Abstract
Objective
The objective of this study was to design and conduct a preliminary evaluation of an intervention to assist parents in decision-making about disclosure of their HIV diagnosis to their children.
Design
This was a pilot randomized controlled trial (RCT) with blinded assessment. Participants were randomized to intervention or treatment-as-usual (TAU) arms.
Setting
The study occurred at an outpatient HIV primary care centre in Shanghai, China.
Participants
Participants were 20 HIV-positive outpatients with at least one child (13–25 years old) who was unaware of the parent’s HIV diagnosis.
Intervention
The nurse-delivered intervention involved three, hour-long, individual sessions over 4 weeks. Intervention content comprised family assessment, discussion of advantages and disadvantages of disclosure, psycho-education about cognitive, social and emotional abilities of children at different developmental stages, and disclosure planning and practicing via role-plays.
Main outcome measure(s)
Primary study outcomes for intervention versus TAU arms were self-reported disclosure distress, self-efficacy and behaviours along a continuum from no disclosure to full disclosure and open communication about HIV.
Results
In all cross-sectional (Wald tests) and longitudinal (general estimating equations) analyses, at both postintervention (4 weeks) and follow-up (13 weeks), effects were in the hypothesized directions. Despite the small sample size, most of these between-arm comparisons were statistically significant, with at least one result for each outcome indicating a ‘large’ effect size.
Conclusion
Our results suggest that nurses are able to deliver a counselling intervention in a clinic setting with the potential to alleviate parental distress around HIV disclosure to their children. Findings warrant future trials powered for efficacy.
Keywords: child, China, disclosure, HIV, parents, randomized controlled trial
Introduction
One of the greatest challenges for persons living with HIV/AIDS (PLWHA) who are parents involves excruciating decisions about whether, when and how to disclose their HIV infection to their children. Many parents understandably resist informing their children of their HIV disease, citing fears of stigma, discrimination and possible rejection; uncertainty about how to disclose; doubts about their children’s ability to keep the information confidential; anxiety about negative psychological consequences for the child; guilt over how the disease was transmitted; and reluctance to contemplate their own possible demise [1–5].
The extent of parental disclosure appears to vary widely within and across settings. One review cited estimates of global parental disclosure of 20–97% in high-income countries and 11–44% in resource-constrained countries [1]. Disclosing parents are more likely to be in poor health, prefer to tell older children and (among mothers) are more likely to tell daughters [6–10].
Findings about the impact of parental disclosure are decidedly mixed [6,8,10–14], with parental reports often more negative than those from children [3,11,12]. Differences may depend as well on age of the child told, if other children were not told, how the disclosure was handled and whether secrecy was demanded [15,16].
Some studies have shown disclosure to be associated with poor family relationship quality, increased family stressors, more problem behaviours, negative psychological effects (e.g. lower self-esteem and mood) and enduring negative memories of the disclosure. The effects may be most pernicious for adolescents, who in one study engaged in more sexual risk acts, smoked more cigarettes and reported more severe substance use and greater emotional distress than their uninformed counterparts [7]. Moreover, the negative impact for parents as well as adolescent children appears to persist for a considerable period of time [7].
On the contrary, some studies have documented no psychological impact of parental HIV disclosure [9], especially on small children, or positive effects such as improved maternal well being, medication adherence, social support, family functioning and child mood [15,17]. Most initial negative effects of disclosure tend to dissipate over time, mediated by a supportive parent–child relationship [11,18]. Moreover, nondisclosure is not necessarily a risk-free alternative: it can be linked to increased fears and anxiety in children and regret over the lack of open communication and the missed opportunity to support their parents [17,19].
The research on parental HIV disclosure derives mostly from samples of HIV-positive mothers in the West, with limited data on disclosure from fathers and either parent in low-resource settings [1,8]. As noted, parental disclosure tends to be rarer in low-resource settings, with forced, planned or unintentional disclosures common yet often regretted by parents and more likely to lead to negative consequences in children [8,20].
In China, a setting with acute HIV stigma [21], the limited relevant research suggests that disclosing HIV/AIDS serostatus to family members is contentious and a cause of considerable psychological distress [22]. Regarding disclosure to children in particular, qualitative findings show that parents report feeling overwhelmed and unequipped to systematically consider relevant factors to make an informed decision. Parents report distress while weighing competing considerations to tell or withhold and avoid confronting the issue to mitigate their distress [23]. These findings are consistent with the broader context of disease disclosure in China, where family members and even patients themselves are often shielded from what is considered the burden of the diagnosis [24–26]. This pattern of limited disclosure is only exacerbated by the acute stigma of HIV in contemporary China [27,28].
Most Chinese parents opt not to disclose their HIV diagnosis, make partial disclosures (reporting that they have some other illness) or tell only their adult-age children in order to garner emotional or financial support [29,30]. Deeply ingrained cultural values promote the value and importance of the family in China, yet also discourage open and direct communication of stigmatizing and sensitive personal information [5]. Findings about the consequences of disclosure in China vary. Some research has shown that HIV disclosure to children in China strengthened family relations and increased assistance with medical care, reinforcing the parent’s willingness to live [32,33]. Although some Chinese children who are aware of their parents' HIV diagnosis exhibit emotional and social dysfunction, others show improved behaviour and focus more intently on their studies [23,29]. Adequate support from caregivers (e.g. grandparents or HIV-positive parents) after disclosure can improve the child’s quality of life and academic performance [19,33].
Practical interventions to address parental distress around HIV disclosure are needed in China. Chinese cultural norms often emphasize family identity and cohesion and underscore the need for family-based programmes [5], which have been shown to be feasible and efficacious for HIV-affected families [34]. We could locate only one formally evaluated intervention study on parental HIV disclosure, the TRACK program [17], which led to positive effects on low-income mothers in the U.S. and greater levels of disclosure to 6–12 year-old children.
In the present study, we describe the development and preliminary evaluation of a theory-informed parental HIV disclosure support intervention delivered at an HIV primary care clinic in Shanghai, China. The intervention was designed to support nondisclosing parents through the decision-making process and alleviate their disclosure-related distress.
As China has a dearth of personnel trained to address mental health needs, who would ideally deliver the intervention, we trained nurses at the clinic as interventionists. This ‘task-shifting’ approach (i.e. the process of redistributing healthcare tasks to less specialized health workers when appropriate [35]) has been endorsed as a means of reducing global inequities in healthcare [26,35] and may facilitate implementation across China and in settings with fewer mental healthcare professionals.
Materials and methods
Procedures
Data were collected from December 2013 through August 2014 at Shanghai Public Health Clinical Center (SPHCC), one of the premier treatment centres for infectious diseases in China. Eligible individuals were Mandarin-speaking patients receiving care at SPHCC, were at least 18 years of age, were confirmed HIV seropositive, had at least one child between 13 and 25 years of age who were not aware of the parent's HIV status and were willing to and physically capable of attending intervention sessions at SPHCC. Cognitively impaired or actively psychotic individuals were excluded. Referrals came from the healthcare providers at SPHCC. Study staff described the trial to all referred patients. Quota sampling ensured an adequate representation of women. Those who were interested and willing to participate provided written informed consent and began their first session immediately or scheduled an appointment for a more convenient date.
At the baseline appointment, participants were randomized to either the control or intervention arm using sequentially numbered, opaque, sealed envelopes containing the intervention assignment, which the staff member opened at the moment of randomization. A block randomization procedure led to equal distribution of men and women within each arm.
Parents in the intervention arm had three counselling sessions with a study nurse of up to 1 h each over 4 weeks. Meeting times were coordinated with the patient’s clinic visits when possible to reduce participant burden. Participants assigned to the control arm received no further intervention beyond the usual care at the clinic. This involved monthly medication pick-ups and any conversations patients initiated with their healthcare providers. All participants continued to receive medical care as usual.
The counselling sessions were delivered by one of four experienced nurses who had been working at SPHCC for 3–25 years, each of whom participated in 40 h of intensive training. The nurse interventionists were supervised at least weekly via online video and instant text messaging by study investigators to ensure fidelity to the intervention protocol. The interventionists also completed a content checklist and a progress note after each session.
At baseline (pre-randomization), immediate postintervention (4 weeks) and follow-up (13 weeks), the participants were given an approximately 1-hour paper-and-pencil assessment survey to complete on their own. Participants in both arms were reimbursed 150 RMB (~$25) for completing each study session, a fee typical for research participation at the site that was not perceived as coercive. For control participants, each study session involved only the assessment survey, whereas intervention participants completed the assessment as well as a counselling session, and were given only one payment of 150 RMB for each visit. Tracking efforts included unscripted reminder phone calls 1 week and 1 day before each scheduled visit. Study staff administering surveys, but not the participants and intervention nurse, were blinded to study arm assignment.
Parental HIV disclosure support intervention
The parental HIV disclosure support intervention was informed conceptually by our Chinese Parental HIV Disclosure Model. This model was based on the Disclosure Process Model of Chaudoir et al. [36], which we adapted for a Chinese cultural context based on qualitative interviews with HIV-positive parents, community advisory board members and HIV care providers [23,37]. Our intervention components drew from Murphy et al.'s model for maternal HIV disclosure as well [17].
The Chinese Parental HIV Disclosure Model [23] comprises three main components that informed our intervention: decision-making (balancing distal factors such as the socio-cultural context with motivations for approaching and avoiding); the disclosure event (content of disclosure and the child’s reaction); and related outcomes (such as the impact on family dynamics and relationships). Relevant distal factors incorporated into the intervention include acknowledgment of social and community-based HIV stigma, low mental health resources for both parental and child functioning, and a strong emphasis on a harmonious family unit with prescribed parental and child roles. Approach and avoidance motivations for disclosure that are unique to the Chinese PLWHA population include fear of revealing mode of HIV transmission and fear of potential consequences from the community (from ‘losing face’ to outright expulsion) if the child cannot keep the parent’s HIV status a secret. Murphy et al.'s model for maternal HIV disclosure highlights the relationship context of the parent, child and family as a target for intervention, emphasizing parenting skills such as parent disclosure self-efficacy, family communication and positive family routines [17]. As routines were not found to be instrumental to disclosure success in Murphy et al.'s work, we concentrated on enhancing parents’ self-efficacy around disclosure and improving family communication that was consistent with the clearly defined roles in Chinese families.
The intervention itself involved three sessions. In Session 1, parents share the story of when and how they were diagnosed and to whom they disclosed including partners and other family members. The nurse interventionist then briefly assesses the parent, child and family’s current strengths and challenges in order to determine a parent’s unique disclosure approach and avoidance motivations and potential mediating processes. The nurse then engages parents in a discussion of the advantages and disadvantages of disclosure. On the basis of the assessment of family functioning, the nurse provides didactic information about useful family communication skills, and parents are asked to practice spending time and having an intentional conversation with the child as a homework assignment. Participants who shared information about their partners were encouraged to share with them the contents of the counselling sessions and to invite them to future sessions.
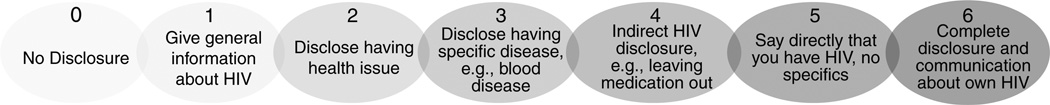
In Session 2, the nurse provides psycho-education to parents as to what they can expect from their children during the disclosure process. Cognizant of the cultural context; the cognitive, social and emotional abilities of children at various stages of development; and research on parental HIV disclosure, we developed a guide for parents on developmental periods spanning the ages of 10–27 years (i.e. early adolescence, middle adolescence, late adolescence and young/emerging adulthood). For children in each developmental period, the guide suggests what they can understand about disclosure (e.g. 14–17 years: ‘Youth have a better understanding of what the diagnosis means for both the present and the future’.); how they might respond (e.g. 14–17 years: ‘Youth may feel responsible for taking care of parent and may worry about not being able to do so’.) and helpful tips for managing the disclosure process with the child (e.g. 14–17 years: ‘Having a discussion about youth’s responsibilities and developing realistic expectations may be helpful in reducing youth’s stress’.). Finally, parents are asked to imagine where they are along a disclosure continuum from no disclosure at all to complete disclosure and open communication about HIV (see Fig. 1 and description in Measures). For homework, parents are asked to carefully consider where they would like to be on the continuum both in the immediate and distant future. Parents are encouraged, if able, to discuss these decisions with their coparents or other sources of social support.
Fig. 1. Disclosure Behaviours Continuum.
In Session 3, parents develop a plan for achieving their desired position along the disclosure continuum, including practicing and role-playing a possible disclosure plan and anticipating and preparing for possible questions and reactions from their child. Nurses assist in troubleshooting potential barriers to carrying out the plan. For homework, parents are encouraged to carry out their plans for the immediate future. (An intervention manual in Mandarin is available from the authors.)
Outcome measures
In addition to standard socio-demographic items on age, sex, number of children, education, employment, income, marital status and sexual orientation, participants completed three measures of intervention outcomes.
Disclosure distress was measured with three items asking participants how stressful it is to contemplate disclosure tasks: ‘How distressed are you about the issue of whether, when, and how to disclose your HIV status to your child?’; ‘How distressed are you about your child’s potential response to disclosure?’; and ‘How distressed are you about your ability to handle your child’s potential response to disclosure?’ Response options ranged from 1 (never/not at all) to 4 (always/extremely). The three individual items scores were averaged to create a mean summary score (Cronbach’s alpha ranged from 0.88 to 0.95 across waves). To minimize the impact of outliers, we dichotomized the variable into ‘higher’ and ‘lower’ levels on the basis of a median split at each wave. Scores exactly on the median were categorized as lower or higher distress to achieve the closest approximation to 50% in each wave [38].
Disclosure self-efficacy was measured with two items: ‘How prepared/ready do you feel about making a decision on whether, when, and how to disclose your HIV status to your child?’; and ‘How prepared/ready do you feel about carrying out the decision you made on whether, when, and how to disclose your HIV status to your child?’ Response options ranged from 1 (not at all) to 4 (completely). The median split procedure described above was employed as well with this variable.
A visual Disclosure Behaviors Continuum ranging from no HIV disclosure whatsoever to full disclosure and open communication about HIV (see Fig. 1) was shown to parents whom were asked to indicate which level best represented their current level of disclosure to their child.
Analytic procedures
Descriptive statistics were first applied to summarize the basic characteristics of the sample at baseline. T-tests and Fisher’s exact tests were used to detect any baseline differences between the intervention and control arms. Due to the small sample size, bootstrapping with additional 3000 samples was used to calculate the standard errors for continuous variables.
We modelled intervention effects across study waves using an intent-to-treat approach in which all randomized participants were included. As the retention rate was 100% with minimal missing values, no analyses or adjustments were included to address missingness. Generalized estimating equations (GEEs) were applied with additional interaction terms between study waves and treatment status. This parameterization allowed us to partition the average treatment effects into comparisons of changes in study arms from baseline to immediate postintervention (4 weeks) and from baseline to follow-up (13 weeks). As the beta-coefficients represented the average change between arms across waves, any potential differences at baseline were taken into account. The disclosure continuum was modelled as a normal continuous variable with a Gaussian link function. Disclosure distress and disclosure self-efficacy were treated as binary variables with logit link functions in the GEE models. The exchangeable working correlation structure was assumed for all the models to account for correlated outcome measures. Due to the small sample size, bootstrapping with additional 3000 samples was used to calculate the standard errors. Wald tests, which follow Chi-square distributions, were applied to compare the average marginal outcomes between study arms at each wave. Although this pilot randomized controlled trial (RCT) was undertaken to ascertain approximate effect sizes and not powered for definitive null hypothesis significance testing, significance levels are nevertheless reported for illustrative purposes.
Results
Participant characteristics
As seen in Table 1, the participants averaged 46.35 (SD = 1.15) years of age, were mainly (70%) male, had one child (75%) and had at least high school level education (70%), About two-thirds worked full time, but only one-third reported household income above the average for Shanghai residents (4000 RMB, approximately US$666/month). About half identified as gay or bisexual, and 85% were in a heterosexual marriage. None of the key demographic variables assessed at baseline differed (at P < 0.10) between the two study arms.
Table 1.
Demographic description of participants by study arm.
| Entire sample (N = 20) Mean (SD) or % |
TAU group (n = 10) Mean (SD) or % |
Intervention group (n = 10) Mean (SD) or % |
|
|---|---|---|---|
| Age (years) | 46.3 (1.2) | 47.1 (1.8) | 45.6 (1.5) |
| Biological sex | |||
| Female | 30% | 30% | 30% |
| Number of children | |||
| 1 | 75% | 70% | 80% |
| 2 | 20% | 20% | 20% |
| 3 | 5% | 10% | 0% |
| Age of the targeted child (SD) | 18.1 (3.6) | 18.1 (3.7) | 18.1 (3.8) |
| Education | |||
| <High school | 32% | 40% | 24% |
| High school | 42% | 40% | 44% |
| >High school | 26% | 20% | 32% |
| Employment/Occupation | |||
| No job | 20% | 20% | 20% |
| Part-time job | 15% | 10% | 20% |
| Full-time job | 65% | 70% | 60% |
| Income | |||
| Below average | 65% | 60% | 70% |
| Above average | 30% | 30% | 30% |
| Do not know | 5% | 10% | 0% |
| Marital status | |||
| Married | 85% | 90% | 80% |
| Sexual orientation | |||
| Gay/Bisexual | 45% | 30% | 60% |
| Heterosexual | 50% | 70% | 30% |
| Refuse to answer | 5% | 0% | 10% |
SD, Standard deviation; TAU, treatment as usual.
Intervention outcomes
The analyses of the effects of the intervention on the three outcome measures (i.e. disclosure distress, disclosure self-efficacy and disclosure behaviours) are summarized in Table 2 and depicted graphically in Fig. 2a–c. As seen in Table 2, descriptive analyses of each outcome indicator were comparable between arms at baseline (and non-significant at P < 0.10), suggesting that the randomization was effective. Results for each outcome are described in detail below.
Table 2.
Cross-sectional and longitudinal outcome analyses of parental HIV disclosure support intervention among N = 20 Chinese parents.
| Total | TAU group | Intervention groupa | Treatment effectb | |||||
|---|---|---|---|---|---|---|---|---|
| Probability of higher disclosure distress | % | (SE) | % | (SE) | % | (SE) | OR | 95% CI |
| Baseline | 50.00 | (0.52) | 50.00 | (0.53) | 50.00 | (0.53) | – | – |
| 4 weeks | 40.00 | (0.50) | 50.00 | (0.53) | 30.00(S) | (0.48) | 0.43 | 0.08–2.28 |
| 13 weeks | 25.00 | (0.44) | 40.00 | (0.52) | 10.00*(L) | (0.32) | 0.17** | 0.03–0.91 |
| Probability of higher disclosure self-efficacy | % | (SE) | % | (SE) | % | (SE) | OR | 95% CI |
| Baseline | 35.00 | (0.49) | 20.00 | (0.42) | 50.00 | (0.53) | – | – |
| 4 weeks | 55.00 | (0.51) | 20.00 | (0.42) | 90.00***(L) | (0.32) | 9.00*** | 2.06–39.29 |
| 13 weeks | 65.00 | (0.49) | 40.00 | (0.52) | 90.00***(L) | (0.32) | 3.38 | 0.45–25.36 |
| Position on disclosure behaviours continuum | Mean | (SE) | Mean | (SE) | Mean | (SE) | Beta | 95% CI |
| Baseline | 0.95 | (0.33) | 0.70 | (0.30) | 1.20 | (0.60) | – | – |
| 4 weeks | 1.75 | (0.19) | 0.80 | (0.32) | 2.70***(L) | (0.21) | 1.40** | 0.31–2.50 |
| 13 weeks | 2.20 | (0.25) | 1.40 | (0.41) | 3.00***(L) | (0.29) | 1.10 | 0.48 to 2.68 |
CI, confidence interval; OR, odds ratio.
The significance levels for P values in this column are based on results of Wald tests.
The significance levels for P values in this column are based on results of Generalized Estimating Equations from baseline to respective end point (4 weeks or 13 weeks).
<0.1.
<0.05.
<0.01.
(S) and (L) refer to small and large effect sizes.
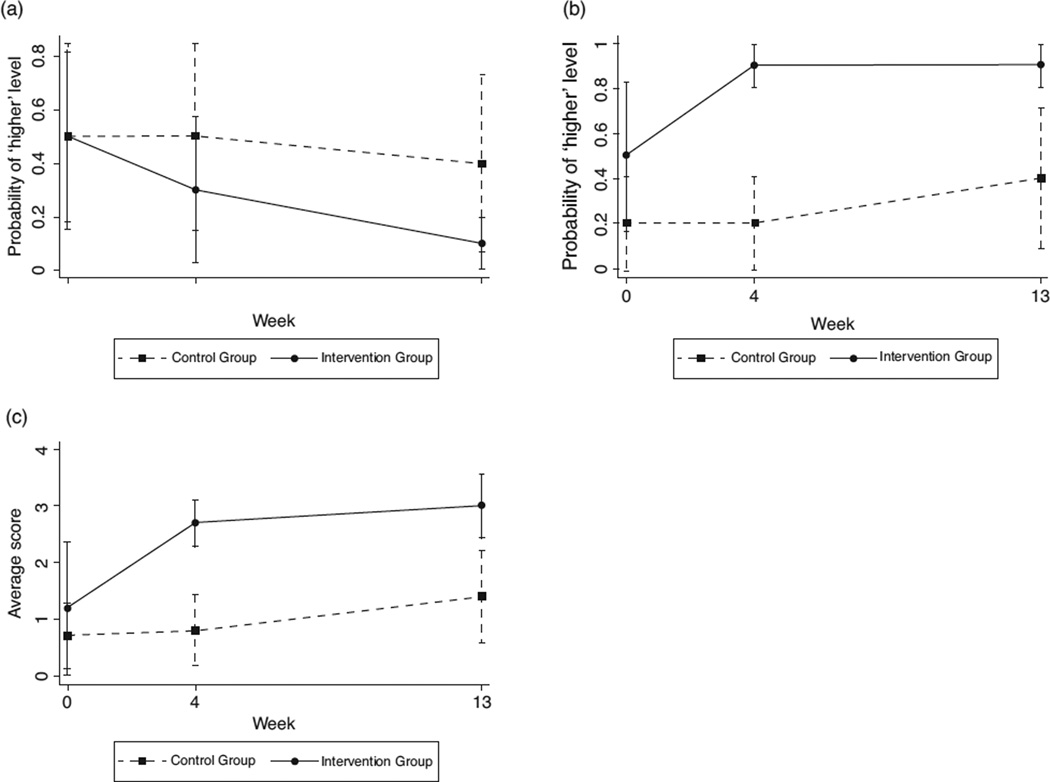
Fig. 2. Adjusted outcomes over time by intervention groups.
(a) Disclosure distress. (b) Disclosure self-efficacy. (c) Disclosure Behaviours Continuum.
Disclosure distress
At baseline, 50% of the participants in both study arms reported ‘higher’ levels of disclosure distress. Over time, however, although participants in the TAU arm generally maintained this level of distress, those in the intervention arm indicated a sharp decrease, to the point at follow-up at which only 10% reporter ‘higher’ levels of distress (, P < 0.10). Longitudinally, the improvement was steeper for intervention than TAU participants from baseline to follow-up [odds ratio (OR) 0.17; 95% confidence interval (95% CI) 0.03–0.91].
Disclosure self-efficacy
At baseline, 20% of TAU participants and 50% of intervention arm participants reported ‘higher’ levels of disclosure self-efficacy. Whereas TAU participants maintained this level throughout the intervention, increasing to 40% at follow-up, ‘higher’ disclosure self-efficacy among the intervention arm participants spiked after the intervention to 90% (, P < 0.01) and remained at this level at follow-up (, P < 0.01). Longitudinally, the improvement was steeper for intervention than TAU participants from baseline to postintervention (OR 9.00; 95% CI 2.06–39.29).
Disclosure continuum
Reports of disclosure behaviours were low at baseline (M/SE = 0.70/0.30 for TAU and 1.2/0.60 for intervention) and progressed respectively during the intervention (to M/SE = 1.40/0.41 and 3.00/0.29 at follow-up). However, the intervention arm versus TAU participants reported significantly greater movement along the continuum at both the postintervention (, P < 0.001) and follow-up cross-sectional analyses, (, P = 0.001) with steeper longitudinal improvement from baseline to postintervention (β = 1.40; 95% CI 0.31–2.50).
Interpreting the means in terms of specific behaviuors (see Fig. 1), we can see that the TAU participants began the study with no disclosure (score of 0 on the continuum) to giving general information about HIV (1) and, on average, did not disclose further during the course of the study. Indeed, data from the follow-up indicated that only one participant got beyond 2 (disclose having health issues), he was at 4 (indirect HIV disclosure; data not shown). On the contrary, the intervention participants began the study at about the same point in terms of disclosure but progressed as the study went on towards a 3, on average disclosing that they have a disease (but not referring to HIV). At follow-up, the intervention participants’ scores ranged from 2 to 5 (saying directly that you have HIV but no specifics), although no one reported full disclosure and open communication about HIV (6).
Discussion
For parents with HIV, decisions around disclosing their diagnosis to their children can be extremely stressful. Only one published study has described an intervention to assist these parents, and that was reported in the U.S. In this study, we described an intervention to assist Chinese parents living with HIV to consider options around disclosure of their diagnosis to their children.
Results from 20 Chinese parents indicated that participation in the intervention was associated overall with less distress around disclosure, great self-efficacy to handle the disclosure process and greater movement along a continuum of disclosure behaviours. The immediate postintervention gains (4 weeks form baseline) appeared to be largely maintained at 13-week follow-up. Although this was a pilot RCT not powered for efficacy, the results of the cross-sectional comparisons as well as the differences in the slope of improvement over time were not only all in the direction of intervention success but most also reached the level of statistical significance. Indeed, the effect sizes from all but one of the cross-sectional comparisons would be considered ‘large’ [39,40].
Despite the limitations of this trial (i.e. small sample size, reliance on self-reported outcomes and short time frame), findings underscore the promise and potential efficacy of the intervention. Our task-shifting strategy, relying on a specially trained nurse interventionist instead of a mental health professional, may be particularly useful in resource-constrained settings. In China, there is a dearth of mental health professionals and nurses are not typically trained to provide mental health counselling for patients. They typically report that they feel unprepared to deal with acute emotional distress in patients and lack training in evidence-based counselling skills with which to intervene. We found nurses at the research site to be interested in the additional training and willing to assume the role of interventionist. Nurses in China, with additional training and supervision, are well positioned to address the psychological needs of their patients.
In our small sample, none of the parents, even after the intervention, reported full disclosure; indeed, their average scores positioned them on the lower end of the continuum, below even making any indirect disclosure about HIV. This nondisclosure level contrasts sharply with what Murphy et al. [17] noted in their U.S. trial, in which 33% of the intervention arm parents disclosed. The minimal disclosure is particularly striking given that the children in our sample, on average, were 18 years of age. Our intervention was designed keeping in mind the acute social stigma of HIV in China and the strong cultural proscriptions against open communication of highly personal information, even among family members. Given this cultural context, the intervention focus was on decreasing the distress around disclosure by improving self-efficacy for a planned, perhaps much later disclosure. Parental HIV disclosure involves intensely personal decisions aimed to balance delicate family relations; thus, any culturally sensitive intervention should aim more to diminish distress than achieve a specific behavioural outcome such as full and open communication around the diagnosis.
Interestingly, none of the participants ever brought a partner with them to the sessions, as they were invited to do. We do not know whether this was due to the need to share childcare responsibilities, making it difficult for both parents to visit the clinic together, or whether they preferred to talk individually with the nurse counsellor. In at least a few cases, we know it was because the participant had not disclosed to the partner. Also, we did not routinely collect data on what was shared with the target child’s other parent about involvement in the study. If more parents had opted to make a full disclosure to their child, we imagine they would want to include the other parent. Future intervention work should consider the possible advantages of involving current partners/other parents more systematically.
A larger trial is needed to fully evaluate the intervention. Should results continue to support the intervention, future work on the best methods for dissemination will be warranted. These might include self-help or peer-facilitated models as well as computer-based programmes, assuring the widest possible access for parents in distress.
Acknowledgments
All authors contributed to the conception and design, drafting and revision of the manuscript, and the approval of the final version. Analysis and interpretation of data were done by J.M.S., J.P.Y., C.C.S. and W.T.C. We gratefully acknowledge our participants, our Community Advisory Board, Red Ribbon Society, Beautiful Life, research consultants Drs. Jessica Haberer, Xiaoming Li, Debra Murphy, Claude Mellins and Deepa Rao, research assistants at University of Washington and the clinic staff at Shanghai Public Health Clinical Center for their support and help with this project. Research reported in this publication was supported by NICHD and NIMH of the NIH under award numbers [R21 HD074141-01], [1F31MH099925-01], [K24MH093243] and [1K23NR 014107]. Additional support was provided by the University of Washington Department of Psychology and University of Washington Center for AIDS Research (CFAR), an NIH-funded programme [P30AI27757].
Footnotes
Conflicts of interest
None.
References
- 1.Clifford G, Craig GM, McCourt C, Barrow G. What are the benefits and barriers of communicating parental HIV status to seronegative children and the implications for Jamaica? A narrative review of the literature in low/middle income countries. West Indian Med J. 2013;62:357–363. doi: 10.7727/wimj.2013.087. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom Delaney R, Serovich JM, Lim J-Y. Reasons for and against maternal HIV disclosure to children and perceived child reaction. AIDS Care. 2008;20:876–880. doi: 10.1080/09540120701767158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawk ST. Disclosures of maternal HIV infection to seronegative children: a literature review. J Soc Pers Relat. 2007;24:657–673. [Google Scholar]
- 4.Lesch A, Swartz L, Kagee A, Moodley K, Kafaar Z, Myer L, Cotton M. Paediatric HIV/AIDS disclosure: towards a developmental and process-oriented approach. AIDS Care. 2007;19:811–816. doi: 10.1080/09540120601129301. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Zhang L, Li X, Kaljee L. Do Chinese parents with HIV tell their children the truth? A qualitative preliminary study of parental HIV disclosure in China. Child Care Health Dev. 2013;39:816–824. doi: 10.1111/j.1365-2214.2012.01394.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee MB, Rotheram-Borus MJ. Parents’ disclosure of HIV to their children. AIDS. 2002;16:2201–2207. doi: 10.1097/00002030-200211080-00013. [DOI] [PubMed] [Google Scholar]
- 7.Rotheram-Borus MJ, Draimin BH, Reid HM, Murphy DA. The impact of illness disclosure and custody plans on adolescents whose parents live with AIDS. AIDS. 1997;11:1159–1164. doi: 10.1097/00002030-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Qiao S, Li X, Stanton B. Disclosure of parental HIV infection to children: a systematic review of global literature. AIDS Behav. 2013;17:369–389. doi: 10.1007/s10461-011-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simoni JM, Davis ML, Drossman JA, Weinberg BA. Mothers with HIV/AIDS and their children: disclosure and guardianship issues. Women Health. 2000;31:39–54. doi: 10.1300/J013v31n01_03. [DOI] [PubMed] [Google Scholar]
- 10.Wiener L, Mellins CA, Marhefka S, Battles HB. Disclosure of an HIV diagnosis to children: history, current research, and future directions. J Dev Behav Pediatr. 2007;28:155–166. doi: 10.1097/01.DBP.0000267570.87564.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy DP, Cowgill BO, Bogart LM, Corona R, Ryan GW, Murphy DA, et al. Parents’ disclosure of their HIV infection to their children in the context of the family. AIDS Behav. 2010;14:1095–1105. doi: 10.1007/s10461-010-9715-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krauss BJ, Letteney S, De Baets AJ, Baggaley R, Okero FA. Caregiver’s HIV disclosure to children 12 years and under: a review and analysis of the evidence. AIDS Care. 2012;25:415–429. doi: 10.1080/09540121.2012.712664. [DOI] [PubMed] [Google Scholar]
- 13.Mellins CA, Brackis-Cott E, Dolezal C, Richards A, Nicholas SW, Abrams EJ. Patterns of HIV status disclosure to perinatally HIV-infected children and subsequent mental health outcomes. Clin Child Psychol Psychiatry. 2002;7:101–114. [Google Scholar]
- 14.Murphy DA, Steers WN, DelloStritto ME. Maternal disclosure of mothers’ HIV serostatus to their young children. J Fam Psychol. 2001;15:441–450. doi: 10.1037//0893-3200.15.3.441. [DOI] [PubMed] [Google Scholar]
- 15.Murphy DA. HIV-positive mothers’ disclosure of their serostatus to their young children: a review. Clin Child Psychol Psychiatry. 2008;13:105–122. doi: 10.1177/1359104507087464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tompkins TL. Disclosure of maternal HIV status to children: to tell or not to tell … that is the question. J Child Fam Stud. 2007;16:773–788. [Google Scholar]
- 17.Murphy DA, Armistead L, Marelich WD, Payne DL, Herbeck DM. Pilot trial of a disclosure intervention for HIV+ mothers: the TRACK program. J Consult Clin Psych. 2011;79:203–214. doi: 10.1037/a0022896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy DA, Marelich WD, Hoffman D. A longitudinal study of the impact on young children of maternal HIV serostatus disclosure. Clin Child Psychol Psychiatry. 2002;7:55–70. [Google Scholar]
- 19.Zhang L, Li X, Zhao J, Zhao G, Kaljee L, Stanton B. Disclosure of parental HIV infection to children and psychosocial impact on children in China: a qualitative study. Asia Pac J Couns Psychother. 2013;4:163–174. doi: 10.1080/21507686.2013.826261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MakhloufObermeyer C, Baijal P, Pegurri E. Facilitating HIV disclosure across diverse settings: a review. Am J Public Health. 2011;101:1011–1023. doi: 10.2105/AJPH.2010.300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Huang L, Wang H, Fennie KP, He G, Williams AB. Stigma mediates the relationship between self-efficacy, medication adherence, and quality of life among people living with HIV/AIDS in China. AIDS Patient Care ST. 2011;25:665–671. doi: 10.1089/apc.2011.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W-T, Starks H, Shiu C-S, Fredriksen-Goldsen K, Simoni J, Zhang F, et al. Chinese HIV-positive patients and their healthcare providers: contrasting Confucian versus Western notions of secrecy and support. ANS Adv Nurs Sci. 2007;30:329–342. doi: 10.1097/01.ANS.0000300182.48854.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JP, Xie T, Shiu C-S, Chen W-T, Simoni JM. Parental HIV disclosure to children in China: considering culture and context. AIDS Behav [Google Scholar]
- 24.Fredriksen-Goldsen KI, Shiu CS, Starks H, Chen WT, Simoni JM, Kim HJ, et al. You must take the medications for you and for me’: family caregivers promoting HIV medication adherence in China. AIDS Patient Care STDS. 2011;25:1–7. doi: 10.1089/apc.2010.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen WT, Shiu CS, Simoni JM, Zhao H, Bao MJ, Lu HZ. In sickness and in health: a qualitative study of how Chinese women with HIV navigate stigma and negotiate disclosure within their marriages/partnerships. AIDS Care. 2011;23(Suppl 1):120–125. doi: 10.1080/09540121.2011.554521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W-T, Shiu C-S, Simoni J, Fredriksen-Goldsen K, Zhang F, Zhao H. Optimizing HIV care by expanding the nursing role: patient and provider perspectives. J Adv Nurs. 2010;66:260–268. doi: 10.1111/j.1365-2648.2009.05165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JP, Leu JX, Simoni JM, Chen WT, Shiu CS, Zhao HX. Please don’t make me ask for help: implicit social support in Chinese individuals living with HIV. AIDS Behav. 2015 doi: 10.1007/s10461-015-1041-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Li X, Stanton B, Fang X, Lin D, Naar-King S. HIV-related knowledge, stigma, and willingness to disclose: a mediation analysis. AIDS Care. 2006;18:717–724. doi: 10.1080/09540120500303403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao S, Li X, Stanton B. Practice and perception of parental HIV disclosure to children in Beijing, China. Qual Health Res. 2014;24:1276–1286. doi: 10.1177/1049732314544967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshioka MR, Schustack A. Disclosure of HIV status: cultural issues of Asian patients. AIDS Patient Care STDS. 2001;15:77–82. doi: 10.1089/108729101300003672. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Sun S, Wu Z, Wu S, Lin C, Yan Z. Disclosure of HIV status is a family matter: field notes from China. J Fam Psychol. 2007;21:307–314. doi: 10.1037/0893-3200.21.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Lin C, Ji G, Sun S, Rotheram-Borus MJ, Ji G. Parents living with HIV in China: family functioning and quality of life. J Child Fam Stud. 2009;18:93–101. doi: 10.1007/s10826-008-9210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu T, Wu Z, Rou K, Duan S, Wang H. Quality of life of children living in HIV/AIDS-affected families in rural areas in Yunnan, China. AIDS Care. 2010;22:390–396. doi: 10.1080/09540120903196883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Ji G, Liang L-J, Ding Y, Tian J, Xiao Y. A multilevel intervention for HIV-affected families in China: together for empowerment activities (TEA) Soc Sci Med. 2011;73:1214–1221. doi: 10.1016/j.socscimed.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Task shifting: global recommendations and guidelines. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 36.Chaudoir SR, Fisher JD, Simoni JM. Understanding HIV disclosure: a review and application of the Disclosure Processes Model. Soc Sci Med. 2011;72:1618–1629. doi: 10.1016/j.socscimed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JP, Simoni JM, Chen W-T, Shiu C-S. Parental HIV disclosure to children in China: considering culture and context. 34th Annual Meeting & Scientific Sessions of the Society of Behavioral Medicine; 2013; San Francisco, CA. [Google Scholar]
- 38.DeCoster J, Iselin AMR, Gallucci M. A conceptual and empirical examination of justifications for dichotomization. Psychol Methods. 2009;14:349. doi: 10.1037/a0016956. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simulat Comput. 2010;39:860–864. [Google Scholar]
- 40.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiat. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]