Abstract
Background
Although psychoactive substances vary in many ways, they have important commonalties, particularly in their ability to lead to an addiction syndrome. The field lacks an updated review of the commonalities and differences in the phenomenology of alcohol, cannabis, tobacco, stimulants, opioids, hallucinogens, sedatives/tranquilizers and inhalants and their related substance use disorders (SUD).
Methods
DSM-IV and DSM-5 SUD diagnostic criteria were reviewed, as was evidence from recent epidemiological and clinical research: psychometric studies (test-retest reliability, latent trait analysis); physiological indicators (tolerance, withdrawal); prevalence and age of onset. Information was incorporated from previous reviews, PubMed and Scopus literature searches, and data from large U.S. national surveys.
Results
Empirical evidence in the form of test-retest reliability and unidimensionality supports use of the same DSM-IV dependence or DSM-5 SUD diagnostic criteria across substances. For most substances, the criteria sets were generally most informative in general population samples at moderate-to-severe levels of SUD. Across substances, two criteria (tolerance and use in hazardous situations) were identified as functioning differently in population subgroups. Since substances have different pharmacological effects, withdrawal is assessed using substance-specific symptoms, while tolerance is not; issues remain with the assessment of tolerance. Alcohol, tobacco, and cannabis were consistently identified as the substances with earliest onset of use, highest prevalence of lifetime use, and highest prevalence of lifetime disorder.
Conclusions
Despite differences between psychoactive substances, the generic DSM criteria set appear equally applicable across substances. Additional studies of tolerance and hazardous use will be useful for future nosologies. Alcohol, cannabis, and tobacco are the substances with the greatest public health impact due to the high prevalence and early onset of their use, and the potential all three substances have to lead to addiction.
Key words or phrases: substance use disorders, phenomenology, item response theory, reliability, DSM
Substance use disorders (SUD) are diagnosed by cognitive, behavioral, and physiological symptoms that accompany repeated substance use despite the occurrence of clinically significant problems. The substances include alcohol, cannabis, tobacco, stimulants, opioids, hallucinogens, sedatives/tranquilizers and inhalants. While these substances vary in many ways (e.g., legality, physiological effects, typical ages of onset), they have important commonalties, particularly in their ability to lead to an addiction syndrome. Since an updated review of the commonalities and differences in the phenomenology of these substances is lacking, we review evidence from recent epidemiologic and clinical studies, covering four areas. (1) Diagnostic criteria for SUD; (2) Psychometric evidence: test-retest reliability and latent trait analysis of SUD; (3) Physiological indicators, i.e., tolerance and withdrawal; and (4) Prevalence and age of onset of substance use and SUD.
1. Diagnostic criteria for SUD
The nosology of SUD was initially based on the “alcohol dependence syndrome” (ADS), combining psychological, biological, and sociological processes whereby drinking develops increasingly greater value relative to other behaviors, becoming unresponsive to consequences of use (Edwards and Gross, 1976), a concept generalized to drugs by the World Health Organization (Edwards et al., 1981). The ADS was seen as one axis of a “bi-axial” set of problems, with a secondary axis consisting of adverse consequences (Edwards et al., 1981, Edwards, 1986). The DSM-III-R and DSM-IV diagnosis of substance dependence was similar to the ADS. DSM-IV provided seven dependence criteria dependence, of which three needed to be met within a 12-month period (American Psychiatric Association, 2000). DSM-III-R and DSM-IV attempted to make the secondary axis (consequences, or abuse) orthogonal to dependence, by only diagnosing abuse in the absence of dependence. DSM-IV provided four abuse criteria, with one required for diagnosis (American Psychiatric Association, 2000). These criteria were largely generic across substances, although cannabis, inhalants and hallucinogens did not have a withdrawal criterion, and nicotine lacked an “abuse” category.
A large body of research into the relationship between dependence and abuse criteria showed that across substances, DSM-IV dependence criteria and three of the four DSM-IV abuse criteria indicated one unidimensional condition (Hasin et al., 2013b). Therefore, in DSM-5, the distinction between dependence and abuse was removed, replaced with one combined “substance use disorder” consisting of eleven criteria to diagnose all SUD (Table 1), of which 2 or more criteria within a 12-month period were required (American Psychiatric Association, 2013). A craving criterion (strong desire for the substance) was added, since it fit well with the dependence and abuse criteria across substances (alcohol (Borges et al., 2011; Casey et al., 2012; Castaldelli-Maia et al., 2015; Cherpitel et al., 2010; Hasin et al., 2012; Keyes et al., 2011a; Mewton et al., 2011a; Mewton et al., 2011b; Preuss et al., 2014); stimulants (Gilder et al., 2014; Hasin et al., 2012); tobacco (Chung et al., 2012; Shmulewitz et al., 2011; Strong et al., 2009; Strong et al., 2012); cannabis, heroin (Hasin et al., 2012); inhalants (Ridenour et al., 2014)), had potential clinical utility, and to enhance consistency with ICD-10 (Hasin et al., 2013b). A withdrawal criterion was added for cannabis given considerable evidence for its existence (Hasin et al., 2013b), and tobacco disorder criteria were aligned with the other substances (American Psychiatric Association, 2013).
Table 1.
DSM-5 substance use disordera criteria (American Psychiatric Association, 2013)
| DSM-5 Criterion | DSM-5 Definition | DSM-5 Substance specific notes | Criterion in DSM-IVb? |
|---|---|---|---|
| Impaired control | |||
| Larger/longer | use substance in larger amounts or over longer period of time than was intended | Dependence | |
| Quit/control | persistent desire to regulate or cut down use, or multiple unsuccessful efforts to quit or decrease use | Dependence | |
| Time spent | lots of time spent obtaining, using or recovering from effects of the substance | Tobacco: often assessed as “chain smoking”; spending lots of time obtaining or recovering is rare | Dependence |
| Craving | an intense desire or urge for the substance; assessed by asking if had such strong desires for the substances that could not think of anything else | Not included | |
| Social impairment | |||
| Neglect roles | recurrent use resulting in failure to fulfill role obligations at home, work, or school | Abuse | |
| Social/personal | continue to use despite social or interpersonal problems caused or exacerbated by effects of the substance; examples include violent arguments, child abuse, physical fights | Abuse | |
| Activities given up | Important social, occupational, or recreational activities given up or reduced because of use | Dependence | |
| Risky use | |||
| Hazardous use | Recurrent use in situations where it is physically hazardous, such as driving, operating machinery | Tobacco: smoking in bed, around flammable chemicals. This may also apply to other substances that are smoked, such as cannabis, opioids, and stimulants. | Abuse |
| Physical/psychological | Continue use despite knowing that use causes or exacerbates a persistent or recurrent physical or psychological problem | Specific disorders for different substances, but underlying similarity is the failure to abstain despite substance use causing difficultly | Dependence |
| Physiological/Pharmacological criteriac | |||
| Tolerance | Requiring a markedly higher dose to achieve desired effect or markedly lower effect with usual dose | Tobacco: often indicated by no longer feeling nauseous or dizzy with use, or more intense effect the first time used during the dayd | Dependence |
| Withdrawal | Substance specific withdrawal symptoms or use of substance or similar substance to avoid or relieve withdrawal symptoms. | Substance specific withdrawal symptoms (Table 5); not included for hallucinogens and inhalants | Dependence |
A DSM-5 SUD is diagnosed by the presence of 2 or more criteria within 12-months, with severity indicated by number of criteria endorsed: 2–3 (mild), 4–5 (moderate), 6 or more (severe).
DSM-IV dependence was diagnosed with the presence of 3 or more criteria occurring within 12-months; abuse was diagnosed with the presence of 1 or more criteria in the absence of dependence, and included a fourth abuse criterion (“continued to use despite legal problems”) that was not included in DSM-5.
physiological/pharmacological criteria that develop during the course of appropriate medical treatment do not count towards a SUD diagnosis. These can count towards a SUD diagnosis if the substances are used inappropriately (other than how they are prescribed). Medical usage may be appropriate for the following substances: cannabis (nausea due to chemotherapy, weight loss in AIDs patients); amphetamine or related stimulants (attention-deficit/hyperactivity, narcolepsy, obesity); opioids (analgesics); hallucinogens (emerging use for major depression (Abdallah et al., 2015)); and sedatives/tranquilizers (sleep disorders, anti-anxiety). Medical usage not indicated for alcohol, tobacco, cocaine, or inhalants.
Some studies use alternate definitions, such as those based on amount of cigarettes smoked (DiFranza et al., 2010).
2. Psychometric evidence
Test-retest reliability
To produce useful scientific results, a measure’s output must be reproducible across independent administrations. Two reviews summarized earlier information on reliability: one for all substance disorders (Hasin et al., 2006) and the other for nicotine dependence (DiFranza et al., 2010). To update results, we searched PubMed and Scopus for (DSM-IV or DSM-5) AND “substance use disorder” AND (reliability or reproducibility), from 2005 or later. English publications through December 2014 were considered and relevant papers were identified from titles and abstracts.
Across substances, DSM-IV dependence showed good to excellent reliability (Pierucci-Lagha et al., 2005, DiFranza et al., 2010, Malison et al., 2011, Hasin et al., 2006), except for hallucinogens and inhalants, which had moderate reliability (Ridenour et al., 2007, Hasin et al., 2006) (Table 2). Similarly, across substances, a combined DSM-IV dependence or abuse category showed good to excellent reliability (Hasin et al., 2006, Pierucci-Lagha et al., 2005, Cottler et al., 2009, Ridenour et al., 2007), except sedatives/tranquilizers (moderate reliability) (Pierucci-Lagha et al., 2005). Dimensional measures of DSM-IV dependence showed good to excellent reliability across substances (Grant et al., 1995, Hasin et al., 1997, Ridenour et al., 2007). DSM-5 SUD reliability was generally good for binary diagnoses (Grant et al., 2015) across substances, while dimensional measures showed good to excellent reliability (Grant et al., 2015). (We comment elsewhere (Hasin et al., 2013a) on methodological reasons that DSM-5 AUD appeared less reliable in one study (Regier et al., 2013)). Additional studies are needed on the reliability of DSM-5 SUD diagnoses in general population and clinical samples.
Table 2.
Test-retest reliability (kappa) for DSM-IV and DSM-5 substance use disorder (SUD) diagnoses.
DSM-IV dependence diagnosed by presence of 3 or more dependence criteria occurring together within a twelve month period (American Psychiatric Association, 2000)
DSM-IV abuse diagnosed by presence of 1 or more abuse criteria in the absence of dependence; N/A for tobacco (American Psychiatric Association, 2000)
DSM-5 substance use disorder diagnosed by 2 or more criteria occurring together within a twelve month period (American Psychiatric Association, 2013)
Latent trait analysis
Latent trait analysis can investigate the relationships between and functioning of indicators (e.g., diagnostic criteria) of a construct that cannot be observed directly (e.g., addiction). For example, factor analysis can determine how many latent factors (or dimensions) best explain underlying correlations between a set of items (diagnostic criteria). For item/criterion sets that form a unidimensional latent trait (one factor), Item Response Theory (IRT) analysis provides further information about the relationship of each criterion to the latent trait, in terms of two parameters, severity and discrimination (Shmulewitz et al., 2011). Item severity is inversely related to prevalence; higher severity is indicated by lower prevalence (only those with a severe trait/disorder will manifest a rare criterion), while higher prevalence indicates low severity. Discrimination indicates how well the item discriminates between individuals with high or low disorder severity. Total discrimination for all criteria in a set across the severity continuum can show where in that continuum the greatest amount of information is found. Additionally, IRT analysis is used to examine differential item functioning (DIF). DIF indicates if criteria show differential endorsement probabilities by demographic or other characteristics, conditional on underlying trait severity. IRT analysis was the principal method used by the DSM-5 SUD workgroup to examine the relationship of abuse to dependence criteria (Hasin et al., 2013b); this literature now numbers over 50 publications.
IRT studies
PubMed and Scopus were searched for English publications available through December 2014 with the terms “item response theory” AND (DSM-IV or DSM-5) AND the substances (alcohol, cannabis or marijuana, cocaine or stimulants, tobacco or nicotine, opioids or heroin, sedatives or tranquilizers, inhalants, hallucinogens). Based on titles or abstracts, we excluded papers not reporting on DSM-IV or DSM-5 SUD criteria sets or those that used IRT for severity scores (not to investigate psychometric evidence). We also excluded a study with incomplete assessment of abuse criteria (Kuerbis et al., 2013a). “Criteria sets” refer to the 11 criteria for DSM-IV abuse or dependence, or for DSM-5 substance use disorder, with 10 criteria for hallucinogens and inhalants (withdrawal is excluded) (Table 1). Heroin and prescription opioids were considered together because they have the same DSM-5 withdrawal syndrome. As Table 3 shows, many studies covered alcohol or cannabis, with fewer studies of less prevalent substances.
Table 3.
Item Response Theory (IRT) studies on DSM-IV/DSM-5 Substance Use Disorder Criteria (adapted from (Hasin et al., 2013b))
| Authors, year | Sample/survey typea | Country | Sample size | Diagnostic instrumentb | Peak informationc | Unidimensional ity shown? |
|---|---|---|---|---|---|---|
| ALCOHOL | ||||||
| Langenbucher et al. 2004 | Adult, clinical | USA | 372 | CIDI–SAM | Moderate | Yes |
| Martin et al. 2006 | Adolescent, clinical | USA | 464 | SCID | Moderate | Yes |
| Saha et al. 2006 | Adult, general population (NESARC) | USA | 20846 | AUDADIS-IV | Moderate-to-severe | Yes |
| Saha et al. 2007 | Adult, general population (NESARC) | USA | 20846 | AUDADIS-IV | Moderate-to-severe | Yes |
| Gelhorn et al. 2008 | Adolescent, mixed | USA | 5587 | CIDI-SAM | Moderate-to-severe | Yes |
| Harford et al. 2009 | Adolescent, general population (NSDUH) | USA | 133231 | Survey-specific instrument | Moderate-to-severe | Yes |
| Wu et al. 2009b | Adult, clinical | USA | 462 | DSM-IV checklist | Moderate | Yes (dependence) |
| Borges et al. 2010 | Adult, ER | Argentina, Mexico, Poland, USA | 3191 | Adapted CIDI | Moderate-to-severe | Yes |
| Cherpitel et al. 2010 | Adult, ER | Arg, Mex, Pol, USA | 5195 | CIDI | Moderate-to-severe | Yes |
| Shmulewitz et al. 2010 | Adult, general population | Israel | 1160 | AUDADIS-IV | Moderate-to-severe | Yes |
| Beseler et al. 2010 | Adolescent, general population | USA | 353 | 11-item self report measure (based on DSM criteria) | N/A | Yes |
| Keyes et al. 2011a | Adult, general population (NLAES) | USA | 18352 | AUDADIS-IV | Moderate-to-severe | Yes |
| Borges et al. 2011 | Adult, ER | Arg, Mex, Pol, USA | 3191 | CIDI | N/A | Yes |
| McCutcheon et al. 2011 | Adult, mixed (COGA) | USA | 8605 | SSAGA | Moderate-to-severe | Yes |
| Mewton et al. 2011a | Adult, general population (NSMHWB) | Australia | 7746 | CIDI Version 2.0 (modified) | Moderate-to-severe | Yes |
| Mewton et al. 2011b | Adolescent, general population (NSMHWB) | Australia | 853 | CIDI Version 2.0 (modified) | Moderate-to-severe | Yes |
| Hagman and Cohn 2011 | Adolescent, general population | USA | 396 | Survey-specific instrument | Moderate-to-severe | Yes |
| Gilder et al. 2011 | Adult, general population (American Indians) | USA | 530 | SSAGA | Moderate | Yes |
| Hasin et al. 2012 | Adult, clinical | USA | 543 | PRISM | Moderate | Yes |
| Rose et al. 2012 | Adolescent, general population (NSDUH) | USA | 9356 | Survey-specific instrument | Moderate | Yes |
| Ehlke et al. 2012 | Adolescent, general population (NSDUH) | USA | 4,605 | Survey-specific instrument | Moderate-to-severe | Yes |
| Casey et al. 2012 | Adult, general population (NESARC) | USA | 22177 | AUDADIS-IV | Moderate-to-severe | Yes |
| Edwards et al. 2013 | Adult, general population | USA | 7454 | SCID | N/A | Yes |
| Kuerbis et al. 2013 | Adult, general population (NSDUH) | USA | 3412 | Survey-specific instrument | Moderate-to-severe | Yes |
| Derringer et al. 2013 | Adult, mixed | USA | 6597 | SSAGA, CIDI-SAM | N/A | Yes (dependence) |
| Hagman and Cohn 2013 | Adult, general population (NSDUH) | USA | Survey-specific instrument | N/A | Yes | |
| Wu et al. 2013 | Adult, clinical | USA | 476 | DSM-IV Checklist | N/A | Yes (dependence) |
| Preuss et al. 2014 | Adult, mixed | Australia, Brazil, Canada, Finland, Japan | 1424 | AUDADIS | N/A | Yes |
| Castaldelli-Maia et al. 2015 | Adult, general (SPMHS) | Brazil | 936 | CIDI | N/A | Yes |
| CANNABIS | ||||||
| Langenbucher et al. 2004 | Adult, clinical | USA | 262 | CIDI-SAM | Moderate | Yes |
| Martin et al. 2006 | Adolescent, clinical | USA | 417 | SCID | Moderate | Yes |
| Gillespie et al. 2007 | Adult, general population | USA | 2337 | SCID | N/A | Yes |
| Lynskey and Agrawal 2007 | Adult, general population (NESARC) | USA | 8933 | AUDADIS-IV | N/A | Yes |
| Hartman et al. 2008 | Adolescent, mixed | USA | 5587 | CIDI-SAM | Moderate-to-severe | Yes |
| Wu et al. 2009b | Adult, clinical | USA | 311 | DSM-IV checklist | Moderate | Yes (dependence) |
| Compton et al. 2009 | Adult, general population (NESARC) | USA | 1603 | AUDADIS-IV | Moderate-to-severe | Yes |
| Mewton et al. 2010 | Adult, general population | Australia | 722 | CIDI | N/A | Yes |
| Piontek et al. 2011 | Adolescent, general population (SHCDDP) | France | 3641 | M-CIDI | Moderate-to-severe | Yes |
| Hasin et al. 2012 | Adult, clinical | USA | 340 | PRISM | Moderate | Yes |
| Wu et al. 2012 | Adult, general population (NSDUH) | USA | 6917 | Survey-specific instrument | Moderate-to-severe | Yes |
| Gizer et al. 2013 | Adult, general population | USA | 1134 | SSAGA | N/A | Yes |
| Derringer et al. 2013 | Adult, mixed | USA | 6597 | SSAGA, CIDI-SAM | N/A | Yes (dependence) |
| Wu et al. 2013 | Adult, clinical | USA | 316 | DSM-IV Checklist | N/A | Yes (dependence) |
| STIMULANTS | ||||||
| Cocaine | ||||||
| Langenbucher et al. 2004 | Adult, clinical | USA | 225 | CIDI-SAM | Moderate | Yes |
| Lynskey and Agrawal 2007 | Adult, general population (NESARC) | USA | 2672 | AUDADIS-IV | N/A | Yes |
| Gillespie et al. 2007 | Adult, general population | USA | 773 | SCID | N/A | Yes |
| Wu et al. 2009a | Adult, clinical | USA | 366 | DSM-IV checklist | Moderate | Yes (dependence) |
| Hasin et al. 2012 | Adult, clinical | USA | 483 | PRISM | Moderate | Yes |
| Saha et al. 2012 | Adult, general population (NESARC) | USA | 2528 | AUDADIS-IV | Moderate | Yes |
| Derringer et al. 2013 | Adult, mixed | USA | 6597 | SSAGA, CIDI-SAM | N/A | Yes (dependence) |
| Wu et al. 2013 | Adult, clinical | USA | 683 | DSM-IV Checklist | N/A | Yes (dependence) |
| Others (amphetamines, etc.) | ||||||
| Lynskey and Agrawal 2007 | Adult, general population (NESARC) | USA | 2025 | AUDADIS-IV | N/A | Yes |
| Gillespie et al. 2007 | Adult, general population | USA | 850 | SCID | N/A | Yes |
| Saha et al. 2012 | Adult, general population (NESARC) | USA | 1750 | AUDADIS-IV | Moderate | Yes |
| Wu et al. 2013 | Adult, clinical | USA | 166 | DSM-IV Checklist | N/A | Yes (dependence) |
| Cocaine or methamphetamine | ||||||
| Gilder et al. 2014 | Adult, general population (American Indians) | USA | 353 | SSAGA | Moderate | Yes |
| TOBACCO | ||||||
| Strong et al. 2009 | Adolescent, general population | USA | 296 | Survey-specific instrument | Moderate-to-severe | Yes (dependence) |
| Saha et al. 2010 | Adult, general population (NESARC) | USA | 7852 | AUDADIS-IV | Moderate | Yes (dependence) |
| Rose and Dierker 2010 | Adolescent, general population (NSDUH) | USA | 2758 | Survey-specific instrument | Moderate-to-severe | Yes (dependence) |
| McBride et al. 2010 | Adult, general population (NESARC) | USA | 6185 | AUDADIS-IV | Moderate | Yes (dependence) |
| Shmulewitz et al. 2011 | Adult, general population | Israel | 727 | AUDADIS-IV | Moderate | Yes |
| Strong et al. 2012 | Adolescent, general population | USA | 556 | Survey-specific instrument | Moderate | Yes (dependence) |
| Chung et al. 2012 | Adolescent, clinical | USA | 471 | SCID | N/A | Yes |
| OPIOIDS | ||||||
| Lynskey and Agrawal 2007 | Adult, general population (NESARC) | USA | 2060 | AUDADIS-IV | N/A | Yes |
| Gillespie et al. 2007 | Adult, general population | USA | 294 | SCID | N/A | Yes |
| Wu et al. 2009c | Adolescent, general population (NSDUH) | USA | 1290 | Survey-specific instrument | N/A | Yes |
| Wu et al. 2009a | Adult, clinical | USA | 354 | DSM-IV checklist | Moderate | Yes (dependence) |
| Wu et al. 2011 | Adult, general population (NSDUH) | USA | 2824 | Survey-specific instrument | N/A | Yes |
| Hasin et al. 2012 | Adult, clinical | USA | 364 | PRISM | Moderate | Yes |
| Saha et al. 2012 | Adult, general population (NESARC) | USA | 1815 | AUDADIS-IV | N/A | Yes |
| Wu et al. 2013 | Adult, clinical | USA | 449 | DSM-IV Checklist | N/A | Yes (dependence) |
| HALLUCINOGENS | ||||||
| Lynskey and Agrawal 2007 | Adult, general population (NESARC) | USA | 2525 | AUDADIS-IV | N/A | Yes |
| Gillespie et al. 2007 | Adult, general population | USA | 643 | SCID | N/A | Yes |
| Wu et al. 2010 | Adolescent, general population (NSDUH) | USA | 1548 | Survey-specific instrument | Moderate-to-severe | Yes |
| Kerridge et al. 2011 | Adult, general population (NESARC) | USA | 2176 | AUDADIS-IV | Moderate-to-severe | Yes |
| SEDATIVES/TRANQUILIZERS | ||||||
| Lynskey and Agrawal 2007 | Adult, general population (NESARC) | USA | 1896 (sed); 1487 (tran) | AUDADIS-IV | N/A | Yes |
| Gillespie et al. 2007 | Adult, general population | USA | 542 | SCID | N/A | Yes |
| Saha et al. 2012 | Adult, general population (NESARC) | USA | 1609 (sed); 1301 (tran) | AUDADIS-IV | Moderate-to-severe | Yes |
| INHALANTS | ||||||
| Lynskey and Agrawal 2007 | Adult, general population (NESARC) | USA | 728 | AUDADIS-IV | N/A | Yes |
| Perron et al. 2010 | Adolescent, clinical | USA | 279 | DIS-IV | N/A | Yes |
| Kerridge et al. 2011 | Adult, general population (NESARC) | USA | 664 | AUDADIS-IV | Moderate-to-severe | Yes |
| Ridenour et al. 2014 | Adolescent, young adult | USA | 162 | CIDI-SAM | N/A | Yes |
abbreviations used for sample/survey types: NESARC: National Epidemiological Survey on Alcohol and Related Conditions (US); NLAES: National Longitudinal Alcohol Epidemiologic Survey (US); NSDUH: National Survey on Drug Use and Health (US); NSMHWB: National Survey of Mental Health and Well-Being (Australia); SPMHS: São Paulo Megacity Health Survey (Brazil); ER: Emergency Room; COGA: Collaborative Study on the Genetics of Alcoholism (US); SHCDDP: Survey on Health and Consumption during the Day of Defense Preparation (France)
abbreviations used for diagnostic instrument: CIDI-SAM: Composite International Diagnostic Interview - Substance Abuse Module; SCID: Structured Clinical Interview for the DSM; AUDADIS-IV: Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV; SSAGA: Semi-Structured Assessment for the Genetics of Alcoholism; PRISM: Psychiatric Research Interview for Substance and Mental Disorders
area of greatest information: moderate, around the mean of the continuum (0); moderate-to-severe, around 1.5 standard deviations above the mean or higher. N/A indicates that no total information curves were shown or total (aggregate) information was not reported on explicitly in the paper.
Unidimensionality across substances
The key empirical evidence necessary to consider combining abuse and dependence criteria in DSM-5 was that for all substances, these items exhibit unidimensionality, i.e., they indicate one underlying latent trait (disorder severity). As reviewed previously (Hasin et al., 2013b), evidence across all substances robustly showed that the 11 criteria (7 DSM-IV dependence criteria, 3 DSM-IV abuse criteria, and craving) indicated one underlying latent construct, with dependence and abuse criteria interspersed across the severity continuum. This supported replacing the two DSM-IV disorders (dependence, abuse) with a single combined disorder in DSM-5. More recent IRT studies continue to consistently indicate unidimensionality for alcohol (Castaldelli-Maia et al., 2015; Preuss et al., 2014, Hagman and Cohn, 2013, Kuerbis et al., 2013b, Ehlke et al., 2012, Rose et al., 2012, Edwards et al., 2013, Wu et al., 2013, Derringer et al., 2013), cannabis (Wu et al., 2013, Gizer et al., 2013, Derringer et al., 2013), cocaine and stimulants (Wu et al., 2013, Derringer et al., 2013, Gilder et al., 2014), opioids (Wu et al., 2013), and inhalants (Ridenour et al., 2014). The unidimensionality evidence also supports dimensional SUD severity scales across all substances; such scales are important in both research and clinical work as they provide information beyond a binary diagnosis (Grant et al., 2015, Hasin et al., 2015).
Defining the region of greatest information
IRT analysis estimates “total information”, which quantifies how well the criteria set as a whole discriminates between individuals with high or low disorder severity. The total or aggregate information curve plotted across the severity continuum shows the precision for the criteria set at each value along the severity continuum (Shmulewitz et al., 2011). A ‘flat’ curve shows equivalent information across all severities, while a ‘peaked’ curve indicates more information about the disorder at the severity level where the ‘peak’ occurs. In all studies with information about these curves, they were peaked, indicating greatest information at a specific severity, with two areas of greatest information: either “moderate” (information ‘peak’ from the mean [0] to about 1.5 SDs above the mean) or “moderate-severe” (information ‘peak’ 1.5 to 2.5 SDs above the mean).
Table 3 shows that regions of greatest information varied little by substance (alcohol, cannabis, opioids, hallucinogens, sedatives/tranquilizers, and inhalants). Instead, differences were found mainly by sample type. General (or mixed) samples tended to provide the most information in the moderate-severe end of the severity continuum, and clinical samples tended to provide the most information at more moderate values. While this could appear counter-intuitive, in clinical samples, the prevalence of all criteria is higher than in the general population, seeming to indicate less severity in these high-severity contexts (analogous to educational testing results only from advanced-placement students who all “get the hard ones right”, in contrast to results from entire schools). An exception to this pattern was stimulants. Clinical studies of cocaine showed greatest information at moderate severity (Hasin et al., 2012, Wu et al., 2009a, Langenbucher et al., 2004), while two general studies also showed moderate severity for stimulants (Gilder et al., 2014, Saha et al., 2012); more studies on stimulants are needed.
Differential Item Functioning (DIF)
DIF occurs when an item’s (criterion’s) parameter estimate differs across population subgroups after accounting for subgroup differences in disorder severity. For example, males generally have higher average AUD severity than females and are more likely to endorse AUD criteria; however, at the same levels of AUD severity, males and females should have the same likelihood of endorsing a criterion, unless it functions differently by sex. Although evidence suggests that across substances, DIF in specific criteria would not lead to differential diagnosis of SUD in population subgroups (Hasin et al., 2013b, Derringer et al., 2013), criteria with DIF across substances suggests that these criteria may not work well in specific subgroups. This information can indicate a poorly functioning criterion for sensitivity analyses and can identify criteria requiring adjustment in future nomenclatures.
The most studied DIF was by age, sex, or race/ethnicity, in the severity parameter. We included studies that reported on DIF results for each criterion (Table 4). DIF was examined most frequently for alcohol, with fewer studies for cannabis, stimulants, tobacco, and opioids, one study for hallucinogens, and no studies for sedatives/tranquilizers or inhalants.
Table 4.
Differential item functioning (DIF) for DSM-IV/DSM-5 Substance Use Disorder Criteria
| Authors, year | Study # | Sex | Age | Race/ethnicity | Criteria tested |
|---|---|---|---|---|---|
| ALCOHOL | |||||
| Martin et al. 2006 | 1 | D6. Activities given up D7. Physical/Psychological A2. Hazardous Use |
Not tested | Not tested | D1-D7 A1-A3 |
| Saha et al. 2006 | 2 | D2. Withdrawal D3. Larger/longer A1. Neglect roles A2. Hazardous use A3. Social/personal |
D1. Tolerance D2. Withdrawal D3. Larger/longer D4. Quit/control D5. Time spent D6. Activities given up A1. Neglect roles |
D1. Tolerance D3. Larger/longer D4. Quit/control A2. Hazardous use |
D1-D7 A1-A3 |
| Harford et al. 2009 | 3 | D7. Physical/Psychological A2. Hazardous use |
(by sex) D1. Tolerance D2. Withdrawal D3. Larger/longer D4. Quit/control D5. Time spent D6. Activities given up D7. Physical/Psychological A1. Neglect roles A2. Hazardous use |
(by sex) D1. Tolerance D2. Withdrawal D4. Quit/control D6. Activities given up D7. Physical/Psychological A1. Neglect roles A2. Hazardous use |
D1-D7 A1-A3 |
| Wu et al. 2009b | 4 | None | None | None | D1-D7 |
| Borges et al. 2010 | 5 | Not tested | Not tested | D2. Withdrawal D3. Larger/longer D4. Quit/control D5. Time spent A1. Neglect roles A3. Social/personal |
D1-D7 A1-A3 |
| Cherpitel et al. 2010 | 6 | Not tested | Not tested | D2. Withdrawal D4. Quit/control A1. Neglect roles |
D1-D7 A1-A3 Craving |
| Shmulewitz et al. 2010 | 7 | A3. Social/personal | None | D1. Tolerance D5. Time spent D7. Physical/Psychological A3. Social/personal |
D1-D7 A1-A3 |
| Keyes et al. 2011a | 8 | None | None | Craving | Craving |
| Mewton et al. 2011a | 9 | A2. Hazardous use Craving |
D1. Tolerance D4. Quit/control A2. Hazardous use |
Not tested | D1-D7 A1-A3 Craving |
| Mewton et al. 2011b | 10 | (by age) None |
D1. Tolerance D4. Quit/control A2. Hazardous use |
Not tested | D1-D7 A1-A3 Craving |
| Gilder et al. 2011 | 11 | D6. Activities given up A2. Hazardous use |
D1. Tolerance A2. Hazardous use |
Not tested | D1-D7 A1-A3 |
| Hasin et al. 2012 | 12 | None | None | None | D1-D7 A1-A3 Craving |
| Ehlke et al. 2012 | 13 | D1. Tolerance A1. Neglect roles A2. Hazardous use |
Not tested | Not tested | D1-D7 A1-A3 |
| Casey et al. 2012 | 14 | A2. Hazardous use | D4. Quit/control | D1. Tolerance D4. Quit/control A2. Hazardous use |
D1-D7 A1-A3 Craving |
| Kuerbis et al. 2013 | 15 | D3. Larger/longer A1. Neglect roles |
D1. Tolerance D4. Quit/control D5. Time spent D7. Physical/Psychological A2. Hazardous use A3. Social/personal |
Not tested | D1-D7 A1-A3 |
| Castaldelli-Maia et al. 2015 | 16 | D2. Withdrawal | D2. Withdrawal D4. Quit/control D7. Physical/Psychological craving |
Not tested | D1-D7 A1-A3 Craving |
| CANNABIS | |||||
| Martin et al. 2006 | 1 | D7. Physical/Psychological A2. Hazardous use |
Not tested | Not tested | D1, D3-D7 A1-A3 |
| Wu et al. 2009b | 4 | None | None | None | D1-D7 |
| Mewton et al. 2010 | 17 | Not tested | None | Not tested | D1-D7 A1-A3 |
| Piontek et al. 2011 | 18 | D1. Tolerance D2. Withdrawal |
Not tested | Not tested | D1-D7 A1-A3 |
| Hasin et al. 2012 | 12 | None | D1. Tolerance D5. Time spent D6. Activities given up D7. Physical/Psychological A1. Neglect roles A2. Hazardous use A3. Social/personal craving |
None | D1, D3-D7 A1-A3 Craving |
| Wu et al. 2012 | 19 | D3. Larger/longer D7. Physical/Psychological |
Not tested | D1. Tolerance D5. Time spent |
D1, D3-D7 A1-A3 |
| Gizer et al. 2013 | 20 | Not tested | Not tested | D2. Withdrawal D7. Physical/Psychological A1. Neglect roles A2. Hazardous use A3. Social/personal |
D1-D7 A1-A3 |
| STIMULANTS | |||||
| Cocaine | |||||
| Wu et al. 2009a | 21 | None | None | D1. Tolerance D6. Activities given up |
D1-D7 |
| Hasin et al. 2012 | 12 | None | None | D1. Tolerance D2. Withdrawal D3. Larger/longer D4. Quit/control D5. Time spent D6. Activities given up D7. Physical/Psychological A1. Neglect roles A2. Hazardous use A3. Social/personal |
D1-D7 A1-A3 Craving |
| Cocaine or methamphetamine | |||||
| Gilder et al. 2014 | 22 | D2. Withdrawal A2. Hazardous use |
D1. Tolerance | Not tested | D1-D7 A1-A3 Craving |
| TOBACCO | |||||
| McBride et al. 2010 | 23 | Not tested | D2. Withdrawal D4. Quit/control D5. Time spent D6. Activities given up D7. Physical/Psychological |
Not tested | D1-D7 |
| Shmulewitz et al. 2011 | 24 | None | A2. Hazardous use | Craving | A1-A3 Craving |
| Strong et al. 2012 | 25 | Not tested | D1. Tolerance D2. Withdrawal D4. Quit/control |
Not tested | D1-D7 Craving |
| Chung et al., 2012 | 26 | Not tested | D1. Tolerance D3. Larger/longer D4. Quit/control A3. Social/personal |
Not tested | D1-D7 A1-A3 Craving |
| OPIOIDS | |||||
| Wu et al. 2009c | 27 | D2. Withdrawal | None | D5. Time spent D7. Physical/Psychological |
D1-D7 A1-A3 |
| Wu et al. 2009a | 21 | None | None | None | D1-D7 |
| Hasin et al. 2012 | 12 | None | None | None | D1-D7 A1-A3 Craving |
| HALLUCINOGENS | |||||
| Wu et al. 2010 | 28 | A2. Hazardous use | A1. Neglect roles | D1. Tolerance D5. Time spent A2. Hazardous use |
D1, D3-D7 A1-A3 |
Notes: Differential item functioning indicates that the criteria show significantly different severity estimates (have different likelihood of endorsement) in the demographic subgroups, at the same level on disorder severity. Criteria are numbered as following: DSM-IV dependence, D1: tolerance; D2: withdrawal; D3: larger/longer; D4: quit/control; D5: time spent; D6: activities given up; D7: physical/psychological; DSM-IV Abuse: A1: neglect roles; A2: hazardous use; A3: social/personal; Craving. The following studies tested DIF for each criterion but only to determine if the criterion DIF led to differential functioning of the entire criteria set, and did not report DIF results for each criterion: alcohol (Saha et al., 2007, Derringer et al., 2013); cannabis (Wu et al., 2009b, Compton et al., 2009, Derringer et al., 2013); stimulants (amphetamines (Saha et al., 2012), (cocaine (Saha et al., 2012, Derringer et al., 2013)); tobacco (Saha et al., 2010); opioids (Saha et al., 2012); hallucinogens (Kerridge et al., 2011); sedatives/tranquilizers (Saha et al., 2012); inhalants (Kerridge et al., 2011).
DIF by substance (Table 4)
In a majority of studies, alcohol, cannabis, stimulants, tobacco and hallucinogens showed DIF by age, sex or race/ethnicity for the criteria assessing tolerance and use in hazardous situations, and similar results were seen for cannabis in studies using related methods (Agrawal and Lynskey, 2007; Delforterie et al., 2015a). Alcohol and tobacco also showed DIF by age or race/ethnicity for difficulty quitting or controlling use. Alcohol, cannabis, stimulants, tobacco and opioids showed DIF inconsistently for the criteria assessing withdrawal, time spent, and continued use despite physical/psychological problems.
A few consistent DIF patterns emerged. Tolerance was more likely to be endorsed by younger participants (Table 4, studies 2, 3, 9–11, 15, 22, 25, 26) perhaps because they begin by using less, then increase use to get the desired effect (Chung et al., 2004, Hartman et al., 2008). Hazardous use was more likely to be endorsed by males (studies 1–3, 9, 11, 13, 14, 22, 28; and (Agrawal and Lynskey, 2007; Delforterie et al., 2015a)) and younger participants (studies 3, 9, 10, 15, 24). Hazardous use may be assessing a distinct dimension of SUD liability (Hasin et al., 2012), or a general tendency to disinhibition or antisocial behaviors more prevalent in younger and male participants (Mewton et al., 2011b, Mewton et al., 2010, Martin et al., 2008), warranting further investigation. Quit/control was less likely to be endorsed by younger participants (studies 2, 3, 9, 10, 14, 15, 25, 26), perhaps reflecting that younger individuals may not try to control use. Overall, alcohol is the only substance with a substantial amount of data on criterion level DIF. Little DIF testing was done for craving (all substances), tobacco abuse, or cannabis withdrawal, new in DSM-5, and warranting further study.
Differences for tobacco
Only seven tobacco IRT studies were available (Table 3). In DSM-IV, tobacco dependence could be diagnosed, but not “abuse”. The limited evidence available showed that the abuse criteria were valid and reliable measures of a tobacco use disorder and that the DSM-5 criteria set provided more information and higher prevalence of a use disorder than DSM-IV dependence (Shmulewitz et al., 2013, Shmulewitz et al., 2011, Chung et al., 2012). These findings led to alignment of the DSM-5 tobacco criteria with those for other substances.
Yet, differences between tobacco and other substances remain. First, DSM-5 suggests different operationalization of some criteria (time spent, hazardous use, tolerance (DiFranza et al., 2010); Table 1). Second, studies were inconsistent on the region of the severity continuum covered by the criteria: some general population studies showed greatest information in the moderate range (Strong et al., 2012, Shmulewitz et al., 2011, McBride et al., 2010, Saha et al., 2010), with others in the moderate-severe range (Rose and Dierker, 2010, Strong et al., 2009), with a wider severity range than other substances (Saha et al., 2010, Strong et al., 2012, Strong et al., 2009). No clinical studies reported total information for tobacco. Third, alternate indices of dependence, i.e., Fagerstrom Test for Nicotine Dependence (FTND; (Heatherton et al., 1991)) and related measures, are widely used (Baker et al., 2012; Hughes, 2006). These assess physical dependence (craving, withdrawal, compulsive use) rather than behavioral, social, or health-related consequences of use; thus, FTND and DSM are considered to indicate related but different domains of dependence (Agrawal et al., 2011; Baker et al., 2012). Recent studies suggest that DSM and FTND criteria together provide more information about tobacco use disorders than either measure alone (Agrawal et al., 2011; Strong et al., 2009; Strong et al., 2012). Studies of DSM-5 tobacco use disorder criteria and diagnoses, and their relationship to the FTND, in general population and clinical samples are warranted.
3. Physiological/pharmacological criteria
Sources of information
Definitions of tolerance and withdrawal were taken largely from expert review papers (Gilpin and Koob, 2008, Koob, 2006, Koob and Volkow, 2010, Koob, 2014). Symptoms of tolerance and withdrawal were derived primarily from DSM-5 (American Psychiatric Association, 2013). Additional information was found by searching PubMed and Scopus English publications from human studies, published or available online through December 2014, for “(DSM-IV or DSM-5) AND tolerance”, and selecting relevant publications based on the titles and abstracts.
Tolerance across substances
For all substances, tolerance occurs when the substance no longer affects an individual as strongly as before. When this happens, the individual needs higher doses of the substance to get the same effect (Gilpin and Koob, 2008, Koob, 2006). This is due to cellular and molecular adaptations to counteract or lessen the substance’s effect, since the body is trying to maintain ‘normal’ functioning in the presence of the substance (Koob and Volkow, 2010). These adaptations reduce sensitivity to further substance use. While tolerance can develop for all substances, the precise mechanisms for tolerance differ across substances, based on how the specific substance acts in the body, primarily the central nervous system (American Psychiatric Association, 2013, Koob and Volkow, 2010, World Health Organization, 2004). For example, tolerance may develop through increased metabolism to clear the substance from the body more quickly (e.g., alcohol, tobacco), or reduced receptor reactivity to the substance (e.g., alcohol, sedatives, tobacco, opioids) (World Health Organization, 2004). Tolerance can develop rapidly for some substances (e.g., sedatives, cannabis, amphetamines), and slowly for others (e.g., inhalants) (World Health Organization, 2004).
Concerns about tolerance as a criterion
The DSM changed-based operationalization of tolerance (needing to use more, or less of an effect, than “before”) is considered problematic across substances (Martin et al., 2008). Since assessment is based on initial or “usual” use levels, tolerance can be endorsed with low levels of use, if small amounts were used (Martin et al., 2008) (e.g., increases from 2 to 3 drinks (Chung et al., 2004, Chung et al., 2001)). This may be the reason tolerance is more likely to be endorsed by younger participants. Conversely, tolerance may not be endorsed at high levels of use (or high disorder severity) if initially high levels were used (e.g., alcohol and cannabis (Martin et al., 2006)), and/or if tolerance developed too quickly (Martin et al., 2008). Additionally, prevalence of tolerance varied across substances (Lynskey and Agrawal, 2007, Hasin et al., 2012, Saha et al., 2012). Lastly, tolerance discriminates poorly between those with or without dependence (alcohol, cannabis (Chung et al., 2004, Chung et al., 2001)), or between those with greater or lesser severity (cannabis, cocaine, amphetamines, opioids (Schuckit et al., 1999)), and does not predict clinical course (alcohol (Hasin et al., 2000)). Therefore, further research investigating inclusion of tolerance in the SUD diagnostic set is warranted.
Withdrawal across substances
Substance use, especially heavy use, leads to tolerance. When regular use ceases or is greatly reduced, the blood or tissue concentration of the substance declines and a re-adjustment is necessary. Withdrawal symptoms indicate this process of readjustment. After the body re-adjusts to the absence of the substance, withdrawal symptoms diminish and eventually cease. These symptoms are adverse across substances, and their avoidance can be one reason for continued use. However, withdrawal symptoms are generally life-threatening only for alcohol, sedatives (e.g, benzodiazepines), and opioids (Koob and Volkow, 2010). In DSM-5, withdrawal is included for alcohol, cannabis, stimulants, tobacco, opioids and sedatives/tranquilizers. The withdrawal criterion is considered positive with endorsement of the required number of substance-specific symptoms, or if the substance or a related substance is used to relieve or avoid symptoms.
Withdrawal by substance
Table 5 presents DSM-5 withdrawal symptoms by substance. All substances show symptoms related to emotional distress, such as depressed or dysphoric mood, anxiety, or insomnia, possibly related to common effects of all substances on the brain systems involved with stress and reward (Koob and Volkow, 2010, Koob, 2014). Other symptoms are more specific to pharmacological effects, and thus differ across substances. Sedating substances (alcohol, sedatives/tranquilizers) have similar withdrawal symptoms: nausea, vomiting, hallucinations, psychomotor agitation, seizures, tremors, sweating, and fast pulse rate. Opioids share some of those symptoms (nausea, vomiting, sweating) but also show other symptoms (lacrimation, diarrhea, yawning, fever, muscle aches). Stimulating substances (stimulants, tobacco) share some withdrawal symptoms (increased appetite, restlessness/agitation), but show some different symptoms, such as fatigue for stimulants and irritability/frustration for tobacco. Similarly, the time period for symptoms to develop (after cessation or reduction of use) and then improve differs across substances (Table 5), since that is partially determined by the amount of time after use the substance is generally biologically available. For example, withdrawal from sedating substances tends to develop within several hours to a few days, and can improve within five days to a week, but some symptoms can last for months. In contrast, withdrawal from tobacco tends to begin within one day, and then improve over two to three weeks. Thus, withdrawal is assessed using substance specific symptoms such that overall, the DSM-5 withdrawal criterion should be a similar indicator across the six substances.
Table 5.
Withdrawal characteristics across substancesa. From (American Psychiatric Association, 2013, Hasin et al., 2006).
| Alcohol | Cannabis | Stimulantsb | Tobacco | Opioidsc | Sedatives/tranquilizers | |
|---|---|---|---|---|---|---|
| Withdrawal symptoms, beginning after cessation of or reduction in heavy or prolonged usec | ||||||
| Sleep problems | ✓ (insomnia) | ✓ (insomnia, disturbing dreams) | ✓ (insmonia, hypersomnia) | ✓ (insomnia) | ✓ (insomnia) | ✓ (insomnia) |
| vivid, unpleasant dreams | ✓ | |||||
| nausea, vomiting | ✓ | ✓ | ✓ | |||
| transient visual, tactile, auditory hallucinations | ✓ | ✓ | ||||
| psychomotor changes | ✓ (agitation) | ✓ (retardation, agitation) | ✓ (agitation) | |||
| anxiety | ✓ | ✓ (or nervousness) | ✓ | ✓ | ||
| seizures | ✓ (generalized tonic-clonic) | ✓ (grandmal) | ||||
| irritability, anger | ✓ (or aggression) | ✓ (or frustration) | ||||
| Changes in weight/appetite | ✓ (decreased appetite, weight loss) | ✓ (increased appetite) | ✓ (increased appetite) | |||
| restlessness | ✓ | ✓ | ||||
| lacrimation, rhinorrhea | ✓ | |||||
| diarrhea | ✓ | |||||
| yawning | ✓ | |||||
| Mood changes | ✓ (depressed) | ✓ (dysphoric) | ✓ (depressed) | ✓ (dysphoric) | ||
| fatigue | ✓ | |||||
| difficulty concentrating | ✓ | |||||
| physical symptoms | ||||||
| abdominal pain | ✓ Any counted as one symptom (when cause discomfort) | |||||
| shakiness/tremors | ✓ (hand tremor) | ✓ (hand tremor) | ||||
| sweating | ✓autonomic hyperactivity (sweating, fast pulse rate) | ✓ pupillary dilation, piloerection, sweating | ✓autonomic hyperactivity (sweating, fast pulse rate) | |||
| fever | ✓ | |||||
| chills | ||||||
| headaches | ||||||
| Muscle aches | ✓ | |||||
| Number of symptoms required | 2 | 3 | 3 (one must be dysphoric mood) | 4 | 3 | 2 |
| Time frame | ||||||
| Onset | Several hours to a few days | Within one week | Few hours to several days | 24 hours | Several minutes to several days (depends on drug type) | Several hours to a few days (depends on drug type) |
| Duration | 4–5 days; anxiety, insomnia, autonomic dysfunction can last 3–6 months | 1–2 weeks; sleep problems > 30 days | 1–2 weeks (Zorick et al., 2010) | 2–3 weeks | One week; chronic symptoms (dysphoria, insomnia) can last for months | Short acting substances, 4–5 days; long acting, a month, can last for several months |
Withdrawal is not included for hallucinogens or inhalants
includes cocaine, amphetamines and related substances, as in DSM-5
includes heroin, analgesics, others, as in DSM-5; withdrawal can also begin after administration of opioid agonist
Cannabis withdrawal
Withdrawal from cannabis was not included in DSM-IV because of lack of evidence, but was added in DSM-5, after supporting evidence became available (Hasin et al., 2013b). Studies showed that cannabis withdrawal is a reliable and valid diagnosis, with a time-limited course after cessation of cannabis use. It is prevalent in both general and clinical samples, and has clinical significance, since withdrawal was associated with difficulty quitting and worse treatment outcomes. Additionally, cannabis withdrawal fit the unidimensional model of cannabis use disorder criteria (Derringer et al., 2013, Gillespie et al., 2007, Gizer et al., 2013, Hartman et al., 2008, Langenbucher et al., 2004, Lynskey and Agrawal, 2007, Mewton et al., 2010, Piontek et al., 2011, Wu et al., 2009b, Wu et al., 2013).
Hallucinogen, inhalant withdrawal
DSM-5 does not include withdrawal for hallucinogens or inhalants due to lack of evidence for a clinically significant withdrawal syndrome (American Psychiatric Association, 2013). A few studies on hallucinogens (Gillespie et al., 2007, Cottler et al., 2009) and inhalants (Ridenour et al., 2007, Perron et al., 2011, Ridenour et al., 2014) suggested that withdrawal symptoms were endorsed, showed acceptable reliability and validity, and fit the underlying disorder latent construct. Yet, a distinct set of symptoms remains to be identified for hallucinogens (Cottler et al., 2009) and inhalants (Ridenour et al., 2014); additional research is needed to determine if identification of such a syndrome is possible (Hasin et al., 2013b).
Exception for medical use
Withdrawal and tolerance are often observed among individuals using substances for medical purposes, such as stimulants, opioids, hallucinogens, sedatives/tranquilizers, and, with the advent of medical marijuana laws, cannabis (Table 1). When substances are used appropriately for supervised medical treatment, these criteria do not count as indicators of a SUD (American Psychiatric Association, 2013). These criteria would count towards a diagnosis if the substance was used in ways other than as prescribed, or for non-therapeutic reasons (American Psychiatric Association, 2013). Although conceptually this distinction appears reasonable, empirical evidence that applying this rule improves the reliability or validity of the criteria or disorder diagnosis is lacking; further studies are warranted. In contrast, tolerance and withdrawal always count towards a diagnosis for non-medically approved substances (alcohol, tobacco, cocaine, inhalants).
4. Prevalence and Age of onset of substance use and SUD
Sources of information
Two sources of information were utilized: literature review and data from US national datasets. Searches were conducted in English publications of human studies, initially restricted to publications dated January 2002-December 2014 from Pubmed, Medline, ProQuest, and Scopus, using the following terms: (“age of/at onset/first use/initiation”) and (alcohol or tobacco or nicotine or stimulant or heroin or cocaine or non-medical or opioid or methamphetamine or inhalant or marijuana or cannabis or k2 or spice or sedative or tranquilizer) and (disorder or abuse or dependence). Earlier publications were included when relevant and needed to supplement scant information. Only US studies were included, since many differences (e.g., legality, availability, and acceptability of substance use) should be considered when comparing patterns across countries, which is beyond the scope of this review. Based on title, abstract, or text, studies were included if they were quantitative, their primary focus was substance use, and substance-specific drug use could be differentiated. While alcohol, cannabis, cocaine, and tobacco had a substantial number of studies reporting on age of onset of use/disorder, several substances (inhalants, other stimulants, hallucinogens, tranquilizers/sedatives) yielded few or no studies (Table 6).
Table 6.
Epidemiology of substance use and use disorders in the United States: prevalence of use, disorder, age at onset
| Authors, Year | Studya | Population | N | Lifetime Use (%) | Age of First Use | Prevalence of disorder, relationship of age to disorderb |
|---|---|---|---|---|---|---|
| ALCOHOL | ||||||
| Bracken et al. 2013 | Clinical sample | Adolescents | 939 | N/R | 13.2 (mean age) | N/R |
| Hingson et al. 2006 | National survey (NESARC) | Adults (Wave 1) | 43093 | N/R | 9% of users began ≤ 14; 17% at 15–16; 32% at 17–18; 15% at 19–20; 28%≥21 | Earlier drinking onset associated with increased dependence |
| Hingson and Zha, 2009 | National survey (NESARC) | Adults (Wave 2) | 39653 | N/R | 7% of users began ≤14; 17% at 15–16; 28% at 17–18; 15% at 19–20; 36%≥21 | Earlier drinking onset associated with increased AUDs |
| Kalaydjian et al. 2009 | National Survey (NCS-R) | Adults | 5692 | 91.7% | 16–17 (median) | Age 21, median age for any AUD |
| Lopez-Quintero et al. 2011 | National survey (NESARC) | Adults (ever used alcohol) | 28907 | 100% | 8% before 14 | Early onset (< 14) not associated with increased dependence; 17% of users met criteria for dependence |
| Moss et al. 2014 | National Survey (Add Health) | Adults (Wave 4) | 4245 | 100% | 15.1 (mean) | Earlier drinking onset associated with abuse and dependence |
| Ridenour et al. 2006 | Longitudinal study (CEDAR) | Adolescents | 590 | N/R | 14.6 (mean) 15.0 (median) |
Prevalence of dependence: 15% of users |
| Sartor et al. 2013 | Female twin study | Adolescents | 3787 | 79%–88% | 15.8 – 16.9 (mean) | AUD symptoms: 30%–39% |
| Trenz et al. 2012 | Community sample (NEURO-HIV Epidemiologic Study) | Adolescents, adults, current drug users | 651 | 71.1% (past 6 months) | 13.4–14.6 (mean) | Early onset of drinking predicted injection drug use |
| CANNABIS | ||||||
| Flory et al. 2004 | Community sample (Project DARE) | Adolescents, young adults | 481 | N/R | 18.5 (mean) | N/R |
| Haberstick et al. 2014 | National longitudinal survey (Add Health) | Young adults | 15,500 | N/R | N/R | Earlier onset of use associated with increased CUD |
| Hasin et al. 2008 | National survey (NESARC) | Adult frequent cannabis users | 2613 | 100% | 16.3 (mean) | N/R |
| Horey et al. 2012 | Clinical sample | Adults in clinical trials for cannabis, cocaine dependence | 242 | N/R | 15.1 (mean) | Mean age at first treatment = 29 |
| Khan et al. 2013 | National survey (NESARC) | Adults with a CUD | 3297 | 100% | 16.7 (mean) | 18.9–19.3 (mean) |
| Le Strat et al. 2014 | National survey (NESARC) | Adults, ever used cannabis | 8068 | 100% | 7% < 14; 83% < 21 | Early onset of use associated with increased CUD |
| Lopez-Quintero et al. 2011 | National survey (NESARC) | Adults, ever used cannabis | 7389 | 100% | 14% < 14 | Early onset (< 14) associated with decreased dependence; 7% of users met criteria for dependence |
| Moss et al. 2014 | National Survey (Add Health) | Adults (Wave 4) | 4245 | 100% | 16 (mean) | Earlier onset of cannabis use associated with increased abuse and dependence |
| Ridenour et al. 2006 | Longitudinal study (CEDAR) | Adolescents | 590 | N/R | 15.7 (mean); 15.5 (median) | Prevalence of dependence: 16% of users |
| Sartor et al. 2013 | Female twin study | Adolescents | 3787 | 44.4%–49.7% | 16 (mean) | CUD symptoms: 21.9%–27.9% |
| Trenz et al. 2012 | Community sample (NEURO-HIV Epidemiologic Study) | Adolescent, adults, current drug users | 651 | 52.4% (Past 6-month use) | 13.4–15.0 (mean) | Early onset of cannabis use did not predict recent injection drug use |
| COCAINE | ||||||
| Bracken et al. 2013 | Clinical Sample | Adolescents | 939 | N/R | 15.1 (mean) | N/R |
| Horey et al. 2012 | Clinical Sample | Outpatients enrolled in clinical trials for cannabis or cocaine dependence | 242 | N/R | 20.9 (mean) | 34.3 (mean age at first treatment) |
| Lopez-Quintero et al. 2011 | National survey (NESARC) | adults who used cocaine at least once | 2259 | 100% | 2% < 14 | Early use onset (< 14) associated with decreased dependence; 17% of users met criteria for dependence |
| Ridenour et al. 2006 | Longitudinal study (CEDAR) | Adolescents | 590 | N/R | 18.9 (mean); 18.5 (median) | Prevalence of dependence: 24% of users |
| Sartor et al. 2014 | Clinical sample | Adults | 9846 | N/R | 19.1–22.6 (mean) | 22.6–33 (mean) |
| INHALANTS | ||||||
| Perron et al. 2009 | National survey (NESARC) | adults who used inhalants at least once | 664 | 100% | 17.5 (mean); 16.2 (median) | Abuse: 17 (mean); Dependence: 16.8 (mean) |
| Wu et al. 2008 | National survey (NESARC) | Adults | 43,093 | 1.7% | 17.5 (mean) | 19% of users met criteria for IUD |
| OPIOIDS | ||||||
| Ridenour et al. 2006 | Longitudinal study (CEDAR) | Adolescents | 590 | N/R | 18.1 (mean) 18.0 (median) |
Prevalence of dependence: 21% of users |
| Sartor et al. 2014 | Clinical sample | Adults | 9846 | N/R | 18.5–23.8 (mean) | 22.5–31.1 (mean) |
| TOBACCO | ||||||
| Lopez-Quintero et al. 2011 | National survey (NESARC) | adults who used tobacco at least once | 15918 | 100% | 31% < 14 | Early onset (< 14) associated with increased dependence; 46% of users met criteria for dependence |
| Moss et al. 2014 | National Survey (Add Health) | Adults (Wave 4) | 4245 | 100% | 17 (mean) | Earlier smoking onset (≤14) predicted dependence |
| Ridenour et al. 2006 | Longitudinal study (CEDAR) | Adolescents | 590 | N/R | 14.5 (mean); 14.5 (median) | Prevalence of dependence: 8% of users |
| Trenz et al. 2012 | Community sample (NEURO-HIV Epidemiologic Study) | Adolescent, adults, current drug users | 651 | Past 6-month use: 89.2% | 13.7–15.0 (mean) | Early onset of smoking (<15 years) did not predict recent injection drug use |
N/R: Not Reported
abbreviations used to define the Study type: NESARC: National Epidemiological Survey on Alcohol and Related Conditions (USA); NCS-R: National Comorbidity Survey Replication (USA); Add Health: National Longitudinal Study of Adolescent to Adult Health (USA); CEDAR: Center for Education and Drug Abuse Research (USA); Project DARE: Drug Abuse Resistance Education (US)
abbreviation used for disorder type: AUD: any alcohol use disorder (abuse or dependence); CUD: any cannabis use disorder; IUD: any inhalant use disorder
To supplement the literature review, we included findings from several U.S. national datasets. For adolescents, prevalence of use of each substance was examined by age in three datasets from 2013: Monitoring The Future (MTF) (Johnston et al., 2014), the National Survey of Drug Use and Health (NSDUH) (Substance Abuse and Mental Health Services Administration, 2014), and the Youth Behavioral Risk Surveillance Survey (YRBS) (Brener et al., 2013). Both MTF and YRBS are nationally representative school-based surveys of adolescents. The NSDUH is a nationally representative household-based survey of the non-institutionalized U.S. population, ages 12 and above. We provide published estimates from MTF (Johnston et al., 2014) and YRBS (Kann et al., 2014); for NSDUH participants age 12–17, prevalence estimates were calculated from the public use dataset using SAS 9.4, adjusting for the complex survey design and sampling weights.
For adults, we examined the median age of onset of use of each substance in two datasets: NSDUH (participants age 18 and older) and Wave 1 of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a nationally representative survey of the civilian, non-institutionalized U.S. adult population in households and group living quarters, conducted 2001–2002 (Grant et al., 2004). Prevalence and median age of onset of use in the NSDUH were calculated from the public use dataset using SAS 9.4, adjusting for the complex sampling design and using sampling weights. We examined prevalence of use and of DSM-IV substance use disorders (abuse, dependence), and median age of onset of use and of SUD in the NESARC using SUDAAN 11.0.1, adjusting for the multi-stage sampling design and using sample weights. Medians are presented as they are less influenced by extreme outliers.
Adolescents: prevalence of use, age of first use
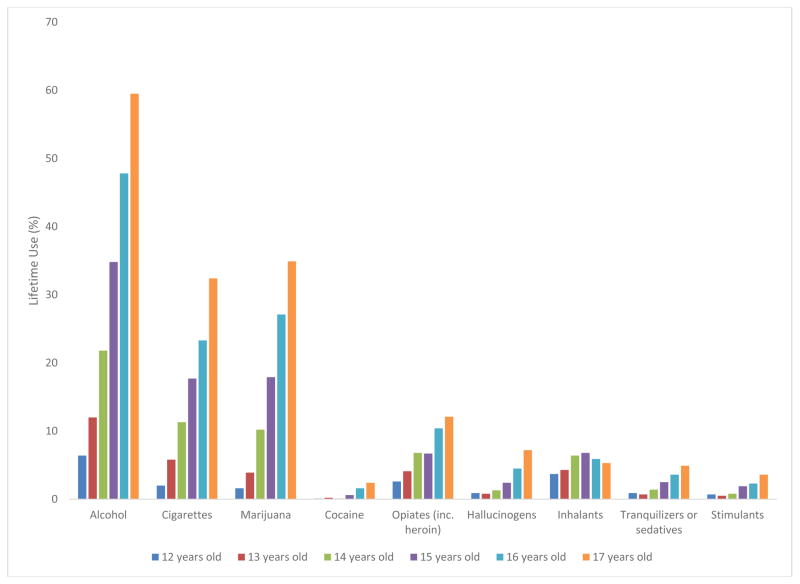
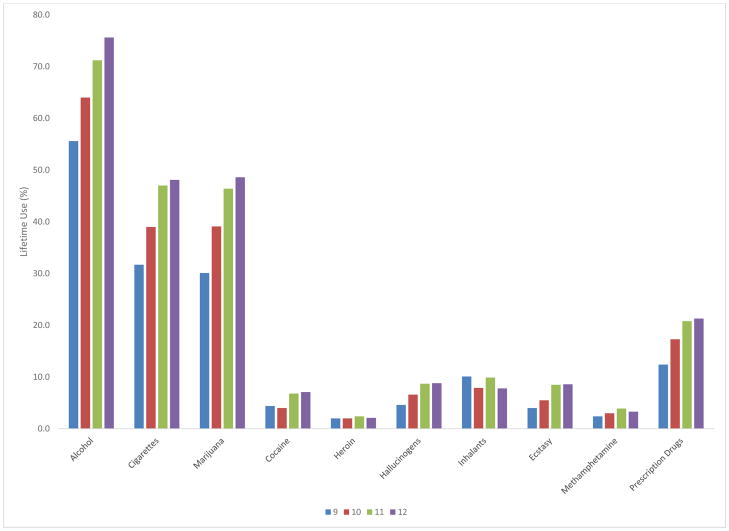
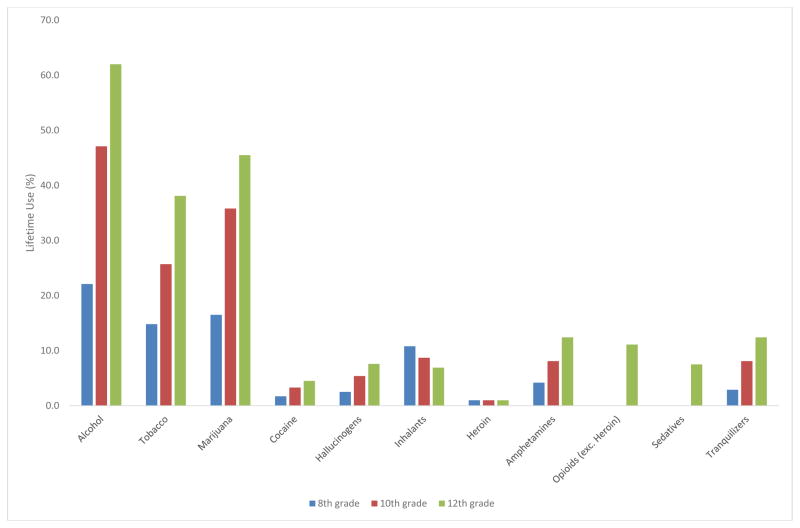
MTF, YRBS and NSDUH consistently showed that adolescents used alcohol, tobacco, and cannabis earliest and most commonly (Figures 1–3). Similarly, in other published studies of adolescents (Table 6), younger ages of onset were reported for alcohol, tobacco and cannabis (Bracken et al., 2013, Ridenour et al., 2006, Sartor et al., 2013). This consistency is probably related to environmental/ecological factors (availability, legal status, social norms), since the pharmacologic properties and subjective effects of these substances differ considerably. In MTF, YRBS, and NSDUH, inhalants showed early onset, but low prevalence. Cocaine, other stimulants, opioids, and tranquilizers/sedatives showed later onset of use and were less common among adolescents.
Figure 1.
Substance use among adolescents (12–17), NSDUH 2013 (N = 17,736)
Figure 3.
Substance use by grade, YRBS, 2013 (N = 13, 633)
Adults: prevalence of use, age of first use
In published studies (Table 6), alcohol, cannabis, and tobacco were the most prevalent substances used. Similarly, both NESARC (Table 7) and NSDUH (Table 8) showed that the prevalence of alcohol, tobacco, and cannabis use was highest, with other substances used less. Overall lifetime substance use was generally higher in NSDUH than NESARC, perhaps due to methodological differences between the studies (self-administered substance use questions in NSDUH, interviewer-administered questions in NESARC) (Grucza et al., 2007). In both the previously published studies (Table 6) and the additional analyses in NESARC (Table 7) and NSDUH (Table 8), among the more prevalent substances, tobacco showed the earliest median age of onset of use, followed by cannabis and alcohol, with onset generally occurring in early to mid-adolescence. Use of inhalants and hallucinogens began at younger ages, and cocaine, opioids, and sedatives/tranquilizers began at older ages. The only difference between the US national surveys in age of first use was for stimulants, reported as earlier in the NESARC than the NSDUH; the reasons for this are unclear. For a more complete understanding of the time course of substance use, further examination of the trajectories of use patterns over time both at a population level (e.g. secular trends) and at the individual level (e.g. longitudinal) is warranted. Further, alcohol and substance use function within larger cultural contexts (Degenhardt et al., 2008); expansion of international research on onset and prevalence of use is warranted.
Table 7.
Prevalence of lifetime substance use and DSM-IV disorders, age of onset of use and disorders, in Wave 1 of the NESARC (N=43,093)
| % Lifetime Abuseb | Among those with abuse | % Lifetime dependencec | Among those with dependence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | % Lifetime Use | Age of First Use | Whole set | Among users | Median age of first use | Median age of abuse onset | Whole set | Among users | Median age of first use | Median age of dependence onset | |
| Median | Range (1%–99%) | ||||||||||
| Alcohol | 82.7 | 17.8 | 8–44 | 17.8 | 21.5 | 17.1 | 19.6 | 12.5 | 15.1 | 16.2 | 20.4 |
| Cannabis | 20.6 | 16.6 | 9–37 | 7.2 | 34.8 | 15.9 | 17.5 | 1.3 | 6.3 | 15.0 | 17.6 |
| Cocaine | 6.2 | 19.8 | 13–42 | 1.8 | 29.8 | 19.3 | 20.2 | 1.0 | 16.0 | 19.7 | 21.9 |
| Stimulants | 4.7 | 17.4 | 11–36 | 1.4 | 30.1 | 17.1 | 18.1 | 0.6 | 13.0 | 16.6 | 18.7 |
| Tobaccoa | 46.9 | 15.3 | 6–31 | N/A | N/A | N/A | N/A | 17.7 | 37.8 | 14.7 | 26.5 |
| Opioids | 4.9 | 19.4 | 10–67 | 1.1 | 23.5 | 17.9 | 17.9 | 0.4 | 8.5 | 18.0 | 18.4 |
| Sedatives/tranquilizers | 5.4 | 19.4 | 8–67 | 1.1 | 20.3 | 17.3 | 17.4 | 0.3 | 6.3 | 17.8 | 17.9 |
| Inhalants | 1.7 | 16.2 | 7–36 | 0.3 | 17.2 | 15.3 | 15.7 | 0.04 | 2.3 | 13.7 | 14.8 |
| Hallucinogens | 5.8 | 17.3 | 12–33 | 1.5 | 25.0 | 16.6 | 17.4 | 0.2 | 4.2 | 15.9 | 17.5 |
Tobacco: cigarette use only, since other forms (cigars, chewing tobacco, snuff) where very rarely used
DSM-IV abuse diagnosed by presence of 1 or more abuse criteria in the absence of dependence; N/A for tobacco (American Psychiatric Association, 2000)
DSM-IV dependence diagnosed by presence of 3 or more dependence criteria occurring together within a twelve month period (American Psychiatric Association, 2000)
Table 8.
Prevalence of lifetime substance use and age of onset of use and disorders, among adult respondents (age 18 and above), in NSDUH 2013 (N=37,424)
| % Lifetime use | Age at First Use, Median | 1% – 99% of age at first use | |
|---|---|---|---|
| Alcohol | 86.8% | 16.3 | 6 – 32 |
| Cannabis | 46.9% | 16.5 | 9 – 41 |
| Cocaine | 15.8% | 19.7 | 12 – 44 |
| Stimulants | 8.3% | 18.4 | 12 – 45 |
| Tobacco (cigarettes) | 66.7% | 15.2 | 6 – 30 |
| Opioids | 2.1% | 19.9 | 8 – 54 |
| Sedatives/tranquilizers | 9.7% | 20.2 | 11 – 60 |
| Inhalants | 8.3% | 16.8 | 7 – 39 |
| Hallucinogens | 16.7% | 17.7 | 12 – 41 |
Adults: prevalence of disorder by substance, age of onset of SUD
Overall, the most commonly diagnosed DSM-IV SUDs (dependence or abuse) were for alcohol (30.3%), tobacco (in DSM-IV, dependence only; 17.7%), and cannabis (8.2%), consistent with the high prevalence of lifetime use of these substances (Table 7). However, among users, any SUD was most prevalent for cocaine (45.8%), stimulants (43.8%), and cannabis (41.1%), followed by tobacco (37.8%), alcohol (36.6%), opioids (32.0%), hallucinogens (29.2%), sedatives/tranquilizers (26.6%), and inhalants (19.5%). For most substances, the age of onset of SUDs was generally late adolescence through young adulthood, in both NESARC (Table 7) and other published studies (Kalaydjian et al., 2009, Khan et al., 2013, Perron et al., 2009), while nicotine dependence was later, about age 26 (Table 7). Across all substances, initiation of use was generally younger among those diagnosed with SUDs (Table 7), and earlier onset of use was generally associated with higher risk or earlier onset of SUD (Table 6) for alcohol (Hingson et al., 2006, Hingson et al., 2009, Moss et al., 2014), tobacco (Lopez-Quintero et al., 2011, Moss et al., 2014), and cannabis (Haberstick et al., 2014, Le Strat et al., 2014, Moss et al., 2014).
Discussion
Review of psychometric studies identified many important similarities across substances. Test-retest reliability of the diagnoses was similar across substances, suggesting that differences in SUD prevalence across substances are not artifacts of a nosology more applicable to one substance than another. IRT studies showed that for all substances, the criteria sets were reliable measures of the underlying condition (SUD severity), providing robust empirical support for generic SUD criteria. Conceptually, generic criteria were supported by similar features across substances, such as commonalities in the neurobiological mechanisms of addiction (Koob, 2006, Koob and Le Moal, 2001), which lead to common behaviors across substances. Questions about whether the same set of diagnostic criteria would be similarly reliable and applicable across substances (Budney, 2006, Hughes, 2006) are addressed by the information provided in this review. Further, in general population samples, the criteria sets provided information at levels of greater severity, a desirable characteristic when the purpose is to identify people with disease (greater severity). To use the criteria sets to identify individuals at risk for developing a SUD, identification of valid and reliable lower severity indicators may be useful. Consistent DIF across substances was identified for two criteria, tolerance and hazardous use, suggesting further investigation into the role of those criteria in the diagnostic criteria set.
Two related issues warrant further comment. First, the most appropriate methodology (IRT) was used consistently to investigate the relationship between dependence and abuse criteria for all substances. Use of a consistent statistical methodology ensured that if results varied, the variation would be due to differences between substances or samples rather than variation in methodology. Having accomplished this task, studies should now investigate the DSM-5 nosology using different methodologies (Hasin, 2015). For example, rest-retest reliability studies are needed. In addition, validity should be evaluated by determining the association of criteria or diagnoses with external validators, including those that are antecedent (e.g., family history), concurrent (which indicate current state) or prospective (which predict prognosis or course). Second, although most IRT studies (Table 3) were in U.S. samples, a number of studies were conducted in other countries, mainly for alcohol (Argentina, Mexico, and Poland (Borges et al., 2011; Borges et al., 2010; Cherpitel et al., 2010); Israel (Shmulewitz et al., 2010); Australia (Mewton et al., 2011a; Mewton et al., 2011b); Australia, Brazil, Canada, Finland, and Japan (Preuss et al., 2014); and Brazil (Castaldelli-Maia et al., 2015)), with two for cannabis (Australia (Mewton et al., 2010) and France (Piontek et al., 2011)) and one for tobacco (Israel (Shmulewitz et al., 2011)). Those studies indicated that the same relationship between dependence and abuse criteria was found in many other countries; unidimensionality results were not limited to U.S. samples. But, in the few studies that investigated criteria functioning between countries, a number of criteria that functioned differently in different countries were identified for alcohol (Borges et al., 2010; Cherpitel et al., 2010) and cannabis (Delforterie et al., 2015a). Further studies should confirm the unidimensionality for all substances across countries, and identify criteria that function differently between countries, to more fully investigate the cross-country applicability of the DSM-5 nosology across substances.
Concerning the physiological criteria, tolerance and withdrawal show many differences and some similarities across substances. First, tolerance can develop for all substances, but problems with operationalization suggest the need for further research into its utility as a SUD criterion. Second, withdrawal can occur for most substances, and a substance-specific set of withdrawal symptoms are necessary. However, further work is needed to determine if withdrawal occurs for hallucinogens and inhalants. Last, tolerance and withdrawal criteria are not considered to indicate a substance use disorder for substances that are used under appropriate medical care (e.g., opioids); research is needed to determine how applying this exception affects diagnostic reliability.
Concerning prevalence and age of onset, alcohol and tobacco use were most prevalent, reflecting their legal status and availability. Cannabis was the most widely used illicit substance. In national surveys, these three substances tended to be used earliest and showed highest prevalence of SUD. Cocaine, stimulants, tranquilizers/sedatives, and inhalants had consistently low prevalence. While age of onset varied somewhat for these substances, it is unclear if this is due to methodological differences in the surveys or differences in secular trends of the age of onset of substance use.
Although the substances have different pharmacological effects, the same SUD or addiction criteria appear to be generally applicable across substances, suggesting an underlying “addictive process” that is common across all substances. This common addictive process is most likely determined in part by genetic factors, as suggested by shared genetic liability across substances (Agrawal et al., 2012, Krueger et al., 2007), and in part by environmental factors, as suggested by shared risk factors for developing all types of SUDs, such as childhood maltreatment (Afifi et al., 2012, Dube et al., 2003, Keyes et al., 2012) and stressful life events (Sinha, 2001, Sinha, 2008). SUDs are a key feature of the “externalizing” axis of psychiatric disorders, as well as antisocial and conduct disorders (Krueger, 1999, Krueger et al., 2007), suggesting that the underlying liability to substance problems may be related to disinhibition, or the inability to refrain from unsafe behaviors, such as problematic or maladaptive substance use (Crowley, 2006).
However, despite the many commonalities across substances, substance-specific genetic and environmental liability factors (Tsuang et al., 1998, Kendler et al., 2003) indicate that individuals often prefer one substance to others. Understanding how the general liability for substance use or disorders is expressed for a particular substance is important for designing appropriate substance specific prevention or intervention programs. A proposed framework for “addiction specificity” (Sussman et al., 2011) lays out the complex relationships between both individual and group level factors that influence which substance an individual is most likely to use (and develop a SUD). An individual’s initial response to a substance may affect future use or disorder (de Wit and Phillips, 2012); e.g., unpleasant flushing response to alcohol decreases the likelihood of alcohol use disorders (Edenberg, 2007) or stimulating effects of alcohol increase risk (King et al., 2014). While an underlying personality “type” appears more susceptible to addiction in general (e.g., impulsivity, aggressiveness (Krueger et al., 2007)), limited evidence suggests that specific personality types were more likely to use specific substances (Milivojevic et al., 2012, Le Bon et al., 2004, Gerra et al., 2008). Abundant evidence indicates that individuals use substances that are used by others around them (parents, peer group). Group level factors may affect availability or ability to acquire specific substances; one study suggests that individuals using opiates would have used alcohol instead in less-permissive environments (Milivojevic et al., 2012). Sociodemographic variables affect choice of substance (Clark et al., 2012), as do social norms, which may also differ by demographics or cultural groups (Keyes et al., 2011b; Keyes et al., 2011c; Keyes et al., 2012; Shmulewitz et al., 2012). Additional research in this area is necessary to understand how these factors all work together to increase liability to general substance use or disorder (commonalities), and to affect risk for a specific substance use or disorder (differences).
In conclusion, through focusing on four areas of SUD phenomenology and epidemiology, we elucidated commonalities and differences across substances. Overall commonalities in the psychometric properties of the criteria sets and diagnoses justify the use of generic diagnostic criteria across substances, as long as specificity is allowed for withdrawal. Having consistent criteria for all SUDs is simpler for clinicians and researchers than different, substance-specific criteria. We identified key areas of research that could influence future nomenclatures, such as validation of the nosology across substances, whether tolerance and hazardous use should be removed or modified, and whether lower severity criteria should be added to aid in screening for substance use problems in the general population. Key differences in prevalence and age of onset of use and use disorders across substances are important for designing age appropriate prevention and intervention programs. Lastly, with a clearer picture of the underlying commonalities across substances, the focus can shift to the differences, and to identifying the reasons individuals choose to use a specific substance, in order to be able to provide the most targeted and effective treatment programs.
Figure 2.
Substance use by grade, MTF, 2013 (N = 41, 700)
Acknowledgments
This work was funded by grant U01AA018111 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (Shmulewitz, PI: Hasin); T32DA031099 from the National Institute on Drug Abuse (NIDA) (Greene, PI: Hasin); and the New York State Psychiatric Institute (Hasin). The authors have no financial or other relationship relevant to this work.
References
- Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–23. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifi TO, Henriksen CA, Asmundson GJ, Sareen J. Childhood maltreatment and substance use disorders among men and women in a nationally representative sample. Can J Psychiatry. 2012;57:677–86. doi: 10.1177/070674371205701105. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Does gender contribute to heterogeneity in criteria for cannabis abuse and dependence? Results from the national epidemiological survey on alcohol and related conditions. Drug Alcohol Depend. 2007;88:300–307. doi: 10.1016/j.drugalcdep.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Scherrer JF, Pergadia ML, Lynskey MT, Madden PA, Sartor CE, Grant JD, Duncan AE, Haber JR, Jacob T, Bucholz KK, Xian H. A latent class analysis of DSM-IV and Fagerstrom (FTND) criteria for nicotine dependence. Nicotine Tob Res. 2011;13:972–981. doi: 10.1093/ntr/ntr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Verweij KJ, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, Nelson EC, Slutske WS, Whitfield JB, Lynskey MT. The genetics of addiction-a translational perspective. Transl Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Author; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Baker TB, Breslau N, Covey L, Shiffman S. DSM criteria for tobacco use disorder and tobacco wthdrawal: a critique and proposed revisions for DSM-5. Addiction. 2012;107:263–275. doi: 10.1111/j.1360-0443.2011.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beseler CL, Taylor LA, Leeman RF. An item-response theory analysis of DSM-IV alcohol-use disorder criteria and “binge” drinking in undergraduates. J Stud Alcohol Drugs. 2010;71:418–23. doi: 10.15288/jsad.2010.71.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges G, Cherpitel CJ, Ye Y, Bond J, Cremonte M, Moskalewicz J, Swiatkiewicz G. Threshold and optimal cut-points for alcohol use disorders among patients in the emergency department. Alcohol Clin Exp Res. 2011;35:1270–6. doi: 10.1111/j.1530-0277.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges G, Ye Y, Bond J, Cherpitel CJ, Cremonte M, Moskalewicz J, Swiatkiewicz G, Rubio-Stipec M. The dimensionality of alcohol use disorders and alcohol consumption in a cross-national perspective. Addiction. 2010;105:240–54. doi: 10.1111/j.1360-0443.2009.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken BK, Rodolico J, Hill KP. Sex, age, and progression of drug use in adolescents admitted for substance use disorder treatment in the northeastern United States: comparison with a national survey. Subst Abus. 2013;34:263–72. doi: 10.1080/08897077.2013.770424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener ND, Kann L, Shanklin S, Kinchen S, Eaton DK, Hawkins J, Flint KH. Methodology of the Youth Risk Behavior Surveillance System--2013. MMWR Recomm Rep. 2013;62:1–20. [PubMed] [Google Scholar]
- Budney AJ. Are specific dependence criteria necessary for different substances: how can research on cannabis inform this issue? Addiction. 2006;101(Suppl 1):125–33. doi: 10.1111/j.1360-0443.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- Casey M, Adamson G, Shevlin M, McKinney A. The role of craving in AUDs: dimensionality and Differential Functioning in the DSM-5. Drug Alcohol Depend. 2012;125:75–80. doi: 10.1016/j.drugalcdep.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Castaldelli-Maia JM, Wang YP, Borges G, Silveira CM, Siu ER, Viana MC, Andrade AG, Martins SS, Andrade LH. Investigating dimensionality and measurement bias of DSM-5 alcohol use disorder in a representative sample of the largest metropolitan area in South America. Drug Alcohol Depend. 2015;152:123–130. doi: 10.1016/j.drugalcdep.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpitel CJ, Borges G, Ye Y, Bond J, Cremonte M, Moskalewicz J, Swiatkiewicz G. Performance of a craving criterion in DSM alcohol use disorders. J Stud Alcohol Drugs. 2010;71:674–84. doi: 10.15288/jsad.2010.71.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Martin CS, Maisto SA, Cornelius JR, Clark DB. Greater prevalence of proposed DSM-5 nicotine use disorder compared to DSM-IV nicotine dependence in treated adolescents and young adults. Addiction. 2012;107:810–8. doi: 10.1111/j.1360-0443.2011.03722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Martin CS, Winters KC, Cornelius JR, Langenbucher JW. Limitations in the assessment of DSM-IV cannabis tolerance as an indicator of dependence in adolescents. Exp Clin Psychopharmacol. 2004;12:136–46. doi: 10.1037/1064-1297.12.2.136. [DOI] [PubMed] [Google Scholar]
- Chung T, Martin CS, Winters KC, Langenbucher JW. Assessment of alcohol tolerance in adolescents. J Stud Alcohol. 2001;62:687–95. doi: 10.15288/jsa.2001.62.687. [DOI] [PubMed] [Google Scholar]
- Clark BC, Perkins A, Mccullumsmith CB, Islam AM, Sung J, Cropsey KL. What does self-identified drug of choice tell us about individuals under community corrections supervision? J Addict Med. 2012;6:57–67. doi: 10.1097/ADM.0b013e318233d603. [DOI] [PubMed] [Google Scholar]
- Compton WM, Saha TD, Conway KP, Grant BF. The role of cannabis use within a dimensional approach to cannabis use disorders. Drug Alcohol Depend. 2009;100:221–7. doi: 10.1016/j.drugalcdep.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Leung KS, Abdallah AB. Test-re-test reliability of DSM-IV adopted criteria for 3,4-methylenedioxymethamphetamine (MDMA) abuse and dependence: a cross-national study. Addiction. 2009;104:1679–90. doi: 10.1111/j.1360-0443.2009.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ. Adolescents and substance-related disorders: research agenda to guide decisions on Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-V) Addiction. 2006;101(Suppl 1):115–24. doi: 10.1111/j.1360-0443.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- De Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev. 2012;36:1565–76. doi: 10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Bruffaerts R, De Girolamo G, Gureje O, Huang Y, Karam A, Kostyuchenko S, Lepine JP, Mora ME, Neumark Y, Ormel JH, Pinto-Meza A, Posada-Villa J, Stein DJ, Takeshima T, Wells JE. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delforterie M, Creemers H, Agrawal A, Lynskey M, Jak S, Van Der Ende J, Verhulst F, Huizink A. Functioning of cannabis abuse and dependence criteria across two different countries: the United States and The Netherlands. Subst Use Misuse. 2015a;50:242–250. doi: 10.3109/10826084.2014.952445. [DOI] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Dick DM, Agrawal A, Bucholz KK, Foroud T, Grucza RA, Hesselbrock MN, Hesselbrock V, Kramer J, Nurnberger JI, Jr, Schuckit M, Bierut LJ, Iacono WG, McGue M. Measurement invariance of DSM-IV alcohol, marijuana and cocaine dependence between community-sampled and clinically overselected studies. Addiction. 2013;108:1767–76. doi: 10.1111/add.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difranza J, Ursprung WW, Lauzon B, Bancej C, Wellman RJ, Ziedonis D, Kim SS, Gervais A, Meltzer B, Mckay CE, O’Loughlin J, Okoli CT, Fortuna LR, Tremblay M. A systematic review of the Diagnostic and Statistical Manual diagnostic criteria for nicotine dependence. Addict Behav. 2010;35:373–82. doi: 10.1016/j.addbeh.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564–72. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Gillespie NA, Aggen SH, Kendler KS. Assessment of a modified DSM-5 diagnosis of alcohol use disorder in a genetically informative population. Alcohol Clin Exp Res. 2013;37:443–51. doi: 10.1111/j.1530-0277.2012.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. The alcohol dependence syndrome: a concept as stimulus to enquiry. Br J Addict. 1986;81:171–83. doi: 10.1111/j.1360-0443.1986.tb00313.x. [DOI] [PubMed] [Google Scholar]
- Edwards G, Arif A, Hadgson R. Nomenclature and classification of drug- and alcohol-related problems: a WHO Memorandum. Bull World Health Organ. 1981;59:225–42. [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1:1058–61. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlke SJ, Hagman BT, Cohn AM. Modeling the dimensionality of DSM-IV alcohol use disorder criteria in a nationally representative sample of college students. Subst Use Misuse. 2012;47:1073–85. doi: 10.3109/10826084.2012.676698. [DOI] [PubMed] [Google Scholar]
- Flory K, Lynam D, Milich R, Leukefeld C, Clayton R. Early adolescent through young adult alcohol and marijuana use trajectories: Early predictors, young adult outcomes, and predictive utility. Development and Psychopathology. 2004;16:193–213. doi: 10.1017/s0954579404044475. [DOI] [PubMed] [Google Scholar]
- Gelhorn H, Hartman C, Sakai J, Stallings M, Young S, Rhee SH, Corley R, Hewitt J, Hopfer C, Crowley T. Toward DSM-V: an item response theory analysis of the diagnostic process for DSM-IV alcohol abuse and dependence in adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47:1329–39. doi: 10.1097/CHI.0b013e318184ff2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Bertacca S, Zaimovic A, Pirani M, Branchi B, Ferri M. Relationship of personality traits and drug of choice by cocaine addicts and heroin addicts. Subst Use Misuse. 2008;43:317–30. doi: 10.1080/10826080701202726. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Gizer IR, Ehlers CL. Item response theory analysis of binge drinking and its relationship to lifetime alcohol use disorder symptom severity in an American Indian community sample. Alcohol Clin Exp Res. 2011;35:984–95. doi: 10.1111/j.1530-0277.2010.01429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder DA, Gizer IR, Lau P, Ehlers CL. Item response theory analyses of DSM-IV and DSM-5 stimulant use disorder criteria in an American Indian community sample. Drug Alcohol Depend. 2014;135:29–36. doi: 10.1016/j.drugalcdep.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Prescott CA, Aggen SH, Kendler KS. Factor and item-response analysis DSM-IV criteria for abuse of and dependence on cannabis, cocaine, hallucinogens, sedatives, stimulants and opioids. Addiction. 2007;102:920–30. doi: 10.1111/j.1360-0443.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health. 2008;31:185–95. [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Gilder DA, Lau P, Wang T, Wilhelmsen KC, Ehlers CL. Contributions of ethnicity to differential item functioning of cannabis abuse and dependence symptoms. J Stud Alcohol Drugs. 2013;74:320–8. doi: 10.15288/jsad.2013.74.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Smith SM, Jung J, Zhang H, Chou SP, Pickering RP, Ruan WJ, Huang B, Saha TD, Aivadyan C, Greenstein E, Hasin DS. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5): Reliability of substance use and psychiatric disorder modules in a general population sample. Drug Alcohol Depend. 2015;148:27–33. doi: 10.1016/j.drugalcdep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC, Dawson DA, Chou PS, Pickering RP. The Alcohol Use Disorder and Associated Disabilities Interview schedule (AUDADIS): reliability of alcohol and drug modules in a general population sample. Drug Alcohol Depend. 1995;39:37–44. doi: 10.1016/0376-8716(95)01134-k. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:361–8. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Abbacchi AM, Przybeck TR, Gfroerer JC. Discrepancies in estimates of prevalence and correlates of substance use and disorders between two national surveys. Addiction. 2007;102:623–9. doi: 10.1111/j.1360-0443.2007.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Young SE, Zeiger JS, Lessem JM, Hewitt JK, Hopfer CJ. Prevalence and correlates of alcohol and cannabis use disorders in the United States: results from the national longitudinal study of adolescent health. Drug Alcohol Depend. 2014;136:158–61. doi: 10.1016/j.drugalcdep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman BT, Cohn AM. Toward DSM-V: mapping the alcohol use disorder continuum in college students. Drug Alcohol Depend. 2011;118:202–8. doi: 10.1016/j.drugalcdep.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman BT, Cohn AM. Using latent variable techniques to understand DSM-IV alcohol use disorder criteria functioning. Am J Health Behav. 2013;37:565–74. doi: 10.5993/AJHB.37.4.14. [DOI] [PubMed] [Google Scholar]
- Harford TC, Yi HY, Faden VB, Chen CM. The dimensionality of DSM-IV alcohol use disorders among adolescent and adult drinkers and symptom patterns by age, gender, and race/ethnicity. Alcohol Clin Exp Res. 2009;33:868–78. doi: 10.1111/j.1530-0277.2009.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CA, Gelhorn H, Crowley TJ, Sakai JT, Stallings M, Young SE, Rhee SH, Corley R, Hewitt JK, Hopfer CJ. Item response theory analysis of DSM-IV cannabis abuse and dependence criteria in adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47:165–73. doi: 10.1097/chi.0b013e31815cd9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D. DSM-5 SUD diagnoses: changes, reactions, remaining open questions. Drug Alcohol Depend. 2015;148:226–229. [PubMed] [Google Scholar]
- Hasin D, Carpenter KM, McCloud S, Smith M, Grant BF. The alcohol use disorder and associated disabilities interview schedule (AUDADIS): reliability of alcohol and drug modules in a clinical sample. Drug and Alcohol Dependendence. 1997;44:133–141. doi: 10.1016/s0376-8716(97)01332-x. [DOI] [PubMed] [Google Scholar]
- Hasin D, Hatzenbuehler ML, Keyes K, Ogburn E. Substance use disorders: Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) and International Classification of Diseases, tenth edition (ICD-10) Addiction. 2006;101(Suppl 1):59–75. doi: 10.1111/j.1360-0443.2006.01584.x. [DOI] [PubMed] [Google Scholar]
- Hasin D, Paykin A, Meydan J, Grant B. Withdrawal and tolerance: prognostic significance in DSM-IV alcohol dependence. J Stud Alcohol. 2000;61:431–8. doi: 10.15288/jsa.2000.61.431. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Auriacombe M, Borges G, Bucholz K, Budney A, Crowley T, Grant BF, O’Brien C, Petry NM, Schuckit M, Wall MM. The DSM-5 field trials and reliability of alcohol use disorder. Am J Psychiatry. 2013a;170:442–3. doi: 10.1176/appi.ajp.2013.13010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Fenton MC, Beseler C, Park JY, Wall MM. Analyses related to the development of DSM-5 criteria for substance use related disorders: 2. Proposed DSM-5 criteria for alcohol, cannabis, cocaine and heroin disorders in 663 substance abuse patients. Drug Alcohol Depend. 2012;122:28–37. doi: 10.1016/j.drugalcdep.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Greenstein E, Aivadyan C, Stohl M, Aharonovich E, Saha T, Goldstein R, Nunes EV, Jung J, Zhang H, Grant BF. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5): Procedural validity of substance use disorders modules through clinical re-appraisal in a general population sample. Drug Alcohol Depend. 2015;148:40–46. doi: 10.1016/j.drugalcdep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry. 2008;69:1354–63. doi: 10.4088/jcp.v69n0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013b;170:834–51. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Edwards EM, Heeren T, Rosenbloom D. Age of drinking onset and injuries, motor vehicle crashes, and physical fights after drinking and when not drinking. Alcohol Clin Exp Res. 2009;33:783–90. doi: 10.1111/j.1530-0277.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–46. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W. Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics. 2009;123:1477–84. doi: 10.1542/peds.2008-2176. [DOI] [PubMed] [Google Scholar]
- Horey JT, Mariani JJ, Cheng WY, Bisaga A, Sullivan M, Nunes E, Levin FR. Comparison of substance use milestones in cannabis- and cocaine-dependent patients. J Addict Dis. 2012;31:60–6. doi: 10.1080/10550887.2011.642753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Should criteria for drug dependence differ across drugs? Addiction. 2006;101(Suppl 1):134–41. doi: 10.1111/j.1360-0443.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: The Institute for Social Research, The University of Michigan; 2014. http://www.monitoringthefuture.org/ [Google Scholar]
- Kalaydjian A, Swendsen J, Chiu WT, Dierker L, Degenhardt L, Glantz M, Merikangas KR, Sampson N, Kessler R. Sociodemographic predictors of transitions across stages of alcohol use, disorders, and remission in the National Comorbidity Survey Replication. Compr Psychiatry. 2009;50:299–306. doi: 10.1016/j.comppsych.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA, Lowry R, Olsen EO, Mcmanus T, Chyen D, Whittle L, Taylor E, Demissie Z, Brener N, Thornton J, Moore J, Zaza S. Youth risk behavior surveillance--United States, 2013. MMWR Surveill Summ. 2014;63(Suppl 4):1–168. http://www.cdc.gov/HealthyYouth/yrbs/index.htm. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kerridge BT, Saha TD, Smith S, Chou PS, Pickering RP, Huang B, Ruan JW, Pulay AJ. Dimensionality of hallucinogen and inhalant/solvent abuse and dependence criteria: implications for the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition. Addict Behav. 2011;36:912–8. doi: 10.1016/j.addbeh.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Eaton NR, Krueger RF, McLaughlin KA, Wall MM, Grant BF, Hasin DS. Childhood maltreatment and the structure of common psychiatric disorders. Br J Psychiatry. 2012;200:107–15. doi: 10.1192/bjp.bp.111.093062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Krueger RF, Grant BF, Hasin DS. Alcohol craving and the dimensionality of alcohol disorders. Psychol Med. 2011a;41:629–40. doi: 10.1017/S003329171000053X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Li G, Hasin DS. Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol Clin Exp Res. 2011b;35:2101–2112. doi: 10.1111/j.1530-0277.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Schulenberg JE, O’Malley PM, Johnston LD, Bachman JG, Li G, Hasin D. The social norms of birth cohorts and adolescent marijuana use in the United States, 1976–2007. Addiction. 2011c;106:1790–1800. doi: 10.1111/j.1360-0443.2011.03485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Schulenberg JE, O’Malley PM, Johnston LD, Bachman JG, Li G, Hasin D. Birth cohort effects on adolescent alcohol use: the influence of social norms from 1976 to 2007. Arch Gen Psychiatry. 2012;69:1304–1313. doi: 10.1001/archgenpsychiatry.2012.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Perez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend. 2013;130:101–8. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75:798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–6. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116:645–66. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbis AN, Hagman BT, Morgenstern J. Alcohol use disorders among substance dependent women on Temporary Assistance with Needy Families: more information for diagnostic modifications for DSM-5. Am J Addict. 2013a;22:402–10. doi: 10.1111/j.1521-0391.2013.12042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbis AN, Hagman BT, Sacco P. Functioning of alcohol use disorders criteria among middle-aged and older adults: implications for DSM-5. Subst Use Misuse. 2013b;48:309–22. doi: 10.3109/10826084.2012.762527. [DOI] [PubMed] [Google Scholar]
- Langenbucher JW, Labouvie E, Martin CS, Sanjuan PM, Bavly L, Kirisci L, Chung T. An application of item response theory analysis to alcohol, cannabis, and cocaine criteria in DSM-IV. J Abnorm Psychol. 2004;113:72–80. doi: 10.1037/0021-843X.113.1.72. [DOI] [PubMed] [Google Scholar]
- Le Bon O, Basiaux P, Streel E, Tecco J, Hanak C, Hansenne M, Ansseau M, Pelc I, Verbanck P, Dupont S. Personality profile and drug of choice; a multivariate analysis using Cloninger’s TCI on heroin addicts, alcoholics, and a random population group. Drug Alcohol Depend. 2004;73:175–82. doi: 10.1016/j.drugalcdep.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Dubertret C, Le Foll B. Impact of age at onset of cannabis use on cannabis dependence and driving under the influence in the United States. Accid Anal Prev. 2014;76C:1–5. doi: 10.1016/j.aap.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Perez De Los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–30. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Agrawal A. Psychometric properties of DSM assessments of illicit drug abuse and dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Psychol Med. 2007;37:1345–55. doi: 10.1017/S0033291707000396. [DOI] [PubMed] [Google Scholar]
- Malison RT, Kalayasiri R, Sanichwankul K, Sughondhabirom A, Mutirangura A, Pittman B, Gueorguieva R, Kranzler HR, Gelernter J. Inter-rater reliability and concurrent validity of DSM-IV opioid dependence in a Hmong isolate using the Thai version of the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA) Addict Behav. 2011;36:156–60. doi: 10.1016/j.addbeh.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Chung T, Kirisci L, Langenbucher JW. Item response theory analysis of diagnostic criteria for alcohol and cannabis use disorders in adolescents: implications for DSM-V. J Abnorm Psychol. 2006;115:807–14. doi: 10.1037/0021-843X.115.4.807. [DOI] [PubMed] [Google Scholar]
- Martin CS, Chung T, Langenbucher JW. How should we revise diagnostic criteria for substance use disorders in the DSM-V? J Abnorm Psychol. 2008;117:561–75. doi: 10.1037/0021-843X.117.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O, Strong DR, Kahler CW. Exploring the role of a nicotine quantity-frequency use criterion in the classification of nicotine dependence and the stability of a nicotine dependence continuum over time. Nicotine Tob Res. 2010;12:207–16. doi: 10.1093/ntr/ntp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon VV, Agrawal A, Heath AC, Edenberg HJ, Hesselbrock VM, Schuckit MA, Kramer JR, Bucholz KK. Functioning of alcohol use disorder criteria among men and women with arrests for driving under the influence of alcohol. Alcohol Clin Exp Res. 2011;35:1985–93. doi: 10.1111/j.1530-0277.2011.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewton L, Slade T, McBride O, Grove R, Teesson M. An evaluation of the proposed DSM-5 alcohol use disorder criteria using Australian national data. Addiction. 2011a;106:941–50. doi: 10.1111/j.1360-0443.2010.03340.x. [DOI] [PubMed] [Google Scholar]
- Mewton L, Teesson M, Slade T. “Youthful epidemic” or diagnostic bias? Differential item functioning of DSM-IV cannabis use criteria in an Australian general population survey. Addict Behav. 2010;35:408–13. doi: 10.1016/j.addbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Mewton L, Teesson M, Slade T, Cottler L. Psychometric performance of DSM-IV alcohol use disorders in young adulthood: evidence from an Australian general population sample. J Stud Alcohol Drugs. 2011b;72:811–22. doi: 10.15288/jsad.2011.72.811. [DOI] [PubMed] [Google Scholar]
- Milivojevic D, Milovanovic SD, Jovanovic M, Svrakic DM, Svrakic NM, Svrakic SM, Cloninger CR. Temperament and character modify risk of drug addiction and influence choice of drugs. Am J Addict. 2012;21:462–7. doi: 10.1111/j.1521-0391.2012.00251.x. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 2014;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011:69–76. doi: 10.2147/SAR.S14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron BE, Howard MO, Maitra S, Vaughn MG. Prevalence, timing, and predictors of transitions from inhalant use to Inhalant Use Disorders. Drug Alcohol Depend. 2009;100:277–284. doi: 10.1016/j.drugalcdep.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Perron BE, Vaughn MG, Howard MO, Bohnert A, Guerrero E. Item response theory analysis of DSM-IV criteria for inhalant-use disorders in adolescents. J Stud Alcohol Drugs. 2010;71:607–14. doi: 10.15288/jsad.2010.71.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80:303–12. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Piontek D, Kraus L, Legleye S, Buhringer G. The validity of DSM-IV cannabis abuse and dependence criteria in adolescents and the value of additional cannabis use indicators. Addiction. 2011;106:1137–45. doi: 10.1111/j.1360-0443.2010.03359.x. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Watzke S, Wurst FM. Dimensionality and stages of severity of DSM-5 criteria in an international sample of alcohol-consuming individuals. Psychol Med. 2014;44:3303–14. doi: 10.1017/S0033291714000889. [DOI] [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, Kupfer DJ. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59–70. doi: 10.1176/appi.ajp.2012.12070999. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Bray BC, Cottler LB. Reliability of use, abuse, and dependence of four types of inhalants in adolescents and young adults. Drug Alcohol Depend. 2007;91:40–9. doi: 10.1016/j.drugalcdep.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Halliburton AE, Bray BC. Does DSM-5 Nomenclature for Inhalant Use Disorder Improve Upon DSM-IV? Psychol Addict Behav. 2014 doi: 10.1037/adb0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Lanza ST, Donny EC, Clark DB. Different lengths of times for progressions in adolescent substance involvement. Addict Behav. 2006;31:962–83. doi: 10.1016/j.addbeh.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JS, Dierker LC. DSM-IV nicotine dependence symptom characteristics for recent-onset smokers. Nicotine Tob Res. 2010;12:278–86. doi: 10.1093/ntr/ntp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JS, Lee CT, Selya AS, Dierker LC. DSM-IV alcohol abuse and dependence criteria characteristics for recent onset adolescent drinkers. Drug Alcohol Depend. 2012;124:88–94. doi: 10.1016/j.drugalcdep.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2006;36:931–41. doi: 10.1017/S003329170600746X. [DOI] [PubMed] [Google Scholar]
- Saha TD, Compton WM, Chou SP, Smith S, Ruan WJ, Huang B, Pickering RP, Grant BF. Analyses related to the development of DSM-5 criteria for substance use related disorders: 1. Toward amphetamine, cocaine and prescription drug use disorder continua using Item Response Theory. Drug Alcohol Depend. 2012;122:38–46. doi: 10.1016/j.drugalcdep.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Compton WM, Pulay AJ, Stinson FS, Ruan WJ, Smith SM, Grant BF. Dimensionality of DSM-IV nicotine dependence in a national sample: an item response theory application. Drug Alcohol Depend. 2010;108:21–8. doi: 10.1016/j.drugalcdep.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82–92. doi: 10.1016/j.drugalcdep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Agrawal A, Lynskey MT, Duncan AE, Grant JD, Nelson EC, Madden PA, Heath AC, Bucholz KK. Cannabis or alcohol first? Differences by ethnicity and in risk for rapid progression to cannabis-related problems in women. Psychol Med. 2013;43:813–23. doi: 10.1017/S0033291712001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Kranzler HR, Gelernter J. Characteristics and course of dependence in cocaine-dependent individuals who never used alcohol or marijuana or used cocaine first. J Stud Alcohol Drugs. 2014;75:423–7. doi: 10.15288/jsad.2014.75.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Daeppen JB, Danko GP, Tripp ML, Smith TL, Li TK, Hesselbrock VM, Bucholz KK. Clinical implications for four drugs of the DSM-IV distinction between substance dependence with and without a physiological component. Am J Psychiatry. 1999;156:41–9. doi: 10.1176/ajp.156.1.41. [DOI] [PubMed] [Google Scholar]
- Shmulewitz D, Keyes K, Beseler C, Aharonovich E, Aivadyan C, Spivak B, Hasin D. The dimensionality of alcohol use disorders: results from Israel. Drug Alcohol Depend. 2010;111:146–54. doi: 10.1016/j.drugalcdep.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmulewitz D, Keyes KM, Wall MM, Aharonovich E, Aivadyan C, Greenstein E, Spivak B, Weizman A, Frisch A, Grant BF, Hasin D. Nicotine dependence, abuse and craving: dimensionality in an Israeli sample. Addiction. 2011;106:1675–86. doi: 10.1111/j.1360-0443.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmulewitz D, Wall MM, Aharonovich E, Spivak B, Weizman A, Frisch A, Grant BF, Hasin D. Validity of proposed DSM-5 diagnostic criteria for nicotine use disorder: results from 734 Israeli lifetime smokers. Psychol Med. 2013;43:2179–90. doi: 10.1017/S0033291712002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmulewitz D, Wall MM, Keyes KM, Aharonovich E, Aivadyan C, Greenstein E, Spivak B, Weizman A, Frisch A, Hasin D. Alcohol use disorders and perceived drinking norms: ethnic differences in Israeli adults. Journal of Studies on Alcohol and Drugs. 2012;73:981–990. doi: 10.15288/jsad.2012.73.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Colby SM, Griesler PC, Kandel D. Linking measures of adolescent nicotine dependence to a common latent continuum. Drug Alcohol Depend. 2009;99:296–308. doi: 10.1016/j.drugalcdep.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Schonbrun YC, Schaffran C, Griesler PC, Kandel D. Linking measures of adult nicotine dependence to a common latent continuum and a comparison with adolescent patterns. Drug Alcohol Depend. 2012;120:88–98. doi: 10.1016/j.drugalcdep.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health, 2013. 2014. [PubMed] [Google Scholar]
- Sussman S, Leventhal A, Bluthenthal RN, Freimuth M, Forster M, Ames SL. A framework for the specificity of addictions. Int J Environ Res Public Health. 2011;8:3399–415. doi: 10.3390/ijerph8083399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenz RC, Scherer M, Harrell P, Zur J, Sinha A, Latimer W. Early onset of drug and polysubstance use as predictors of injection drug use among adult drug users. Addict Behav. 2012;37:367–72. doi: 10.1016/j.addbeh.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- World Health Organization. [Accessed February 2, 2015];Neuroscience of Psychoactive Substance Use and Dependence: summary. 2004 Available: http://www.who.int/substance_abuse/publications/en/Neuroscience_E.pdf.
- Wu LT, Howard MO, Pilowsky DJ. Substance use disorders among inhalant users: results from the National Epidemiologic Survey on alcohol and related conditions. Addict Behav. 2008;33:968–73. doi: 10.1016/j.addbeh.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Pan JJ, Blazer DG, Tai B, Brooner RK, Stitzer ML, Patkar AA, Blaine JD. The construct and measurement equivalence of cocaine and opioid dependences: a National Drug Abuse Treatment Clinical Trials Network (CTN) study. Drug Alcohol Depend. 2009a;103:114–23. doi: 10.1016/j.drugalcdep.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Pan JJ, Blazer DG, Tai B, Stitzer ML, Brooner RK, Woody GE, Patkar AA, Blaine JD. An item response theory modeling of alcohol and marijuana dependences: a National Drug Abuse Treatment Clinical Trials Network study. J Stud Alcohol Drugs. 2009b;70:414–25. doi: 10.15288/jsad.2009.70.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Pan JJ, Yang C, Reeve BB, Blazer DG. An item response theory analysis of DSM-IV criteria for hallucinogen abuse and dependence in adolescents. Addict Behav. 2010;35:273–7. doi: 10.1016/j.addbeh.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Ringwalt CL, Yang C, Reeve BB, Pan JJ, Blazer DG. Construct and differential item functioning in the assessment of prescription opioid use disorders among American adolescents. J Am Acad Child Adolesc Psychiatry. 2009c;48:563–72. doi: 10.1097/CHI.0b013e31819e3f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Swartz MS, Pan JJ, Burchett B, Mannelli P, Yang C, Blazer DG. Evaluating brief screeners to discriminate between drug use disorders in a sample of treatment-seeking adults. Gen Hosp Psychiatry. 2013;35:74–82. doi: 10.1016/j.genhosppsych.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Woody GE, Yang C, Pan JJ, Blazer DG. Abuse and dependence on prescription opioids in adults: a mixture categorical and dimensional approach to diagnostic classification. Psychol Med. 2011;41:653–64. doi: 10.1017/S0033291710000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Woody GE, Yang C, Pan JJ, Reeve BB, Blazer DG. A dimensional approach to understanding severity estimates and risk correlates of marijuana abuse and dependence in adults. Int J Methods Psychiatr Res. 2012;21:117–33. doi: 10.1002/mpr.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 2010;105:1809–1818. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]