Abstract
Frontal fibrosing alopecia (FFA) is a form of scarring hair loss that is characterized by hair follicle destruction in a fronto-temporo-parietal distribution. Its etiology is unknown; however, most authors presently favor an immune pathogenesis. Associated autoimmune connective tissue diseases have been reported in patients with FFA. We present a case of FFA in a woman with primary biliary cirrhosis and polymyalgia rheumatica, suggesting an association between these clinical entities and supporting a potential autoimmune etiology of FFA.
Key Words: Alopecia, Hair disorder, Hair loss, Immune-mediated disease, Lichen planopilaris, Frontal fibrosing alopecia, Primary biliary cirrhosis, Polymyalgia rheumatica
Established Facts
Frontal fibrosing alopecia (FFA) is a clinical variant of lichen planopilaris characterized by frontotemporal hair loss.
The pathogenesis of FFA is unknown, but some have postulated it to be an immune-mediated disorder.
Autoimmune diseases share numerous features and coexist with significant frequency in the same individuals.
Novel Insights
The concomitant presentation of FFA, primary biliary cirrhosis (PBC), and polymyalgia rheumatica (PMR) has never been previously reported.
The coexistence of FFA with other autoimmune and immune-mediated diseases, such as PBC and PMR, may not be coincidental and is likely underreported.
Introduction
Frontal fibrosing alopecia (FFA) is a form of scarring hair loss that is characterized by hair follicle destruction in a fronto-temporo-parietal distribution [1]. FFA is most commonly detected in postmenopausal women, and thus a hormonal etiology has been suggested [1]. However, histologically FFA appears to be a lymphocyte-mediated alopecia. Thus, most authors presently favor an immune pathogenesis and often categorize FFA as a clinical variant of lichen planopilaris [2]. Associated autoimmune connective tissue diseases have been reported in patients with FFA [3,4]. We present a case of FFA in a woman with primary biliary cirrhosis (PBC) and polymyalgia rheumatica (PMR), suggesting an association between these clinical entities and supporting a potential autoimmune etiology of FFA [2]. To our knowledge, there is one reported case of PBC in a patient with FFA [5] and no reported case of PMR in a patient with FFA.
Case Report
A 59-year-old Caucasian woman with a 2-year history of progressive frontal hairline recession and eyebrow thinning was referred to the Hair Disorders Clinic at the Wake Forest Department of Dermatology. She reported no other associated symptoms. At the time of presentation, she was using clobetasol 0.05% foam for scalp hair loss, topical tacrolimus ointment for eyebrow hair loss, and prednisone 20 mg for PMR and ursodiol for PBC. She also had received intralesional triamcinolone injections (5 mg/ml) to her scalp in the past. Clinical examination showed band-like scarring alopecia of the frontotemporal hairline, with perifollicular erythema, follicular prominence, and scale (fig. 1, 2). The hair pull test was negative. The eyebrows were scant with few intact terminal hairs. There were no other skin, oral mucosal, or nail findings. Outside medical records reported a history of oral lichen planus, which had resolved without treatment. The patient's clinical presentation was indicative of FFA and further histological examination was deemed unnecessary for diagnosis. In addition to her current therapy, the patient was started on topical minoxidil 5% applied daily, finasteride 2.5 mg daily, and doxycycline 100 mg twice daily.
Fig. 1.
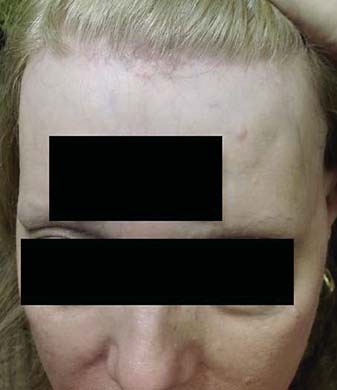
Frontal hairline showing recession with perifollicular erythema and mild follicular hyperkeratosis.
Fig. 2.
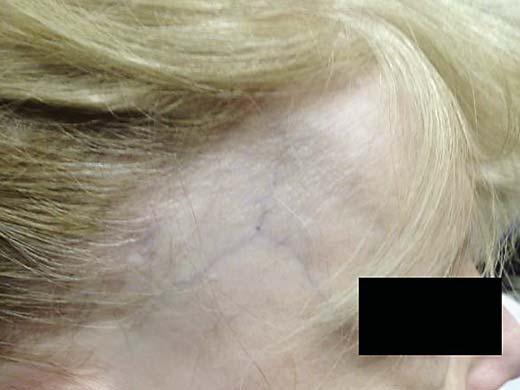
Right scalp showing symmetric frontotemporal alopecia with concomitant thinning of the eyebrows.
Discussion
FFA, PBC, and PMR are described as autoimmune and/or immune-mediated diseases because they are caused by a disrupted immune response to some components of hair follicles, bile ducts, and joint synovium, respectively [1,6,7]. PBC is an autoimmune disease characterized by injury of small and medium-sized bile ducts, leading to liver cirrhosis and liver failure [6]. Ninety-five percent of patients with PBC have antimitochondrial antibodies on serological testing [6]. Patients with PBC, particularly women, have a higher concomitant likelihood of autoimmune disease, with ∼50% reporting an additional autoimmune process [6]. Associated autoimmune disorders and their frequencies include Sjögren's syndrome (7-34%), Hashimoto's thyroiditis (11-13%), and rheumatoid arthritis (3-8%) [6]. PMR is an inflammatory, presumably autoimmune, rheumatic condition characterized by aching and morning stiffness in the shoulders, hip girdle, and neck. An association between PMR and PBC has been reported, but the frequency of concomitant disease presentation is not known [7,8,9].
Autoimmune and immune-mediated diseases share numerous features and coexist with significant frequency in the same individuals. For this reason, we believe that the presentation of FFA, as in this patient with PBC and PMR, is not coincidental and is likely underreported. FFA has been previously associated with other immune-mediated disorders, such as lupus erythematosus, Hashimoto's thyroiditis, and rheumatoid arthritis [3,4]. Additionally, the coexistence of PBC in patients with lichen planus and oral lichen planus has been described in the literature [10,11,12]. Koulentaki et al. [13] reported that the incidence rates of cutaneous lichen planus and oral lichen planus in patients with PBC were 10.2% (5/29 cases) and 8.2% (4/49), respectively. As previously mentioned, FFA is considered by many to be variant of lichen planopilaris [2], which may be further substantiated by our patient's history of oral lichen planus.
The association of FFA with PBC and/or PMR may support the theory that the pathophysiology of FFA is primarily T cell-mediated. Biopsied tissue in FFA shows a lymphocytic infiltrate characteristically at the level of the infundibulum and isthmus, sparing the lower portion of the hair follicle [1]. The infiltrate shows a T cell predominance, staining positive for CD45RO, which is a pan- T cell marker. Additional staining shows prominent CD4 positivity as well as CD8, CD43, and CD3. Antibodies to CD20 for B lymphocytes stain less than 5% of the lymphocytic infiltrate [2]. Similarly, the lymphocytic infiltrate of PMR and PBC consists mainly of T lymphocytes, which are almost all CD45RO-positive [14,15]. In PBC, the autoreactive lymphocytes are predominantly CD4+ and CD8+ T cells, which are specific for peptides encoded by the E2 components of pyruvate dehydrogenase complexes (PDC-E2) [15]. Further immunophenotyping studies of FFA can help elucidate the etiology of FFA as an autoimmune disease.
Another connection is that both PBC and PMR occur with increased frequency in women, as does FFA. The idea that there is a hormonal role in the etiology of these diseases has been raised [1,16]. Some PBC genetic research proposes that immune tolerance is related to genes on the X chromosome [16]. One study suggested that women with PBC are more likely to have monosomy of the X chromosome compared to healthy controls or those with hepatitis C [17]. To our knowledge, the role of immune tolerance genes on the X chromosomes of patients with FFA has not been proposed or examined yet, and thus may be another area for future study.
Additionally, genome-wide associated studies for PBC and PMR point to a possible genetic susceptibility through polymorphism of the HLA-DR8 and HLA-DPB1 genes [18,19] for PBC and the HLA-DRB1*04 gene for PMR [20]. An associated HLA gene has not been discovered for FFA yet. A commonality of HLA polymorphism between FFA, PBC, and/or PMR may further suggest genetic overlap between these diseases.
The potential association of PBC with FFA is important when considering treatment options for FFA. Certain medications such as hydroxychloroquine, mycophenolate mofetil, and methotrexate, which are occasionally used to treat FFA, cannot be used in patients with liver disease. Therefore physicians should be aware of the potential association between these diseases and should screen patients for signs and symptoms of liver dysfunction and obtain liver biochemical and function tests, including alkaline phosphatase, antimitochondrial antibodies, and antinuclear antibodies, prior to starting these medications.
Statement of Ethics
This study did not require approval by the institute's committee on human research. The patient gave consent for the use of anonymized photographs for research purposes.
Disclosure Statement
A.J. McMichael: Advisory board or panel: Chair of Dermatology at Wake Forest Baptist Health, President of Skin of Color Society. Consultant: Galderma, Guthey Renker, eResearch Technology, Inc., Johnson & Johnson, Keranetics, Merck & Co., Inc., Merz Pharmaceuticals, Procter & Gamble, Samumed, Incyte. Grants/research support: Allergan, Procter & Gamble, Samumed. Other financial or material support (royalties, patents, etc.): UptoDate, Informa Healthcare. A.N. Eginli and C.W. Bagayoko have no conflicts of interested to declare.
References
- 1.Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770–774. [PubMed] [Google Scholar]
- 2.Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59–66. doi: 10.1016/s0190-9622(97)70326-8. [DOI] [PubMed] [Google Scholar]
- 3.Del Rei M, Pirmez R, Sodre CT, Tosti A. Coexistence of frontal fibrosing alopecia and discoid lupus erythematosus of the scalp in 7 patients: just a coincidence? J Eur Acad Dermatol Venereol. 2016;30:151–153. doi: 10.1111/jdv.12642. [DOI] [PubMed] [Google Scholar]
- 4.Banka N, Mubki T, Bunagan MJ, McElwee K, Shapiro J. Frontal fibrosing alopecia: a retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int J Dermatol. 2014;53:1324–1330. doi: 10.1111/ijd.12479. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Belmonte MR, Navarro-López V, Ramírez-Boscà A, Martínez-Andrés MA, Molina-Gil C, González-Nebreda M, Asín-Llorca M. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64–69. doi: 10.1111/jocd.12125. [DOI] [PubMed] [Google Scholar]
- 6.Gatselis NK, Zachou K, Koukoulis GK, Dalekos GN. Autoimmune hepatitis, one disease with many faces: etiopathogenetic, clinico-laboratory and histological characteristics. World J Gastroenterol. 2015;21:60–83. doi: 10.3748/wjg.v21.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke AK, Hamilton E, Williams R. Polymyalgia rheumatica and primary biliary cirrhosis. Br Med J. 1979;1:1147. doi: 10.1136/bmj.1.6171.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagnerie F, Taillan B, Euller-Ziegler L, Ziegler G. Primary biliary cirrhosis, temporal arteritis (giant cell arteritis) and polymyalgia rheumatica in a single patient. Scand J Rheumatol. 1988;17:231–232. doi: 10.3109/03009748809098790. [DOI] [PubMed] [Google Scholar]
- 9.Chaouat D, Belange G, Gompel H. Polymyalgia rheumatica in primary biliary cirrhosis: value of the search for antimitochondrial antibodies in rhizomelic pseudo-polyarthritis and Horton disease. Rev Med Interne. 1994;15:775–776. doi: 10.1016/s0248-8663(05)81407-9. [DOI] [PubMed] [Google Scholar]
- 10.Nagao Y, Sata M. Disappearance of oral lichen planus after liver transplantation for primary biliary cirrhosis and immunosuppressive therapy in a 63-year-old Japanese woman. Hepat Mon. 2014;14:e16310. doi: 10.5812/hepatmon.16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oleaga JM, Gardeazabal J, Sanz de Galdeano C, Diaz PJ. Generalized lichen planus associated with primary biliar cirrhosis which resolved after liver transplantation. Acta Derm Venereol. 1995;75:87. doi: 10.2340/000155557587. [DOI] [PubMed] [Google Scholar]
- 12.Seehafer JR, Rogers RS, 3rd, Fleming CR, Dickson ER. Lichen planus-like lesions caused by penicillamine in primary biliary cirrhosis. Arch Dermatol. 1981;117:140–142. [PubMed] [Google Scholar]
- 13.Koulentaki M, Ioannidou D, Stefanidou M, Maraki S, Drigiannakis I, Dimoulios P, Melono JM, Tosca A, Kouroumalis EA. Dermatological manifestations in primary biliary cirrhosis patients: a case control study. Am J Gastroenterol. 2006;101:541–546. doi: 10.1111/j.1572-0241.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 14.Meliconi R, Pulsatelli L, Uguccioni M, Salvarani C, Macchioni P, Melchiorri C, Focherini MC, Frizziero L, Facchini A. Leukocyte infiltration in synovial tissue from the shoulder of patients with polymyalgia rheumatica. Quantitative analysis and influence of corticosteroid treatment. Arthritis Rheum. 1996;39:1199–1207. doi: 10.1002/art.1780390719. [DOI] [PubMed] [Google Scholar]
- 15.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan MM, Bianchi DW. Primary biliary cirrhosis: for want of an X chromosome? Lancet. 2004;363:505–506. doi: 10.1016/S0140-6736(04)15576-1. [DOI] [PubMed] [Google Scholar]
- 17.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, Watnik M, Gershwin ME, Podda M. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533–535. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 18.Underhill J, Donaldson P, Bray G, Doherty D, Portmann B, Williams R. Susceptibility to primary biliary cirrhosis is associated with the HLA-DR8-DQB1*0402 haplotype. Hepatology. 1992;16:1404–1408. doi: 10.1002/hep.1840160616. [DOI] [PubMed] [Google Scholar]
- 19.Underhill JA, Donaldson PT, Doherty DG, Manabe K, Williams R. HLA DPB polymorphism in primary sclerosing cholangitis and primary biliary cirrhosis. Hepatology. 1995;21:959–962. [PubMed] [Google Scholar]
- 20.Pipitone N, Salvarani C. Update on polymyalgia rheumatica. Eur J Intern Med. 2013;24:583–589. doi: 10.1016/j.ejim.2013.03.003. [DOI] [PubMed] [Google Scholar]


