Abstract
Objective
No standardized treatment strategies exist for patients with gynecologic malignancies complicated by brain metastases. Identification of poor outcome characteristics, long-term survival indicators, and molecular markers could help individualize and optimize treatment.
Methods
This retrospective cohort study included 100 gynecologic cancer patients with brain metastases treated at our institution between January 1990 and June 2009. Primary outcome was overall survival (OS) from time of diagnosis of brain metastases. We used univariate and multivariate analyses to evaluate associations between OS and clinical factors. We used immunohistochemistry to examine expression of five molecular markers in primary tumors and brain metastases in a subset of patients and matched controls. Statistical tests included the Student’s paired t-test (for marker expression) and Kaplan-Meier test (for correlations).
Results
On univariate analysis, primary ovarian disease, CA-125 < 81 units/mL at brain metastases diagnosis, and isolated versus multi-focal metastases were all associated with longer survival. Isolated brain metastasis remained the only significant predictor on multivariate analysis (HR 2.66; CI 1.19–5.93; p=0.017). Expression of vascular endothelial growth factor A (VEGF-A) was higher in metastatic brain samples than in primary tumors of controls (P<0.0001). None of the molecular markers were significantly associated with survival.
Conclusions
Multi-modality therapy may lead to improved clinical outcomes, and VEGF therapy should be investigated in treatment of brain metastases.
INTRODUCTION
Metastatic brain lesions are uncommon in gynecologic malignancies; they occur in 0.3–0.9% of uterine corpus cancers [1–3], 0.3–11.6% of ovarian cancers [4–9], and 0.4–2.3% of cervical cancers [10–13]. Although rare, such lesions have become a focus of interest as advances in multimodality treatments are prolonging survival among gynecologic cancer patients, thereby allowing a larger percentage of patients to live long enough to develop distant metastases [4, 14–17]. Such patients require evaluation for additional therapy options and present a new need for an extensive treatment risk/benefit analysis.
In most cases, the presence of metastatic brain lesions carries a grave prognosis. Previously, these patients were given supportive or palliative treatment, usually including chemotherapy, and were expected to survive only a few months. Improvements in both surgical technique and radiation therapy have led to additional options, and long-term survival is now possible in rare cases. Additionally, ovarian, endometrial, and cervical cancer patients in whom the brain is the first and only site of recurrent disease may achieve long-term benefits from treatment [3, 7, 9, 18, 19].
Molecular characteristics of tumors are becoming increasingly important in diagnosis, treatment, and prognosis of many malignancies. Immunohistochemistry (IHC) in gynecologic tumors and the subsequent brain metastases may identify a prognosticator and potentially targetable biomarker. We focused on five specific markers based on previous data and departmental experience: ephrin type-A receptor 2 (EphA2), estrogen receptor (ER), progesterone receptor (PR), multidrug resistance protein 1 (MDR1), and vascular endothelial growth factor A (VEGF-A).
EphA2 is a tyrosine kinase inhibitor involved in many cancer-related pathways including activation of focal adhesion kinase, suppression of integrin function, and activation of the extracellular signal-regulated kinases cascade. High levels of EphA2 correlate with aggressive features in ovarian carcinoma and brain metastasis in lung cancer [20, 21]. Similarly, in endometrial and ovarian cancers, ER and PR status can be associated with adverse prognostic factors such as lymphovascular space invasion, and these receptors can serve as targets for treatment [22, 23]. MDR1 is a permeability glycoprotein in the superfamily of ATP-binding cassette transporters. These receptors are responsible for decreased accumulation of drugs, such as the anticancer drugs doxorubicin and vinblastine, in multidrug-resistant cells. Consistent with its function at the blood-brain barrier, MDR1 is associated with increased risk of brain metastasis in ovarian cancer [22]. Lastly, VEGF-A has gained clinical relevance in treatment of gynecologic malignancies as we have seen success with the anti-angiogenic drug bevacizumab [24, 25]. High expression of VEGF-A has also been associated with increased risk of central nervous system metastases in cancers that have a high propensity for brain metastases, such as non-small-cell lung cancer and melanoma [26, 27].
Here, we sought to identify patient characteristics, disease features, and treatment modalities that associate with overall survival as well as evaluate expression of specific molecular markers with the hypothesis that they may be unique to gynecologic cancer patients with brain metastases.
PATIENTS and METHODS
Study population
An Institutional Review Board-approved retrospective chart review included all patients with ovarian, fallopian tube, primary peritoneal, endometrial, cervical, vulvar, and vaginal cancers who were diagnosed with brain metastases between January 1, 1990 and June 30, 2009 at Washington University School of Medicine/Barnes-Jewish Hospital. Subjects were identified using ICD-9 codes for symptoms suggestive of brain metastases (seizures, altered mental status, other new onset neurological deficits, and hospice) to query the gynecologic oncology billing department. We included patients over age 18 with brain metastases originating from any gynecologic malignancy except gestational trophoblastic disease. Additional demographic and clinical data were extracted from both inpatient and outpatient paper and electronic medical records.
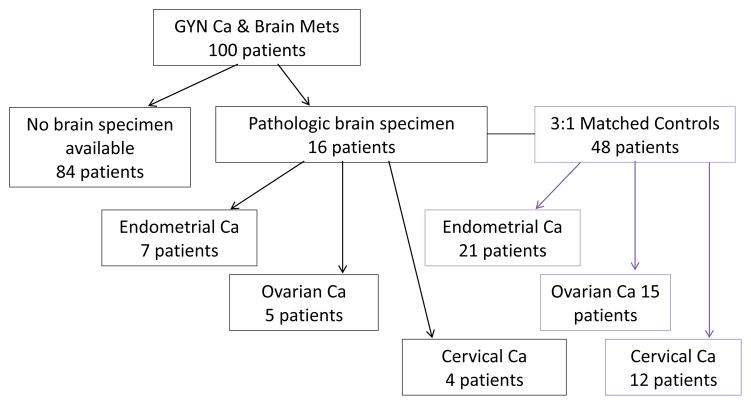
A central pathology review was conducted to confirm original cancer diagnosis. Brain metastases were confirmed by radiology reports, hospital charts, and pathology reports where possible. We obtained all available pathologic brain and primary tissue specimens of eligible subjects entered into the study. Brain biopsy specimens were available for only sixteen of the patients as many underwent biopsy at outside facilities or did not have remaining sample available for staining. We matched, at a 3:1 ratio, control patients within the same study period to brain samples from these 16 subjects (Fig. 1). Of the 16 patients for whom we had brain biopsies, we were able to obtain specimens from their primary malignancy in 5 cases. Control subjects were women diagnosed with gynecologic cancers with metastatic disease but who never experienced brain metastases. Patients were matched by type of cancer, age at time of original diagnosis, race, stage, and year of treatment.
Figure 1.
Study Design
100 patients had brain metastases secondary to gynecologic cancer. For 84 patients there was no brain specimen available, 16 patients had pathologic brain tissue specimens available through our pathology department and were used for further molecular analyses. Of the 16 patients, 7 had endometrial cancer, 5 had ovarian, and 4 had cervical cancer. The sixteen cases of brain metastases were matched to 48 controls matched for type of cancer, age, race, stage, and year of treatment. For 5 of the 16 patients, matched brain and primary tissue samples were also available.
Immunohistochemistry
Formalin-fixed, paraffin-embedded samples of primary tumors and brain metastases were stained as previously described [21, 28–31]. Briefly, for EphA2, sections were deparaffinized and then probed with a monoclonal anti-EphA2 antibody (MedImmune, Gaithersburg, MD) overnight at 4°C. Next, the slides were rinsed with phosphate-buffered saline-Tween 20, incubated with biotinylated linked antimouse IgG secondary antibody (Dako) for 30 minutes, incubated with a ready-to-use avidin-biotin complex method reagent (Dako) for 5 to 15 minutes, and then counterstained with Mayer hematoxylin (1:10) for 35 to 60 seconds. ER and PR immunostaining of paraffin sections was performed on a Ventana BenchMark ULTRA IHC Staining Module (Oro Valley, AZ) using pre-diluted anti-ER (SP1) and anti-PR (1E2) antibodies (FDA-approved method). MDR-1 staining was performed by using the Biogenex Super Sensitive Detection Kit (Biogenex Laboratories, San Ramon, CA) on the BioGenex i6000 Austostainer.[30] For VEGF-A, slides were incubated with rabbit polyclonal anti-VEGF antibody (1:50; Santa Cruz Biotechnology) at 4 °C overnight. Slides were then incubated in rabbit horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature, incubated with 3,3′-diaminobenzidine (Phoenix Biotechnologies) for 7 to 10 min, and then counterstained with Gill’s no. 3 hematoxylin (Sigma) for 20 to 30 seconds.[31]
All samples were reviewed by a board-certified pathologist (M.E.P.) and two gynecologic oncologists (N.K., P.T.) who were all blinded to the clinical outcomes of the patients. Protein expression was determined semi-quantitatively by assessing the percentage of stained cells and the staining intensity. The percentage of positive cells was scored as follows: 0 points, 0–5%; 1 point, 6–25%; 2 points, 26–50%; and 3 points, >50%. The staining intensity was scored as follows: 1 point, weak intensity; 2 points, moderate intensity; and 3 points, strong intensity. Points were added, and an overall score between 0 and 6 was assigned: negative (overall score = 0), weak (1 or 2), moderate (3 or 4), or strong (5 or 6)24. The scores of all reviewers were averaged for each slide and there was high inter-rater reliability.
Statistical analysis
Statistical analyses included chi-square tests with Fischer’s exact tests for comparison of patient characteristics and Student’s t-test with Bonferroni correction for analysis of protein expression in tissue samples. Kaplan-Meier curves were generated for survival, and the log-rank statistic was used for comparisons. We used the Cox proportional hazards model to determine the impact of multiple covariates on the prognosis of brain metastases from gynecologic malignancies. The location of primary disease, CA-125 at brain metastases, and isolated (single focus of disease) versus multiple foci of brain metastases were regarded as candidate prognostic factors. The results of the multivariate analysis are expressed as hazards ratios with 95% confidence intervals (CIs) (SPSS). P-values of <0.05 were considered to indicate statistical significance.
RESULTS
One hundred subjects with ovarian (N=49), uterine (N=32), or cervical (N=19) cancer diagnosed with brain metastases were identified during the 19-year study period (Table 1). The median age at diagnosis was 56.6 years (range 28.3–82.5). The majority of the patients were white (82%) with stage III/IV disease (68%) at initial presentation. The majority of subjects received combination therapy consisting of either two or three modalities (n= 55, 55.4%); 20 (19.6%) subjects received no therapy (Table 1). The most frequent therapy given, either alone or in combination, was whole brain radiation therapy (n= 70, 69.3%). Median follow-up time was 38 months for the entire cohort.
Table 1.
Patient demographic/clinical characteristics
| Variable | No. of patients |
|---|---|
| Primary Site | |
| Ovary/fallopian tube/primary peritoneal | 49 |
| Uterine/endometrial | 32 |
| Cervix | 19 |
| Vulvar/vaginal | 0 |
| Age at initial diagnosis | |
| Mean ± SD (range) | 56.7 ± 10.7 (28.3–82.48) |
| Age at diagnosis of brain metastases | |
| Mean ± SD (range) | 59.4 ± 10.5 (32.2–85.1) |
| Race | |
| White | 82 |
| Black | 12 |
| Other | 4 |
| Unknown | 2 |
| BMI | |
| Mean ± SD (range) | 28.8 ± 7.1 (18.0–54.0) |
| Tumor Stage | |
| I | 17 |
| II | 11 |
| III | 39 |
| IV | 29 |
| Unknown | 4 |
| Histology | |
| Adenocarcinoma | 9 |
| Clear Cell | 3 |
| Endometrioid | 19 |
| Serous | 38 |
| Squamous | 12 |
| Mixed | 8 |
| Other | 10 |
| Unknown | 1 |
| Grade | |
| Low (I or II) | 26 |
| High (III) | 63 |
| Unknown | 11 |
| Cytoreduction | |
| Optimal | 34 |
| Suboptimal | 12 |
| Unknown or n/a | 54 |
| Time from initial diagnosis to brain metastases, mean months ± SD (range) | 33.6 ± 30.4 (0–164.8) |
| Brain metastases at initial diagnosis | |
| Present | 4 |
| Absent | 96 |
| Clinical presentation of brain metastases (10 patients had >1 presenting symptom) | |
| Seizures | 4 |
| Altered Mental Status | 32 |
| Fall | 4 |
| Weakness | 10 |
| Headache | 38 |
| None/Other | 22 |
| CA-125 at time of diagnosis of brain metastases | |
| Median (range) | 78.0 (6–5748) |
| Treatment for brain metastases | |
| Chemotherapy | 3 |
| Radiation | 22 |
| Combination | 55 |
| Surgery + Radiation | 6 |
| Surgery + Radiation + Chemo | 7 |
| Radiation + Chemo | 28 |
| Radiation + Gamma Knife | 3 |
| Chemo + Gamma Knife | 5 |
| Surgery + Radiation + Gamma Knife | 1 |
| Surgery + Chemo + Gamma Knife | 2 |
| Surgery + Radiation + Chemo + Gamma Knife | 1 |
| Radiation + Chemotherapy + Gamma Knife | 2 |
| Expectant Management | 20 |
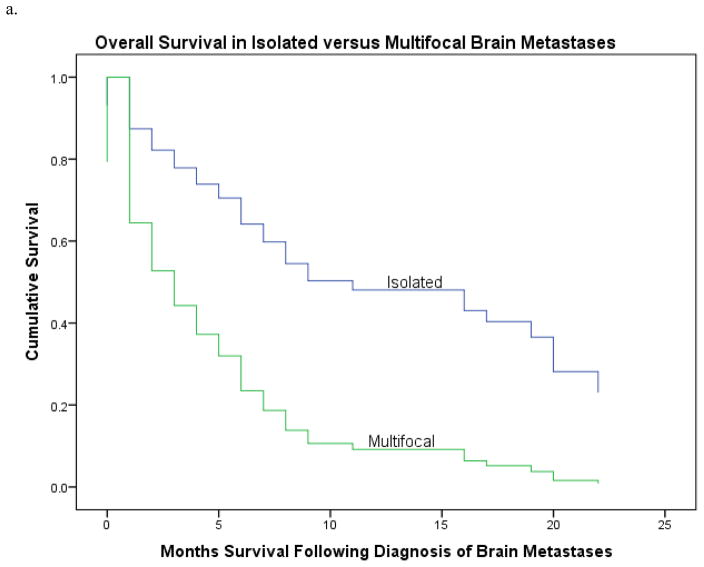
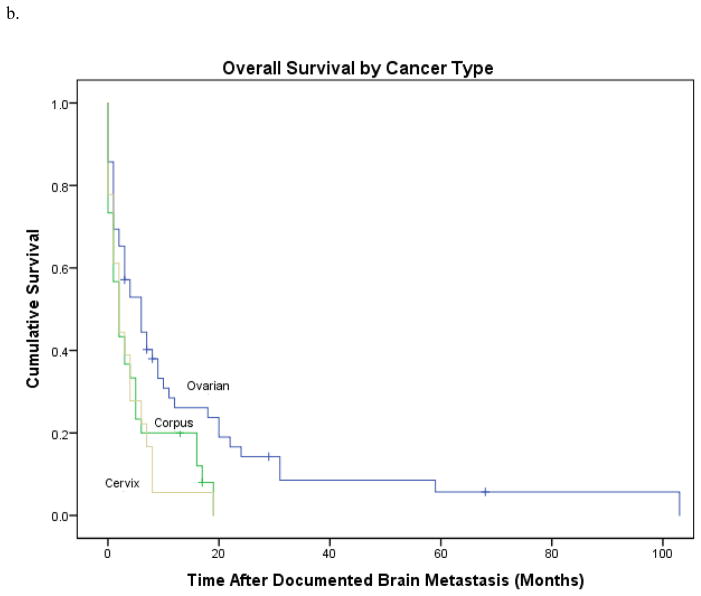
In univariate analysis (Table 2), patients with isolated brain metastasis had a median survival of 17 months versus 2 months in those with multifocal disease, HR 0.33; CI 0.18–0.63, p=0.001 (Fig 2a). A lower CA-125 level (<81 units/mL) at time of brain metastases correlated with a longer survival (HR 0.54; 95% CI 0.3, 0.95; P=0.03). When subdividing by original diagnosis, patients with ovarian cancer had longer overall survival (original diagnosis to last follow-up) than those with uterine or cervical primaries 52 versus 19 or 23 months, respectively (HR 3.28; CI 31.6–44.4, p=0.002) (Fig. 2b). Patients with ovarian primary malignancies also experienced a longer interval from time of diagnosis of brain metastases to last follow-up: 6 versus 2 months, HR 0.70; CI 1.63–4.37, p=0.01 (Fig. 2c). Patients presenting with seizures, falls, or weakness had a worse prognosis than patients presenting with altered mental status, headaches, or other symptoms (HR 0.54; 95% CI 0.32, 0.91; P=0.02). Patients treated with single-agent therapy had poorer survival than those treated with multimodality therapy (2 versus 14 months; HR 6.0; 95% CI 1.62, 5.9; P<0.001) (Table 2). Only isolated brain metastasis remained significantly associated with survival on multivariate analysis, HR 2.66; CI 1.19–5.93; p=0.02.
Table 2.
Univariate and Multivariate analysis of prognostic factors on overall survival
| Variable | Univariate | Multivariate |
|---|---|---|
| Isolated versus Multifocal Brain Metastases | HR 0.33; CI 0.17–0.63, p=0.001 | HR 2.66; CI 1.19– 5.93; p=0.017 |
| CA-125 Level | HR 0.54; CI 0.30–0.95; p=0.03 | ns |
| Ovarian versus Uterine versus Cervical primary | HR 3.28; CI 31.6–44.4, p=0.002 | ns |
| Presenting Symptom: seizures, falls, or weakness vs. all other groups | HR 0.54; CI 0.32–0.91, p=0.02 | ns |
| Single agent vs. multimodal therapy | HR 6.0; CI 1.62–5.9, p<0.001 | ns |
ns=not significant
Figure 2.
A. Survival in patients from diagnosis to last follow-up, divided by isolated versus multifocal brain metastases. Median survival in patients with isolated brain metastasis was 17 months, median in patients with multifocal brain metastases was 2 months, p=0.001.
B. Survival subdivided by original diagnosis. Patients with ovarian cancer had longer overall survival (original diagnosis to last follow-up) than those with uterine or cervical primaries 52 versus 19 or 23 months, respectively, p=0.002.
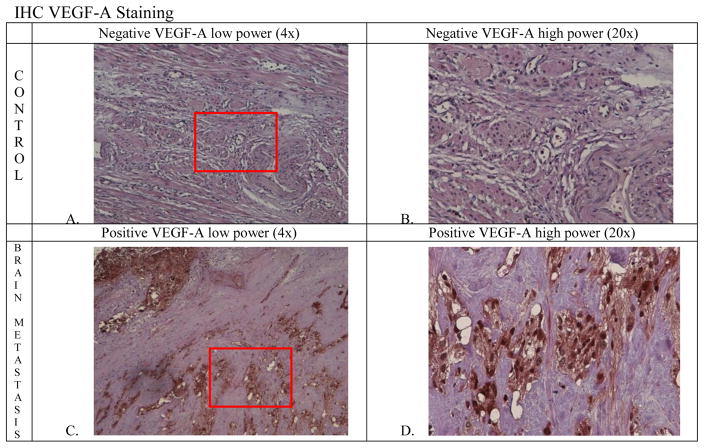
Sixteen patients with brain metastases (5 ovarian, 7 uterine, 4 cervical) were matched to 48 controls. Compared to the primary tumors of controls, metastatic brain specimens of cases displayed higher expression of VEGF-A (P<0.0001). Figure 2 shows representative examples of stained primary tumors and brain metastases. In five cases, we were able to stain the primary tumors and metastatic brain tissues from the same patients. In these cases, the levels of VEGF-A expression in the brain metastases were higher than in the primary tumors, but the differences were not statistically significant (P=0.10). Expression levels of PR (P=0.045), EphA2 (P=0.48), ER (P=0.16), and MDR-1 (P=0.39) were similar between brain metastases of cases and primary tumors of controls. Overall, none of the molecular markers were significantly associated with survival.
DISCUSSION
In general, patients with brain metastases from gynecologic malignancies face poor prognoses. In this study, improved survival was associated with isolated brain metastasis, ovarian primary, low CA-125 level, presenting symptoms other than seizure, falls, or weakness, and multimodal treatment. As in previous reports on gynecologic cancer patients with brain metastases, most patients were treated with cranial surgery, radiotherapy, chemotherapy, and combinations of these [3, 5, 32–36]. When the systemic disease is controlled, surgical removal and/or cranial radiotherapy seem to be good options for treating brain metastases [3, 32]. In addition, chemotherapy following these treatments has been shown to improve outcomes in patients with brain metastases from ovarian cancer [36, 37]. Consistent with prior findings, multimodal therapy proved most beneficial in our study and should be strongly considered in cases of brain metastases from gynecologic malignancies [35, 36, 38, 39].
As brain metastases are uncommon events, treatment of these patients is neither consistent nor standardized, and reports within the literature are limited by small sample sizes. For endometrial cancer, fewer than 100 cases have been reported. One recent study of 22 patients at a single institution showed that survival was better with use of multimodal therapy than with whole brain radiation therapy (WBRT) alone [38]. Two smaller studies corroborate these findings [3, 34].
Similarly, only 104 cases of ovarian cancer with brain metastases have been reported [32]. Treatment was shown to vary widely between patients; a few more than half received WBRT alone or in combination with chemotherapy or surgery. A meta-analysis of all cases reported in the literature as of 2001 indicated that patients receiving multimodal therapy had better survival than those who received no treatment or single modality treatment [32]. Additionally, subgroups of patients with particular characteristics may benefit from one type of therapy over another.
Literature regarding brain metastases in cervical cancer is limited to case reports. The most recent study, from 1997, discusses a patient with a solitary metastatic brain lesion who remained disease-free six years after craniotomy and surgical resection [13]. Similar to endometrial and ovarian cancer patients with brain metastases, median survival of cervical cancer patients with central nervous system metastases was better with the combination of surgery and WBRT than with WBRT alone [18, 34].
Several randomized clinical trials have compared surgery plus WBRT to WBRT alone for patients with isolated lesions, demonstrating a survival benefit in patients who undergo surgery in addition to WBRT. Factors that correlated significantly with increased survival in addition to surgical treatment were the absence of extracranial disease, or presence of stable extracranial disease, longer time to the development of the brain metastasis, and younger age [40–42].
The Radiation Therapy Oncology Group (RTOG) conducted a Phase III study which included 333 patients with one to three brain metastases (maximum diameter 4 cm) who were randomly assigned to WBRT with or without gamma knife. Survival was significantly longer with gamma knife in patients with a single brain metastasis. When considering the entire study population, survival was similar between groups with a higher incidence of grade 3 or 4 toxicity in the gamma knife arm [43]. Similar results were found by Kondziolka, et al [44]. Tumor size is the most common criterion for choosing surgery over radiosurgery as lesions larger than 3 cm are less favorable for gamma knife and surgery is usually preferable, though this is also influenced by location [45, 46].
Consistent with previous reports [35, 38], most of the patients reviewed in this study were experiencing symptoms when brain lesions were diagnosed. Given the low incidence of brain metastases in gynecologic cancer patients, it is not surprising that routine cranial radiographic evaluation is not performed in the absence of symptoms; adding brain imaging to routine surveillance has not been shown to be an effective use of resources and is not part of current ASCO or NCCN guidelines. However, the patients reviewed in our study suffered worse outcomes when they presented with seizures, falls, or weakness than when they presented with milder symptoms such as headaches, which was the most frequently reported symptom (37% of patients). Thus, heighted awareness regarding sentinel symptoms may lead to earlier detection of brain metastasis and improved prognosis.
Angiogenesis is necessary for the growth and development of solid tumors [47, 48]. VEGF is a potent and specific pro-angiogenic factor that functions by promoting endothelial cell migration and proliferation [49]. Similar to findings in non-small cell lung cancer (NSCLC) [50], we found that metastatic brain specimens expressed higher levels of VEGF than control primary tumors. However, given concern for potential increase in the risk of intracerebral hemorrhage (ICH) patients with CNS metastases were historically excluded from bevacizumab trials.
To assess this risk, Besse et al. conducted a safety analysis of approximately 13,000 patients from randomized and nonrandomized trials conducted in patients with breast, NSCLC, pancreatic, renal cell, or colorectal cancer analyzed the incidence of ICH with bevacizumab [51]. Patients with central nervous system (CNS) metastases were at similar risk of developing ICH, independent of bevacizumab therapy. Similarly, an evidence-based review of patients with NSCLC and brain metastases concluded that neither bevacizumab nor sunitinib/sorafenib increased the risk of ICH in patients with treatment-emergent, pretreated, or untreated occult brain metastases [52]. ICH appears to be uncommon even in highly vascular tumors such as glioblastoma [53, 54]. Therefore, it would seem that in with a history of treated nonhemorrhagic brain metastases probably should not be excluded from systemic therapy with a VEGF inhibitor as long as they are not on concurrent anticoagulation. In practice, during local therapy for brain metastases antiangiogenic therapy is commonly held.
There are ongoing Phase II trials of bevacizumab for brain metastases in breast cancer (NCT02185352) and other solid tumors (NCT01898130) [55]. Based on the current literature, anti-VEGF therapy should be considered as part of the multimodal treatment approach in gynecologic cancer patients with brain metastases.
To our knowledge, this is the largest review of single-institution patients with brain metastases from gynecologic malignancies to include both clinicopathologic features and immunohistochemical analysis. The large number of cases allowed us to draw conclusions regarding presenting symptoms, CA-125 levels, and optimal treatment. The large number also enabled us to locate sixteen brain biopsy specimens from gynecologic oncology patients for immunohistochemical analysis.
We do acknowledge several weaknesses of this retrospective study. Given the rarity of brain metastases in gynecologic malignancies, we evaluated different tumor sites and histologies together. This may have introduced bias due to potentially different responses to treatment, though we were able to show that patients with ovarian primaries have improved survival consistent with findings from Nasu, et al[35]. Another limitation was that the primary tumor specimens were not available for 11 of the patients for whom we had brain biopsies; such lack of specimens is common in large, tertiary care centers because patients often undergo primary surgery elsewhere. There was also a small sample size of patients for whom brain biopsy specimens were available for IHC; such numbers preclude meaningful generalization regarding outcomes of possible targeted therapy. Finally, generalizability of our findings is limited as treatments evolved over 19 years at a single institution.
In conclusion, this retrospective study evaluated the clinicopathologic and immunohistochemical characteristics of brain metastases from gynecologic malignancies. Using multivariate analysis to evaluate prognostic factors, we found three independent favorable prognostic factors: CA-125 level < 81 units/ml, presenting symptoms other than seizures, falls, or weakness, and multimodal therapy. Given the increased expression of the marker VEGF-A in brain metastasis specimens, further investigation in the role of anti-angiogenic (VEGF-A therapy) in treatment of brain metastases should be further evaluated in addition to aggressive multimodal therapy.
Figure 3.
A. Control specimen from ovarian cancer patient at low power (4x), B. Control at high power (20x), C. Brain specimen at low power (4x), D. Brain specimen at high power (20x).
Highlights.
Identification of poor outcome characteristics and long-term survival indicators.
Increased VEGF-A expression in metastatic brain samples compared to controls.
Multi-modality therapy was associated with improved clinical outcomes.
Footnotes
Conflict of Interest:
Dr. Hagemann reports that research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aalders JG, Abeler V, Kolstad P. Recurrent adenocarcinoma of the endometrium: a clinical and histopathological study of 379 patients. Gynecol Oncol. 1984;17(1):85–103. doi: 10.1016/0090-8258(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 2.Gien LT, et al. Brain metastases from endometrial carcinoma: a retrospective study. Gynecol Oncol. 2004;93(2):524–8. doi: 10.1016/j.ygyno.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Cormio G, et al. Brain metastases from endometrial carcinoma. Gynecol Oncol. 1996;61(1):40–3. doi: 10.1006/gyno.1996.0093. [DOI] [PubMed] [Google Scholar]
- 4.Larson DM, et al. Central nervous system metastases in epithelial ovarian carcinoma. Obstet Gynecol. 1986;68(6):746–50. [PubMed] [Google Scholar]
- 5.Cohen ZR, et al. Brain metastases in patients with ovarian carcinoma: prognostic factors and outcome. J Neurooncol. 2004;66(3):313–25. doi: 10.1023/b:neon.0000014516.04943.38. [DOI] [PubMed] [Google Scholar]
- 6.D’Andrea G, et al. Solitary cerebral metastases from ovarian epithelial carcinoma: 11 cases. Neurosurg Rev. 2005;28(2):120–3. doi: 10.1007/s10143-004-0363-4. [DOI] [PubMed] [Google Scholar]
- 7.Bruzzone M, et al. Cerebral metastases secondary to ovarian cancer: still an unusual event. Gynecol Oncol. 1993;49(1):37–40. doi: 10.1006/gyno.1993.1082. [DOI] [PubMed] [Google Scholar]
- 8.Ricke J, Baum K, Hosten N. Calcified brain metastases from ovarian carcinoma. Neuroradiology. 1996;38(5):460–1. doi: 10.1007/BF00607277. [DOI] [PubMed] [Google Scholar]
- 9.Hardy JR, V, Harvey J. Cerebral metastases in patients with ovarian cancer treated with chemotherapy. Gynecol Oncol. 1989;33(3):296–300. doi: 10.1016/0090-8258(89)90515-5. [DOI] [PubMed] [Google Scholar]
- 10.Kumar L, Tanwar RK, Singh SP. Intracranial metastases from carcinoma cervix and review of literature. Gynecol Oncol. 1992;46(3):391–2. doi: 10.1016/0090-8258(92)90239-f. [DOI] [PubMed] [Google Scholar]
- 11.Saphner T, et al. Neurologic complications of cervical cancer. A review of 2261 cases. Cancer. 1989;64(5):1147–51. doi: 10.1002/1097-0142(19890901)64:5<1147::aid-cncr2820640530>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Cormio G, et al. Brain metastases from cervical carcinoma. Tumori. 1996;82(4):394–6. doi: 10.1177/030089169608200420. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud-Ahmed AS, et al. Tumor distribution and survival in six patients with brain metastases from cervical carcinoma. Gynecol Oncol. 2001;81(2):196–200. doi: 10.1006/gyno.2001.6140. [DOI] [PubMed] [Google Scholar]
- 14.Mayer RJ, Berkowitz RS, Griffiths CT. Central nervous system involvement by ovarian carcinoma: a complication of prolonged survivial with metastatic disease. Cancer. 1978;41(2):776–83. doi: 10.1002/1097-0142(197802)41:2<776::aid-cncr2820410253>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Stein M, et al. Involvement of the central nervous system by ovarian carcinoma. Cancer. 1986;58(9):2066–9. doi: 10.1002/1097-0142(19861101)58:9<2066::aid-cncr2820580917>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Dauplat J, Nieberg RK, Hacker NF. Central nervous system metastases in epithelial ovarian carcinoma. Cancer. 1987;60(10):2559–62. doi: 10.1002/1097-0142(19871115)60:10<2559::aid-cncr2820601035>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Melichar B, et al. Brain metastases of epithelial ovarian carcinoma responding to cisplatin and gemcitabine combination chemotherapy: a case report and review of the literature. Gynecol Oncol. 2004;94(2):267–76. doi: 10.1016/j.ygyno.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Tangjitgamol S, et al. Role of surgical resection for lung, liver, and central nervous system metastases in patients with gynecological cancer: a literature review. Int J Gynecol Cancer. 2004;14(3):399–422. doi: 10.1111/j.1048-891x.2004.14326.x. [DOI] [PubMed] [Google Scholar]
- 19.Pothuri B, et al. Craniotomy for central nervous system metastases in epithelial ovarian carcinoma. Gynecol Oncol. 2002;87(1):133–7. doi: 10.1006/gyno.2002.6792. [DOI] [PubMed] [Google Scholar]
- 20.Kinch MS, Moore MB, Harpole DH., Jr Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9(2):613–8. [PubMed] [Google Scholar]
- 21.Thaker PH, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10(15):5145–50. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo K, et al. Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecol Oncol. 2014;133(3):473–9. doi: 10.1016/j.ygyno.2014.03.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berek JHN. Practical Gynecologic Oncology. 5. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 24.Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 25.Tewari KS, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–43. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rofstad EK, Halsor EF. Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res. 2000;60(17):4932–8. [PubMed] [Google Scholar]
- 27.Hanrahan EO, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2010;28(2):193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamat AA, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12(6):1707–14. doi: 10.1158/1078-0432.CCR-05-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamat AA, et al. EphA2 overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer. 2009;115(12):2684–92. doi: 10.1002/cncr.24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuo K, et al. Multidrug resistance gene (MDR-1) and risk of brain metastasis in epithelial ovarian, fallopian tube, and peritoneal cancer. Am J Clin Oncol. 2011;34(5):488–93. doi: 10.1097/COC.0b013e3181ec5f4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamat AA, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007;13(24):7487–95. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 32.McMeekin DS, et al. Ovarian cancer metastatic to the brain: what is the optimal management? J Surg Oncol. 2001;78(3):194–200. doi: 10.1002/jso.1149. discussion 200–1. [DOI] [PubMed] [Google Scholar]
- 33.Lee YK, et al. Gamma-knife radiosurgery as an optimal treatment modality for brain metastases from epithelial ovarian cancer. Gynecol Oncol. 2008;108(3):505–9. doi: 10.1016/j.ygyno.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Mahmoud-Ahmed AS, et al. The effect of radiation therapy on brain metastases from endometrial carcinoma: a retrospective study. Gynecol Oncol. 2001;83(2):305–9. doi: 10.1006/gyno.2001.6384. [DOI] [PubMed] [Google Scholar]
- 35.Nasu K, et al. Clinicopathologic features of brain metastases from gynecologic malignancies: a retrospective study of 139 cases (KCOG-G1001s trial) Gynecol Oncol. 2013;128(2):198–203. doi: 10.1016/j.ygyno.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Cormio G, et al. Changes in the management and outcome of central nervous system involvement from ovarian cancer since 1994. Int J Gynaecol Obstet. 2011;114(2):133–6. doi: 10.1016/j.ijgo.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez GC, et al. Improved palliation of cerebral metastases in epithelial ovarian cancer using a combined modality approach including radiation therapy, chemotherapy, and surgery. J Clin Oncol. 1992;10(10):1553–60. doi: 10.1200/JCO.1992.10.10.1553. [DOI] [PubMed] [Google Scholar]
- 38.Chura JC, et al. Multimodal therapy improves survival in patients with CNS metastasis from uterine cancer: a retrospective analysis and literature review. Gynecol Oncol. 2007;107(1):79–85. doi: 10.1016/j.ygyno.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Anupol N, et al. Evaluation of prognostic factors and treatment modalities in ovarian cancer patients with brain metastases. Gynecol Oncol. 2002;85(3):487–92. doi: 10.1006/gyno.2002.6653. [DOI] [PubMed] [Google Scholar]
- 40.Patchell RA, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 41.Vecht CJ, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33(6):583–90. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 42.Noordijk EM, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29(4):711–7. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 43.Andrews DW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 44.Kondziolka D, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427–34. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 45.Ewend MG, et al. Guidelines for the initial management of metastatic brain tumors: role of surgery, radiosurgery, and radiation therapy. J Natl Compr Canc Netw. 2008;6(5):505–13. doi: 10.6004/jnccn.2008.0038. quiz 514. [DOI] [PubMed] [Google Scholar]
- 46.Gaspar LE, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):17–32. doi: 10.1007/s11060-009-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–95. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175(3):409–16. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29(6 Suppl 16):10–4. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 50.Jubb AM, et al. Vascular phenotypes in primary non-small cell lung carcinomas and matched brain metastases. Br J Cancer. 2011;104(12):1877–81. doi: 10.1038/bjc.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Besse B, et al. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 2010;16(1):269–78. doi: 10.1158/1078-0432.CCR-09-2439. [DOI] [PubMed] [Google Scholar]
- 52.Sandler A, et al. An evidence-based review of the incidence of CNS bleeding with anti-VEGF therapy in non-small cell lung cancer patients with brain metastases. Lung Cancer. 2012;78(1):1–7. doi: 10.1016/j.lungcan.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Kreisl TN, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–5. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman HS, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 55.Lin X, DeAngelis LM. Treatment of Brain Metastases. J Clin Oncol. 2015;33(30):3475–84. doi: 10.1200/JCO.2015.60.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]