Abstract
Background
Calcific uremic arteriolopathy (CUA), also referred to as calciphylaxis, is a rare and serious complication of kidney failure with limited treatment options. Kidney transplantation (KTX) restores kidney function and is hence a potential treatment option for CUA. We present 3 patients who had their CUA lesions successfully healed after urgent KTX.
Methods
Data were retrospectively retrieved from hospital records at our national transplant center.
Results
All 3 patients had previously been kidney transplanted and had experienced graft loss and were in stage 5 kidney failure when CUA developed. One patient was on warfarin treatment for pulmonary embolism. Skin lesions developed in the lower limbs in all 3 patients. Multidisciplinary care including intensified hemodialysis did not induce any clinically relevant improvement of the lesions. The recipients were enlisted on a clinically urgent waitlist for KTX and received a deceased donor kidney after 2 to 4 weeks. All recipients experienced good graft function. The lesions healed completely within 6 weeks in 2 patients. In the third patient, partial healing occurred after 2 months and complete healing was achieved 4 months after transplantation.
Conclusions
These cases indicate that urgent KTX may contribute to an efficient treatment for end-stage renal disease patients with CUA.
Calcific uremic arteriolopathy (CUA), also termed calciphylaxis, is a rare condition most often occurring in end-stage renal disease (ESRD) patients and typically in patients on dialysis. A hallmark of this condition is arteriolar medial calcification and development of subcutaneous necrosis and ulcerations, particularly in the lower limbs and abdomen.1,2 The 1-year mortality is reported to be as high as 40 to 80%.3,4 Risk factors for development of CUA are hyperparathyroidism, diabetes mellitus, obesity, hypercoagulative disorders, increased calcium-phosphate product and medication with systemic steroids, iron and warfarin.1-5 The current treatment strategy yields poor outcomes despite multiple interventions including intensified dialysis.6 Restoring kidney function by kidney transplantation (KTX) may be another approach. Two previous case reports describe regression of CUA after KTX, as long as 67 and 128 months after transplantation. At our national transplant center, we accept patients with CUA to be waitlisted for deceased donor KTX as clinically urgent. During the last 3 years, we have transplanted 3 patients in this category for rescue therapy of CUA ulcers.
PATIENT CASES
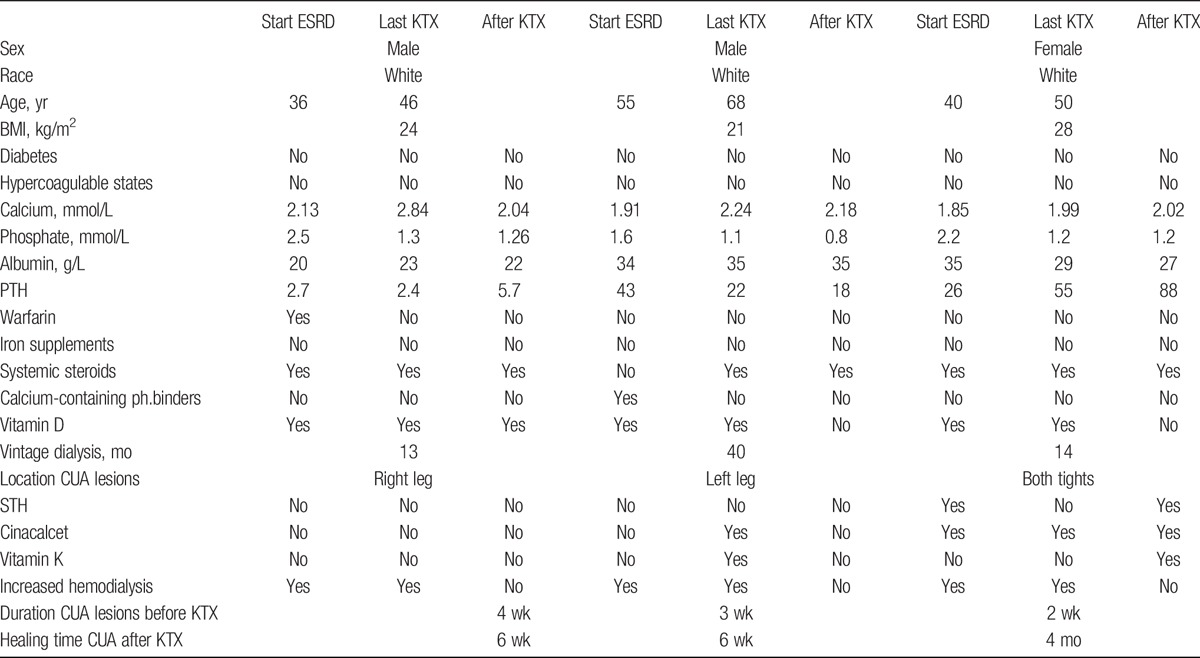
Table 1 shows patient characteristics, laboratory test findings and relevant medication in relation to time of KTX in all 3 patients.
TABLE 1.
Laboratory parameteres from time of developing ESRD, time of KTX and 14 days after KTX.
All patients had normal immunological risk and were transplanted with deceased donor kidneys. They all received standard immunosuppressive regimen with basiliximab induction, followed by maintenance immunosuppression consisting of twice daily tacrolimus (trough levels, 3-7 μg/L), mycophenolate mofetil 750 mg twice daily and steroids. Our steroid regimen consists of 250 mg SoluMedrol at day 0 and daily prednisolone dose of 20 mg/d × 1, tapered to 10 mg/d after 1 month, and further down to 5 mg/d after 6 months.
Patient 1
This 46-year-old male patient was known with Crohn disease and kidney failure due to secondary amyloidosis. He did not suffer from diabetes or hypercoagulable disorders. His weight and nutritional status was normal. His first kidney graft had failed after 12 years due to recurrence of amyloidosis, and he was reestablished in hemodialysis for 4 months when severe pain and ulcerations evolved in the right leg. His immunosuppression, including prednisolone 5 mg × 1, was continued. The calcium and phosphate levels were adequate. He had been treated with warfarin due to pulmonary embolism for 3 months when ulcerations evolved; skin biopsy confirmed the CUA diagnosis.
Warfarin was withdrawn and replaced by low molecular weight heparin.
The dialysis treatment was intensified. Sodium-thiosulphate (STH) was not given, neither cinacalcet nor vitamin K.
After 1 month without any signs of recovery, he was listed for urgent KTX and received a transplant 4 weeks later. He needed 4 sessions of hemodialysis due to initially delayed graft function, but later the kidney function rapidly improved to normal and the skin lesions started to heal. His skin lesions healed completely after 6 weeks. At present, 2.5 years later, he is in good condition, his creatinine level is 80 μmol/L and no new skin lesions have appeared.
Patient 2
This 68-year-old man had kidney failure due to nephrosclerosis. He had previously lost 2 kidney grafts and his dialysis vintage was in total 40 months. His first transplant failed after 8 years due to chronic rejection and his second graft failed after about a year without ever obtaining desired function. His maintenance immunosuppression, including prednisolone 5 mg × 1, was continued. He had normal weight- and nutritional status and he did not suffer from diabetes or hypercoagulative disorders. Active vitamin D and calcium-containing phosphate binders had regulated his secondary hyperparathyroidism adequately. He never was treated with warfarin. A few weeks after he was reestablished in hemodialysis he presented with painful skin ulcers in the left leg. Skin biopsy confirmed CUA. He received intensified dialysis, cinacalcet treatment, and vitamin K substitution, but did not receive STH. Calcium-containing phosphate binder was withdrawn.
After 1 month without improvement, he was listed for urgent transplantation and received a deceased donor kidney 3 weeks later. There was immediate good graft function, and his ulcers started to heal shortly after KTX, and were completely resolved within 6 weeks. At present, 13 months after transplantation, plasma creatinine value is 90 μmol/L, and no new skin lesions have developed.
Figure 1 shows the left leg of patient 2 4 days and 22 days after KTX.
FIGURE 1.
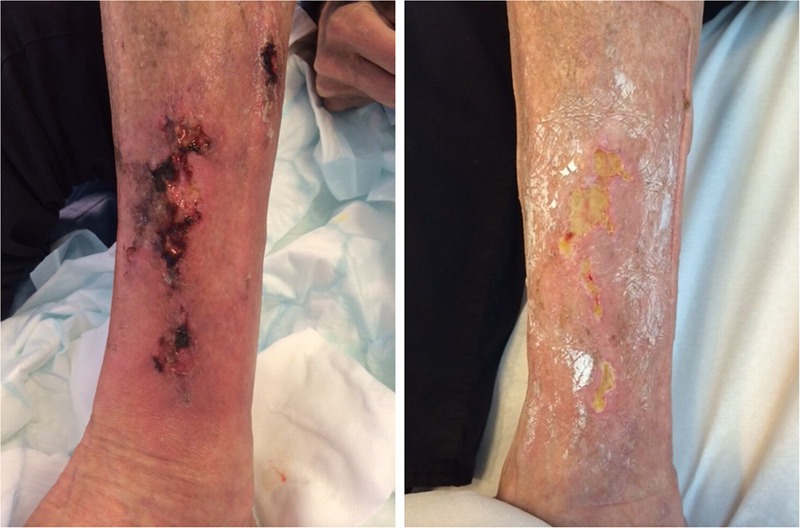
The left leg of patient 24 days and 22 days after KTX.
Patient 3
This 50-year-old woman was known with Crohn’s disease, short bowel syndrome and ESRD suspected due to AA amyloidosis. Her BMI was 28 kg/m2, and her nutrition status was normal. She did not suffer from diabetes or hypercoagulable disorders. She complained of severe pain in both thighs when her first kidney graft had started to fail after 9 years due to chronic rejection. She was not yet in need for hemodialysis. At first, there were no visible skin lesions but these developed 8 months later when she had supplements of magnesium, calcium, active vitamin D, and cinacalcet due to secondary hyperparathyroidism. When CUA was diagnosed by skin biopsy, she was enlisted for urgent KTX and intensified dialysis treatment was started.
She received a graft 2 weeks later, and postoperatively, she suffered from wound infections and diarrhea related to comorbid conditions. The first month, plasma creatinine levels were 220 μmol/L as the ulcers became worse and new lesions developed. This despite meticulously wound care and close surveillance. Treatment with STH, vitamin K, and cinacalcet as well as repeated sessions with hyperbaric oxygen treatment were started.
Two months after transplantation, some signs of healing were seen and 4 months after KTX, the lesions were fully resolved when plasma creatinine had stabilized at 120 μmol/L. Later, the graft function has fluctuated with episodes of diarrhea and secondary electrolyte deficiencies. She has had recurrent skin infections in the thighs and legs, but no new CUA lesions.
DISCUSSION
There are reports of CUA in patients with normal kidney function5 but all 3 of our patients had failing kidney grafts and were considered candidates for KTX when they were in need for renal replacement therapy. They were enlisted as clinically urgent for rescue of CUA and all received a kidney transplant within 4 weeks. There was subsequent healing within a few weeks in 2 of the patients and 4 months in the third patient.
Two previously published cases7,8 are in line with our findings. They were transplanted 2 and 6 months after CUA was diagnosed and experienced complete remission 6 and 12 months later, respectively. Although the time to healing was conceivably longer in these reports than in our study, it may be speculated that CUA lesions become more severe with time and thus complicate the healing process after transplantation. In any case, it is conceivable that a good kidney function after transplantation is a key factor for success for cure of such ulcers. This notion is substantiated by reports of CUA appearing in kidney transplant recipients with delayed- or reduced graft function.9-11 The last of our presented patients did not have levels of creatinine below 120 μmol/L before 3 months had passed after transplantation, and it was not until the renal function had been restored to that level that the CUA started to heal properly.
All patients experienced severe pain in their CUA lesions before KTX, and the pain was not relieved before restoration of kidney function after KTX. None of the patients were followed up by radiotracer uptake on nuclear bone scans of CUA lesions, a method that could have provided clues whether lesions were showing subclinical signs of improvement before KTX.
Adjustment of medications used to treat mineral bone disorders and secondary hyperparathyroidism was performed in all patients before KTX.
The most significant risk factor for development of CUA in addition to kidney failure is use of warfarin.2 One of our patients had warfarin withdrawn and the other patients had supplementation of vitamin K, because supplementation of vitamin K may play a role in treating this condition.12 The patient who had warfarin withdrawn did not receive supplementation of vitamin K, but warfarin withdrawing alone seemed not sufficient to reverse the condition. The role of vitamin K probably has a role in both development and treatment in this condition and should be subject for further investigation in future studies. Two of our reported patients had ESRD caused by AA amyloidosis, which is a rare cause in Norway. Amyloidosis is often present in the vasculature of subcutaneous fat. However, it is unlikely that amyloidosis had a role for the occurrence of CUA because amyloidosis was not present in any of the skin biopsies taken of the CUA lesions.
The use of systemic steroids could have contributed in the development of our patients CUA lesions as all were treated with prednisolone 5 mg a day as part of maintenance immunosuppression. STH has not been part of the treating tradition in our center, even though this is the most common intervention to treat CUA.2 None of our patients was treated by STH before transplantation but 1 had this administered 5 times a week after transplantation as the condition did not improve. The clinical effect was sparse until the kidney graft obtained better function.
All patients had priority transplantation, as medical treatment did not improve CUA. We cannot predict how the lesions would have progressed if they remained on the ordinary waitlist treated by intensified dialysis. What we do know is that they all received medical therapy and wound care for several weeks without results before enlisted as clinically urgent for KTX. Given these observations and the knowledge of the poor prognosis with the use of conservative measures, we believe that swift restoration of kidney function by KTX was of central importance for the good outcomes in these 3 patients.
Priority candidates on the waiting list receive the first crossmatch negative kidney available. This is of course a priority issue because it increases the waiting time for other patients. However, at our center, the waitlist is relative short with average waiting time less than a year, and thus this practice appears ethically sound.13 Another approach may of course be to use a live donor kidney, but this was not an option for our patients.
Extensive ulcerations may be considered as a contraindication for transplantation. Despite such ulcerations, urgent KTX appeared as a safe and efficient procedure for our kidney failure patients with CUA and should be considered as a treatment option of this condition.
Footnotes
Published online 25 October, 2016.
The authors declare no funding or conflicts of interest.
E.N. contributed to the writing of the abstract and the article and collecting of clinical variables and revision of the literature. D.O.D. participated by critical revision of the article. I.M.S. participated by critical revision of the article. A.Å. participated by critical revision of the article. A.V.R. participated by critical revision of the article. A.H. participated by critical revision of the article.
REFERENCES
- 1.Budisavljevic MN, Cheek D, Ploth DW. Calciphylaxis in chronic renal failure. J Am Soc Nephrol. 1996;7:978–982. [DOI] [PubMed] [Google Scholar]
- 2.Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis and outcome. J Am Acad Dermatol. 2007;56:569–579. [DOI] [PubMed] [Google Scholar]
- 4.Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210–2217. [DOI] [PubMed] [Google Scholar]
- 5.Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531–537. [DOI] [PubMed] [Google Scholar]
- 7.Ahnsan U. Resolution of calciphylaxis after successful kidney transplantation. Abstract NKF. 2009 Spring Clinical Meetings.
- 8.Bhat S, Hegde S, Bellovich K, et al. Complete resolution of calciphylaxis after kidney transplantation. Am J Kidney Dis. 2013;62:132–134. [DOI] [PubMed] [Google Scholar]
- 9.Smith S, Inaba A, Murphy J, et al. A case report: radiological findings in an unusual case of calciphylaxis 16 years after renal transplantation. Skeletal Radiol. 2013;42:1623–1626. [DOI] [PubMed] [Google Scholar]
- 10.Vanparys J, Sprangers B, Sagaert X, et al. Chronic wounds in a kidney transplant recipient with moderate renal impairment. Acta Clin Belg. 2013;68:128–131. [DOI] [PubMed] [Google Scholar]
- 11.Brewster UC, Perazella MA. Calcific uremic arteriolopathy in a transplanted kidney. Am J Med Sci. 2005;329:102–103. [DOI] [PubMed] [Google Scholar]
- 12.Levy R. Potential treatment of calciphylaxis with vitamin K(2): Comment on the article by Jacobs-Kosmin and DeHoratius. Arthritis Rheum. 2007;57:1575–1576. [DOI] [PubMed] [Google Scholar]
- 13.The Norwegian Renal Registry. Annual Report 2014. http://www.nephro.no/nnr/AARSM2014.pdf. Published 2014. Accessed Septed 23, 2016.


