López-Narváez ML, González-Castro TB, Juárez-Rojop I, Pool-García S, Genis A, Ble-Castillo J, Fresán A, Nicolini H. Association between the SAT-1 Gene and Suicidal Behavior in Mexican Population. Journal of Psychiatry. 2015;S1:005. [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268:873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentani A, Kuipers SD, Ter Horst GJ, Den Boer JA. Selective chronic stress-induced in vivo ERK1/2 hyperphosphorylation in medial prefrontocortical dendrites: implications for stress-related cortical pathology? Eur J Neurosci. 2002;15:1681–1691. doi: 10.1046/j.1460-9568.2002.02000.x. [DOI] [PubMed] [Google Scholar]
- Turecki G. The molecular bases of the suicidal brain. Nat Rev Neurosci. 2014;15:802–816. doi: 10.1038/nrn3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–278. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen JP, Bos MP, Herrmann-Erlee MP. Parathyroid hormone-induced ornithine decarboxylase activity in fetal rat osteoblasts. J Bone Miner Res. 1989;4:485–492. doi: 10.1002/jbmr.5650040406. [DOI] [PubMed] [Google Scholar]
- Vemana S, Pandey S, Larsson HP. Intracellular Mg2+ is a voltage-dependent pore blocker of HCN channels. Am J Physiol Cell Physiol. 2008;295:C557–C565. doi: 10.1152/ajpcell.00154.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Geijer T, Rozanov V, Wasserman J. Suicide attempt and basic mechanisms in neural conduction: relationships to the SCN8A and VAMP4 genes. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:116–119. doi: 10.1002/ajmg.b.30128. [DOI] [PubMed] [Google Scholar]
- Weiger T, Hermann A. Polyamines block Ca(2+)-activated K+ channels in pituitary tumor cells (GH3) J Membr Biol. 1994;140:133–142. doi: 10.1007/BF00232901. [DOI] [PubMed] [Google Scholar]
- Weiger TM, Langer T, Hermann A. External action of di- and polyamines on maxi calcium-activated potassium channels: an electrophysiological and molecular modeling study. Biophys J. 1998;74:722–730. doi: 10.1016/S0006-3495(98)73997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci U S A. 2004;101:3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World report on disability. 2011
- Williams K, Kashiwagi K, Fukuchi J, Igarashi K. An acidic amino acid in the N-methyl-D-aspartate receptor that is important for spermine stimulation. Mol Pharmacol. 1995;48:1087–1098. [PubMed] [Google Scholar]
- Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol. 1994;45:803–809. [PubMed] [Google Scholar]
- Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, Brebner K, Liu L, Weinberg J, Christie BR, Phillips AG, Wang YT. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci U S A. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995;14:1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li Z, Wu Z, Chen J, Wang Z, Peng D, Hong W, Yuan C, Yu S, Xu Y, Xu L, Xiao Z, Fang Y. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Psychopharmacology (Berl) 2014a;231:685–693. doi: 10.1007/s00213-013-3297-0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xue G, Zhang W, Wang L, Li H, Zhang L, Lu F, Bai S, Lin Y, Lou Y, Xu C, Zhao Y. Akt and Erk1/2 activate the ornithine decarboxylase/polyamine system in cardioprotective ischemic preconditioning in rats: the role of mitochondrial permeability transition pores. Mol Cell Biochem. 2014b;390:133–142. doi: 10.1007/s11010-014-1964-z. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zheng X, Paupard MC, Wang AP, Santchi L, Friedman LK, Zukin RS, Bennett MV. Spermine potentiation of recombinant N-methyl-D-aspartate receptors is affected by subunit composition. Proc Natl Acad Sci U S A. 1994;91:10883–10887. doi: 10.1073/pnas.91.23.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomkowski AD, Hammes L, Lin J, Calixto JB, Santos AR, Rodrigues AL. Agmatine produces antidepressant-like effects in two models of depression in mice. Neuroreport. 2002;13:387–391. doi: 10.1097/00001756-200203250-00005. [DOI] [PubMed] [Google Scholar]
- Zschenderlein C, Gebhardt C, von Bohlen Und Halbach O, Kulisch C, Albrecht D. Capsaicin-induced changes in LTP in the lateral amygdala are mediated by TRPV1. PLoS One. 2011;6:e16116. doi: 10.1371/journal.pone.0016116. [DOI] [PMC free article] [PubMed] [Google Scholar]