Abstract
HIV sequence diversity and the propensity of eliciting immunodominant responses targeting variable regions of the HIV proteome are hurdles in the development of an effective AIDS vaccine. An HIV-derived conserved element (CE) p24gag plasmid DNA (pDNA) vaccine is able to redirect immunodominant responses to otherwise subdominant and often more vulnerable viral targets. By homology to the HIV immunogen, seven CE were identified in SIV p27Gag. Analysis of 31 rhesus macaques vaccinated with full-length SIV gag pDNA showed inefficient induction (58% response rate) of cellular responses targeting these CE. In contrast, all 14 macaques immunized with SIV p27CE pDNA developed robust T cell responses recognizing CE. Vaccination with p27CE pDNA was also critical for the efficient induction and increased the frequency of Ag-specific T cells with cytotoxic potential (granzyme B+ CD107a+) targeting subdominant CE epitopes, compared with the responses elicited by the p57gag pDNA vaccine. Following p27CE pDNA priming, two booster regimens, gag pDNA or codelivery of p27CE+gag pDNA, significantly increased the levels of CE-specific T cells. However, the CE+gag pDNA booster vaccination elicited significantly broader CE epitope recognition, and thus, a more profound alteration of the immunodominance hierarchy. Vaccination with HIV molecules showed that CE+gag pDNA booster regimen further expanded the breadth of HIV CE responses. Hence, SIV/HIV vaccine regimens comprising CE pDNA prime and CE+gag pDNA booster vaccination significantly increased cytotoxic T cell responses to subdominant highly conserved Gag epitopes and maximized response breadth.
Introduction
Vaccine-induced protection against HIV has been difficult to obtain, partly because the high viral mutation rate generates a broad repertoire of viable alternatives to the epitopes targeted by the immune system of the host, thereby eluding the immune system. To address the problem of viral variability, many approaches have been tested including strategies that use consensus, center-of-tree or ancestral sequences, multiple strains or mosaic immunogens, immunogens consisting of known epitopes, and chimeric molecules expressing a selection of the most conserved epitopes from different clades of HIV (1–22). In our approach (20–22), we designed a plasmid DNA (pDNA) vaccine to focus the immune response to conserved elements (CE) of the HIV-1 proteome based on stringent conservation, broad HLA-coverage, and association with HIV control (19, 23–25). The p24Gag protein was chosen because CTL responses to Gag, particularly to its proteolytic processing product p24Gag, have been associated with better control of HIV viremia (19, 23, 26–31). Similarly, a direct correlation between Gag-specific T cell responses prior to infection and control of acute viremia was found in DNA-vaccinated macaques (32). Others found that the inclusion of Gag in the vaccine was critical for the control of viremia using recombinant vesicular stomatitis virus (33, 34), and recombinant human CMV (35, 36) vaccines.
We identified seven CE that represent 54% of the HIV-1 p24Gag protein, and are found in nearly every HIV-1 (M group) strain observed to date throughout the world (19, 23–25). Because this vaccine excludes variable elements of the viral protein, the development of vaccine-induced cellular responses targeting immunodominant decoy epitopes, encoded by variable regions capable of mutating to avoid immune recognition without substantial cost to viral fitness, is prevented. Thus, CE vaccines are targeted to the presumed weakness of the virus. We demonstrated that the HIV p24CE pDNA vaccine efficiently primes cellular responses targeting regions that are poorly immunogenic when present within the complete Gag protein (20–22). However, CE responses can be significantly boosted by vaccination with pDNA expressing the entire p55Gag protein. This demonstrates that the p55Gag protein is processed into peptides containing the CE epitopes, and that these epitopes are indeed presented by MHC but are subdominant in the presence of peptides from the variable regions of Gag. These findings also demonstrate that p24CE pDNA priming alters the immunodominance hierarchy seen in HIV infection and other vaccine modalities. Thus, a vaccine regimen combining p24CE pDNA prime followed by a boost with p55Gag pDNA may help solve a major obstacle in HIV vaccine development by altering the hierarchy of epitope recognition, and inducing cell-mediated responses to potentially protective, and otherwise subdominant, highly conserved epitopes.
To further expand the CE vaccine platform, a CE pDNA vaccine derived from SIV p27Gag was developed by analogy to the HIV p24CE pDNA. Here, we describe the immunogenicity of this SIV vaccine in rhesus macaques. In addition, we report the development of a novel pDNA vaccine regimen that includes CE prime/CE+gag pDNA codelivery booster vaccination. This improved vaccine regimen induces a higher magnitude of cytotoxic cellular responses with significantly broader epitope recognition than the regimen including only gag pDNA booster vaccination for both SIV and HIV.
Materials and Methods
Plasmids
Plasmids SIV p27CE1 (plasmid 262S) and p27CE2 (plasmid 263S) contain the RNA/codon-optimized p27CE genes inserted into a pCMVkan vector between the human CMV promoter and bovine growth hormone (BGH) polyadenylation signal (37). Both proteins contain the GM-CSF signal peptide at the N terminus. Insertion of a FLAG tag at the C terminus of p27CE1 and p27CE2 generated plasmids 264S and 265S, respectively. The SIV p57gag-pro (plasmid 256S) produces SIVmac239 p57Gag and the protease (Pro) from an RNA/codon optimized gene cloned into pCMVkan. The SIV p57gag (plasmid 206S) and MCP3-p39gag (plasmid 209S) express the complete SIVmac239 p57Gag protein and the p39Gag processing intermediate (p19Gag+p27Gag). HIV p24CE1/2 (plasmid 306H) is a dual promoter plasmid generated to express the p24CE1 gene from the human CMV promoter and the p24CE2 gene from the simian CMV promoter in the opposite transcriptional orientation using the BGH polyA signal for p24CE1 and the SV40 polyA signal for p24CE2 (21). HIV-1 p55gag pDNA (plasmid 114H) expresses the full-length p55Gag protein from the HXB2 strain. Endotoxin-free DNAs (Qiagen, Valencia, CA) were prepared according to the manufacturer’s protocol.
SIV p27CE DNA expression upon transient transfection
HEK293T cells (60-mm plates seeded with 106 cells) were transfected by the calcium phosphate DNA coprecipitation procedure using 0.5 μg of plasmid DNA together with 6.5 μg of Bluescript as carrier DNA. Six hours after transfection, the medium was replaced with 3 ml of DMEM containing 2% FCS. After 2 d, supernatants and cells were collected, and the cells were lysed in 0.5 ml of 0.5× RIPA buffer (Boston BioProducts, Ashland, MA). Protein expression was analyzed by Western immunoblots using 12% NaDodSO4 polyacrylamide gels (NuPAGE Bis-Tris, NuPAGE; Invitrogen, Life Technologies, Carlsbad, CA) and blotted on to nitrocellulose membranes that were then probed with a mouse anti-SIVp27Gag Ab KK64 (38) (dilution 1:1000), followed by anti-mouse IgG-HRP labeled Ab (1:10,000 dilution; GE Healthcare, Piscataway, NJ). As control, the membranes were probed with anti-actin Ab (clone C4; EMD Millipore, Billerica, MA) at a dilution of 1:10,000. The bands were visualized using the ECL Prime Western blotting detection system (GE HealthCare).
Vaccination of rhesus macaques
This study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Indian rhesus macaques were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International at the Advanced BioScience Laboratories (Rockville, MD) and were approved by the Institutional Animal Care and Use Committee (Office of Laboratory Animal Welfare assurance number A3467-01 and U.S. Department of Agriculture certificate number 51-R-0059), or were housed and cared for at the National Institutes of Health (Bethesda, MD) in accordance with the Guide for Care and Use of Laboratory Animals report number NIH 82-53 in a biosafety level 2 National Institute of Allergy and Infectious Diseases facility. The outbred animals were tested for selected MHC haplotypes (Supplemental Table I). The CE priming vaccinations used a mixture of 1 mg each of SIV p27CE1 and p27CE2 pDNA. The gag pDNA booster vaccination used 1 mg of SIV p57gag pDNA. The CE+gag pDNA booster vaccination used 1 mg each of p57gag and MCP3-p39gag pDNA, SIV p27CE1 and p27CE2 pDNA. PBMC from SIV gag pDNA–only immunized macaques described elsewhere (32, 39, 40) were included in this study. The HIV pDNA vaccinated animals received a prime p24CE1/2 (4 mg) expressing the p24CE1 and p24CE2 proteins, followed by booster vaccinations using codelivery of p24CE1/2 (2 mg) and p55gag pDNA (2 mg). All DNA vaccine mixtures contained 0.2 mg of expression-optimized macaque IL-12 DNA (plasmid AG157) (41). The pDNA vaccine was formulated in 0.6 ml of sterile water and was delivered via i.m. injection at two different sites (0.3 ml at each site) followed by in vivo electroporation using the Elgen 1000 device (macaques L986, R108, R677, R682, R683, R684 Fig. 4A; macaques RH01A through RHOCM Fig. 5C; and macaques 5698 through 5702 Fig. 8C) and the CELLECTRA 5P device (macaques T129 through T152 Fig. 4A) (Inovio Pharmaceuticals, Plymouth Meeting, PA). Blood samples were collected on the day of each vaccination and 2 wk later.
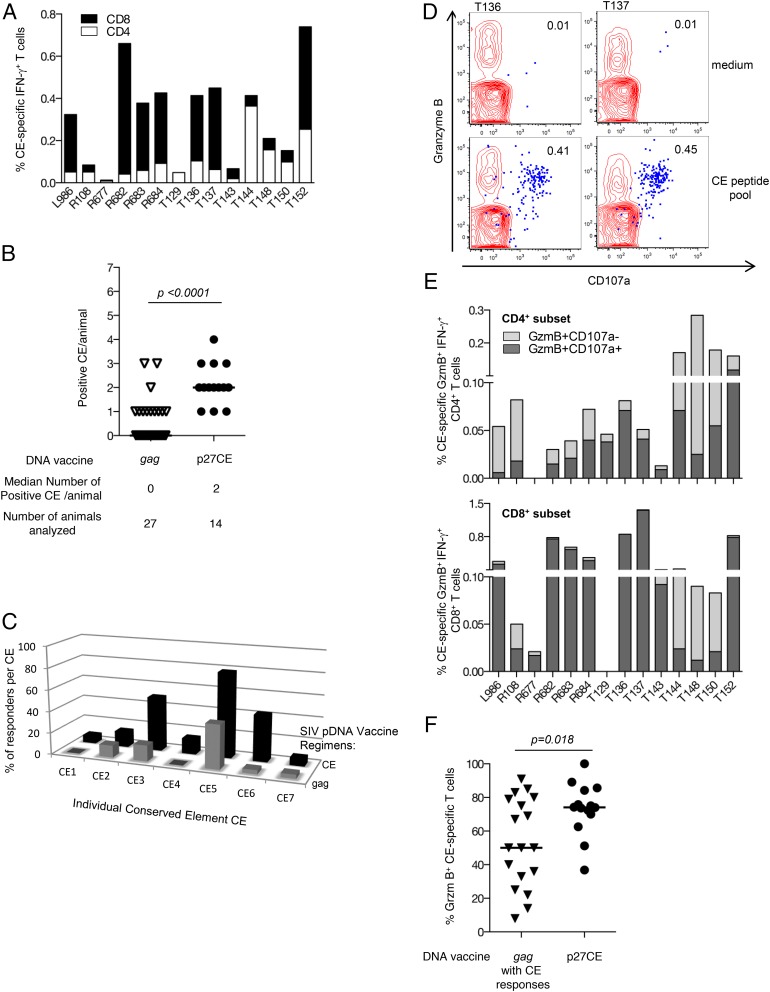
FIGURE 4.
SIV p27CE pDNA vaccine induces robust CE-specific cytotoxic T cell responses in macaques. (A) Rhesus macaques (n = 14) received three vaccinations with SIV p27CE pDNA (mixture of p27CE1 DNA and p27CE2 DNA) at 0, 2, and 4 mo, except animals L986 and R108, that received two vaccinations (0 and 2 mo). PBMC were collected 2 wk after the last vaccination and stimulated with a peptide pool consisting of a mixture of 15-mers overlapping by 11 aa and 10-mers overlapping with nine aa. The frequency of the CE-specific CD4+ (open bars) and CD8+ (filled bars) IFN-γ-producing T cells was determined. The DNA vaccine in macaques L986, R108 through R684 was administered using the Elgen 1000 electroporation device, and in macaques T129 through T152 using the CELLECTRA 5P device. No difference in the level or nature of the induced CE-specific T cell responses was found using these electroporation devices, which allowed the combination of the animals in one treatment group. (B) p27CE pDNA vaccine induces broader responses to CE. The scatter plot shows the number and range of CE recognized by each macaque immunized with the p27CE pDNA compared with macaques vaccinated by full-length gag pDNA (from Fig. 2). The median number of recognized CE and the number of animals analyzed are listed at the bottom. (C) Comparison of the breadth of the CE-specific responses. Mapping of CE-specific responses induced by the gag pDNA (gray bars) and CE pDNA (black bars) vaccines. The bars show the percentage of the vaccinated animals recognizing each specific CE. (D) Plot overlays show the granzyme B content and degranulation activity (CD107a+) from unstimulated (upper plots) and peptide stimulated (lower plots) T lymphocytes from two vaccinated animals. The IFN-γ+ T cells are shown in blue. (E) Frequency of CE-specific cytotoxic T cell responses. The frequency of CE-specific IFN-γ+ CD4+ (top panel) and CD8+ (bottom panel) subsets of T cells harboring granzyme B (GzmB+) and able to degranulate upon Ag-stimulation (CD107a+) are shown. (F) Comparison of the frequency of granzyme B+ CE-specific IFN-γ–producing T cells from p27CE pDNA vaccinated animals (n = 14) and the subgroup of gag pDNA immunized macaques which developed CE-specific responses (n = 18; from Fig. 2B). Th median and p values (t test) are indicated.
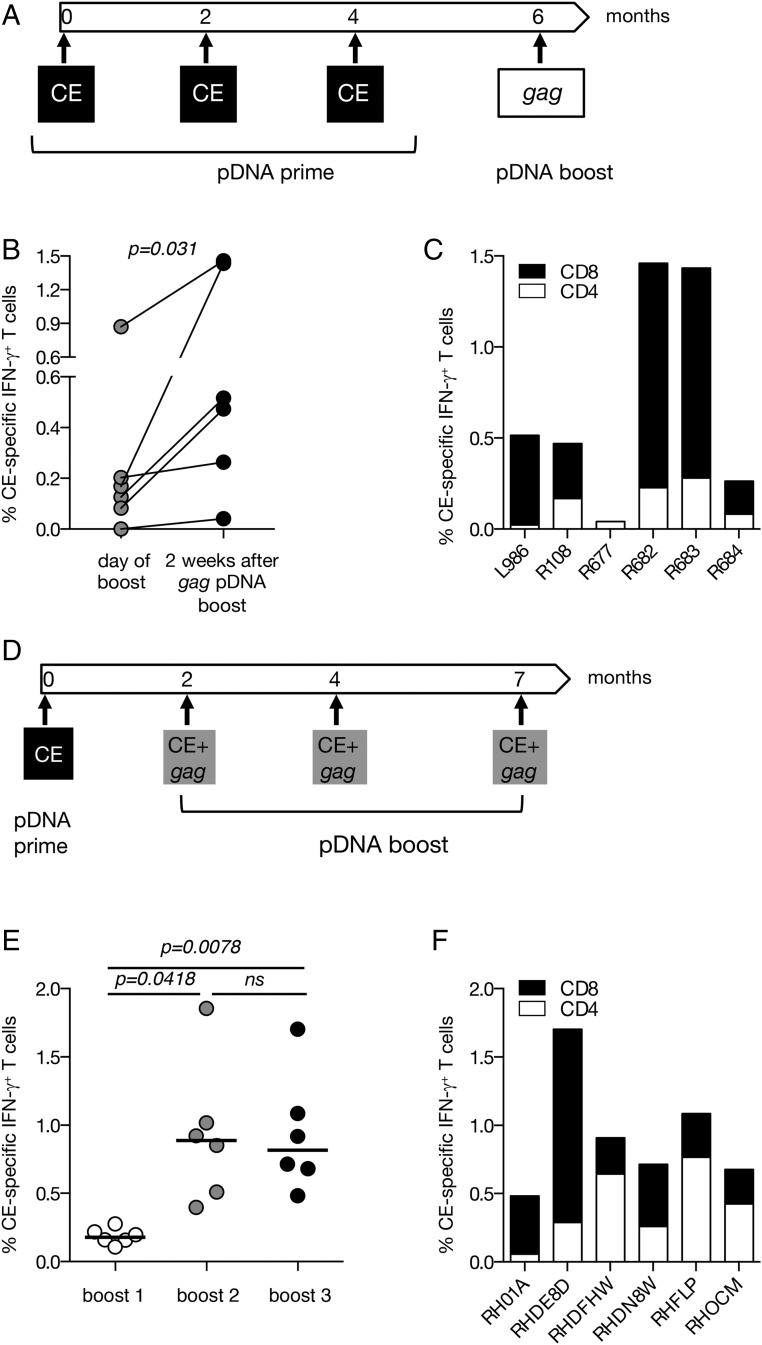
FIGURE 5.
SIV p27CE pDNA prime-boost vaccination regimens. (A) The cartoon depicts the p27CE pDNA prime/gag pDNA boost vaccination regimen. (B) Plot shows the comparison between the levels of the CE-specific responses measured at the day of the gag pDNA booster vaccination and 2 wk later in six macaques described in Fig. 4. The p value is from a paired t test. (C) The frequency of p27CE-specific IFN-γ+ T cell responses is shown for CD4+ (open bars) and CD8+ (filled bars) T cells after the gag pDNA booster vaccination. (D) The cartoon depicts the p27CE pDNA prime and p27CE+gag pDNA booster vaccination regimen. (E) Comparison of the CE-specific cellular responses measured 2 wk after each p27CE+gag pDNA booster vaccination. The p values are from ANOVA (Dunnett’s test). (F) CE-specific T cell responses in the vaccinated macaques (n = 6) were measured 2 wk after the last p27CE+gag pDNA booster vaccination. The frequency of the IFN-γ+ p27CE-specific responses is shown for CD4+ (open bars) and CD8+ (filled bars) T cells.
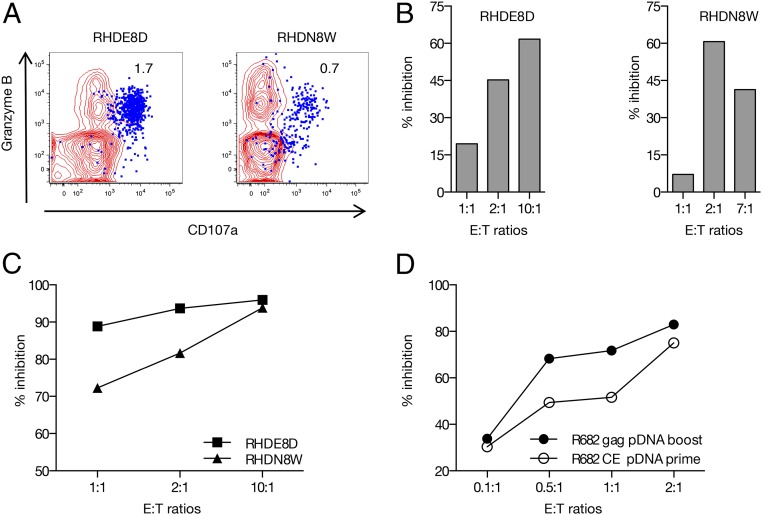
FIGURE 8.
CE-specific cytotoxic T cells reduce viral replication. (A) Plot overlays show the granzyme B content and degranulation activity (CD107a+) of the CE-specific T cells (in blue) upon peptide stimulation. Numbers within the plots indicate the percentage of IFN-γ+ T cells. (B) Inhibition of SIV replication (in percent of the negative control samples) measuring p27Gag in the culture supernatants in the presence of increasing amounts of CD8+ T cells from two representative macaques after the last p27CE+gag booster vaccination. (C) Reduction in the frequency of SIV infected cells by increasing amounts of autologous CD8+ T cells (collected after the last vaccination from RHDE8D and RHDN8W) as measured by intracellular p27Gag staining followed by flow cytometry. (D) Reduction in the frequency of SIV infected cells by increasing amounts of autologous CD8+ T cells, collected after CE pDNA prime (open circles) and gag pDNA (solid circles) from macaque R682, and measured as in (C).
Intracellular cytokine staining
Ficoll-hypaque isolated PBMC were cultured in 96-well plates in the presence of various peptide pools from SIV and HIV, respectively, at a final concentration of 1 μg/ml for each peptide. Peptide pools covering all CE or each seven individual CE were prepared combining 15-mer peptides overlapping by 11 aa and 10-mer peptides overlapping by nine aa (Infinity Biotech Research & Resource). Analysis of Gag-specific responses was performed using pools of 15-mer peptides. Ag-specific T cells were measured by intracellular cytokine staining followed by polychromatic flow cytometry as detailed elsewhere (32, 39, 42) using the following mixture of cell surface Abs: CD3-APCCy7 (clone SP34-2), CD4-V500 (clone L200), CD95-FITC (clone DX2) (BD Pharmingen, San Diego, CA), CD8-Alexa Fluor-405 (clone 3B5; Invitrogen), CD28-PerCP Cy5.5 (clone CD28.2; BioLegend, San Diego, CA). Ten minutes after addition of peptides, the CD107a-eFluor 660 (clone eBioH4A3; eBioscience, San Diego, CA) Ab was added. After cell permeabilization, intracellular staining was performed using IFN-γ-PE Cy7 (clone B27; BD Pharmingen) and granzyme B-PE Abs (clone GB12; Invitrogen). As negative and positive controls, PBMC were cultured in medium without peptide pools or stimulated with PMA and calcium ionophore (Sigma, St. Louis, MO). Peptide-stimulated samples were considered positive if the responses were 2 fold higher than that of unstimulated medium-only control and greater than 0.01 after subtracting the medium control value. Samples were acquired on an LSR II or Fortessa flow cytometer (BD Biosciences, San Jose, CA), and the data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Virus inhibition assay
Autologous cryopreserved PBMC were used to perform a virus inhibition assay essentially as previously described (43). Briefly, PBMC samples obtained before the study start and after the last vaccination were magnetically sorted into CD8-depleted PBMC (target cells) and positively selected CD8+ T cells (effector cells) using a non-human primate-specific CD8 Microbead kit (Miltenyi Biotec), according to the manufacturer’s instructions. The purity of the effector CD8+ population was monitored by flow cytometry and found to be >95%. Both target and effector cell populations were cultured in complete RPMI medium [RPMI 1640 supplemented with 10% FCS, antibiotics and 50 IU human recombinant IL-2 (Peprotech)] at a density of 2 × 106 cells/ml. The target cells were stimulated with 5 μg/ml Con A during the 24 h prior to infection with SIVmac239. For viral infection, the CD8-depleted PBMC were washed and exposed to SIVmac239 at a multiplicity of infection of 0.1 in a final volume of 0.5 ml for 2 h at 37°C. After incubation with the virus, the target cells were washed twice with complete RPMI medium, resuspended in complete medium at a density of 106 cells/ml, and 105 cells/well were seeded in a 96-well plate. Different effector/target ratios were tested in duplicate by seeding different amounts of CD8+ T cells in a final volume of 200 μl/well. On days 4, 7, and 11, 100 μl supernatant was removed for p27Gag determinations and an equal volume of fresh medium was added to each well. SIV p27Gag concentration was measured in the stored supernatants by SIV p27Gag ELISA (Zeptometrix). On day 11, the cells were harvested and stained with labeled CD3, CD4, and CD8 Abs. To identify infected cells, the surface stained cells were permeabilized with fixation/permeabilization buffer (eBioscience), and incubated with 0.4 μg of allophycocyanin-conjugated anti-SIV p27Gag Ab [55-2F12, National Institutes of Health AIDS Reagent Program (44)] for 30 min at 4°C. After the incubation, the cells were washed twice and analyzed by flow cytometry. Virus inhibition was determined by the reduction of p27Gag in supernatant (ELISA) or the frequency of SIV infected cells (flow cytometry) of target cells cultured in the presence of increasing amounts of autologous CD8+ T cells. Viral inhibition is presented as the percentage reduction of the values obtained with negative control samples.
Statistical analyses
The statistical analyses were carried out with GraphPad Prism version 6.0 for MacOS X (GraphPad Software, La Jolla, CA).
Results
Identification of conserved regions in SIV Gag protein
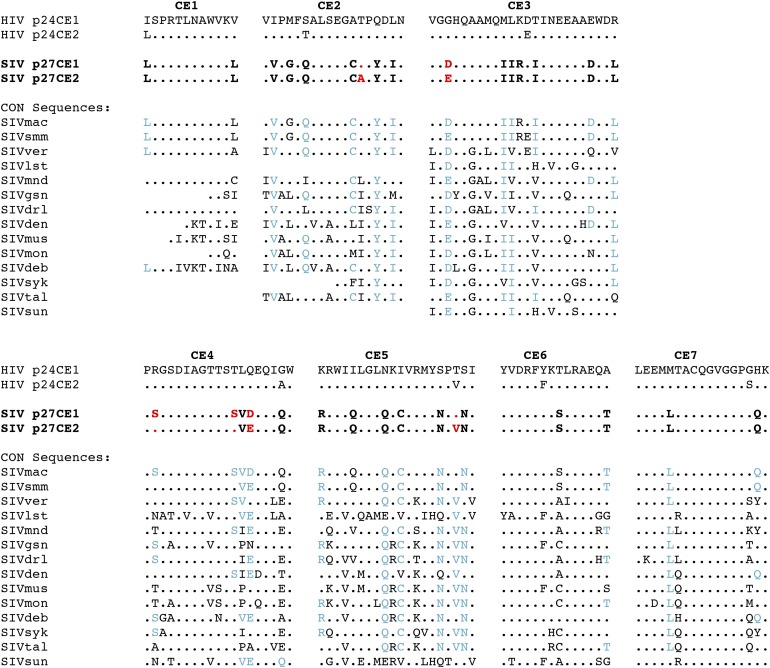
We designed an SIV-based CE vaccine by analogy to our reported work on conserved element HIV p24Gag. Because SIVmac was generated upon SIVsmm transmission from captive sooty mangabey monkeys into rhesus macaques with subsequent spread within primate centers (45), no sequences of SIVmac evolution in the wild are known, and, in general, very few examples of other SIV sequences are available from wild populations (see Fig. 1 legend). Therefore, the available data does not provide reliable guidance to define truly conserved sequences within SIV Gag. Thus, the SIV sequences for inclusion in this vaccine were chosen based on an alignment of available SIV sequences (n = 790 from 14 species), dominated by experimental SIVmac evolution, and to a lesser extent by SIVsmm sequences (Fig. 1), and guided by homology to HIV. Significant homology between HIV and SIV CE was found, especially in CE1, CE4, CE6, and CE7. The sites at which the SIV CE differed from that of HIV-1 were often conserved across distantly related SIV strains (Fig. 1, sites shown in blue type). As with the HIV CE constructs, we generated two versions to encompass polymorphic sites, which together represent nearly all SIV strains. Only one toggle amino acid site was used per CE, except for CE4, in which two additional amino acids were substituted because those amino acid variants were always found together in the database. No toggled amino acid was included for SIV CE1, CE6 or CE7 due to the high degree of conservation observed in those segments among available SIV sequences.
FIGURE 1.
Derivation of SIV p27Gag CE and conservation relative to HIV-1 and SIV strains from multiple species. All sequences were compared with HIV-1 p24CE1 (20), with a dot indicating homology. Toggle positions that distinguish SIV p27CE1 and p27CE2 are shown in red type. Amino acid differences that distinguished the SIV and HIV-1 CE but were conserved in other SIV strains are shown in blue type. A protocol of including only one toggle site per CE was adhered to except for CE4, in which two additional amino acids were substituted because those amino acid variants were always found together in the database. No toggled amino acid was included for CE1, CE6 or CE7 due to the complete conservation observed in those segments among available SIV sequences. The sequences shown correspond to the consensus of those obtained from the Los Alamos HIV sequence database. Blank positions indicate that sequences corresponding to the CE region were not available. SIVmac (species of origin: macaque), n = 495; SIVsmm (sooty mangabey), n = 272; SIVver (vervet), n = 3; SIVlst (l’Hoest’s), n = 4; SIVmnd (mandrill), n = 3; SIVgsn (greater spot-nosed), n = 2, one of two sequences matched HIV p24CE1 at position 9 of CE2 and position 1 of CE3; SIVdrl (drill), n = 2, one of two sequences matched HIV p24CE1 at position 11 of CE4; SIVden (Dent’s Mona); n = 1; SIVmus (mustached), n = 1; SIVmon (mona) n = 1; SIVdeb (De Brazza’s), n = 2; SIVsyk (Sykes), n = 1; SIVtal (talapoin), n = 2, one of two sequences matched HIV p24CE1 at position 20 of CE3 and at position 6 of CE5; SIVsun (sun-tailed), n = 1.
Poor induction of cellular immune responses to CE upon vaccination with full-length gag pDNA
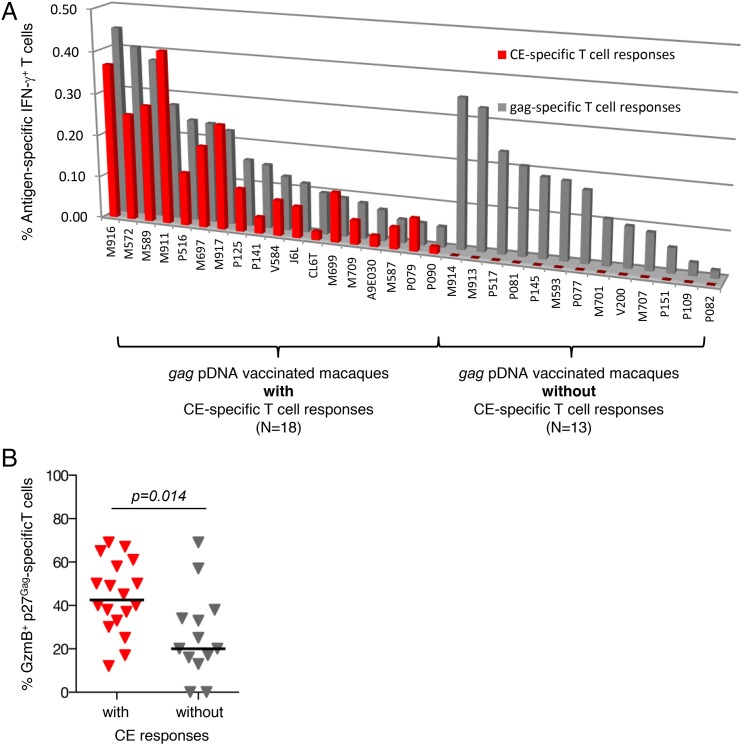
Samples from 31 macaques, immunized with SIV gag pDNA by intramuscular/electroporation delivery as part of other studies, were used to analyze whether the gag pDNA-induced cellular responses target the epitopes encoded by the conserved elements identified within p27Gag protein. Upon PBMC stimulation with Gag-peptides, p27Gag-specific T cell responses (range 0.06–0.5% of IFN-γ producing T lymphocytes) were found in all animals (Fig. 2A). To examine responses to CE, PBMC were stimulated with a CE-specific peptide pool consisting of a mixture of 15-mer (overlapping by 11 aa) and 10-mer (overlapping by nine aa) peptides spanning the seven CE to maximize the detection of CE-specific CD4+ and CD8+ T cell responses, a protocol applied throughout this work. Despite robust p27Gag-specific responses, only 18 of these macaques (58%) showed T cell responses targeting CE (Fig. 2A). No difference in the magnitude of p27Gag-specific T cell responses was observed between the two groups of animals with positive or negative CE immunity (Supplemental Fig. 1A), demonstrating that there is no link between the ability to mount CE and p27Gag responses. In addition, no correlation between the magnitude of CE and p27Gag responses was found among the SIV gag pDNA vaccinated animals (Fig. 2A). The ability to mount CE responses was independent of the CD4+/CD8+ ratio of Gag-specific IFN-γ+ T cells (Supplemental Fig. 1B), which showed no difference between the two subgroups of animals.
FIGURE 2.
Cellular responses in gag pDNA vaccinated macaques. (A) PBMC from rhesus macaques (n = 31) immunized with pDNA encoding the full-length SIV p57Gag protein were analyzed by flow cytometry for Ag-specific responses targeting the complete p27Gag (gray bars) protein or epitopes encoded by the CE (red bars). The values are plotted by decreasing p27Gag T cell responses and sorted according to the presence (n = 18) or absence (n = 13) of CE responses. Of note, animals P516 and P517 were analyzed with a peptide pool covering p39Gag that spans both the N terminal p19Gag and the p27Gag. (B) p27Gag-specific T cell responses were evaluated for their cytotoxic potential. The frequency of granzyme B+ Gag-specific T cells was determined among IFN-γ–producing p27Gag T cells comparing the subgroup of gag pDNA immunized macaques with (n = 18) and without (n = 13) CE-specific responses. The median and p values (t test) are indicated.
T cell responses to individual CE were mapped, using peptide subpools specific for each CE, in 14 of the 18 animals with positive CE responses (Table I). CE5 was recognized by the majority of these animals (11 of 14), followed by CE3 and CE2 (four and three animals, respectively), whereas CE1 and CE4 were not recognized by any of these macaques. Interestingly, most (10 of 14) of the macaques that responded to CE elicited responses to a single CE. These data are similar to our findings with HIV p55gag DNA (p55Gag is the homolog of SIV p57Gag) vaccinated macaques (21), where only ∼50% of the animals developed CE-specific responses with very limited breadth coverage. Taken together, these data suggest an immunodominance hierarchy favoring variable regions, what we refer to as decoy responses, which prevents the development of T cell responses to the highly conserved elements within Gag.
Table I. Mapping of cellular immune responses to individual CE in a subset of SIV gag pDNA vaccinated macaques with CE-specific T cell responses.
| Macaques(n = 14) | % CE-Specific IFN-γ+ T Cell Responses | Responses to Individual CE | No. of Positive CE per Animal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CE1 | CE2 | CE3 | CE4 | CE5 | CE6 | CE7 | |||
| M911 | 1.07 | + | 1 | ||||||
| M697 | 0.87 | + | + | + | 3 | ||||
| M917 | 0.50 | + | 1 | ||||||
| M699 | 0.38 | + | 1 | ||||||
| M589 | 0.18 | + | 1 | ||||||
| M916 | 0.16 | + | + | 2 | |||||
| P516 | 0.13 | + | 1 | ||||||
| P125 | 0.10 | + | + | 2 | |||||
| V584 | 0.08 | + | 1 | ||||||
| J6L | 0.07 | + | 1 | ||||||
| M587 | 0.06 | + | + | + | 3 | ||||
| P141 | 0.04 | + | 1 | ||||||
| M709 | 0.03 | + | 1 | ||||||
| A9E030 | 0.03 | + | 1 | ||||||
We next compared the cytotoxic potential of the Gag-induced responses by measuring the frequency of granzyme B+ IFN-γ+ p27Gag–specific T cells among the subgroups of animals with or without CE-specific immunity. As shown in Fig. 2B, the percentage of cytotoxic T cells was significantly higher in gag pDNA vaccinated macaques, which developed responses targeting CE (p = 0.014, Mann-Whitney U test), suggesting that T cells targeting the conserved elements are functionally more cytotoxic than those targeting the variable regions. To overcome these limitations (exclusion of responses to subdominant epitopes, induction of less cytotoxic T cells), a pDNA vaccine encoding only CE from SIV p27Gag was generated and tested.
Generation of SIV p27CE DNA vaccine
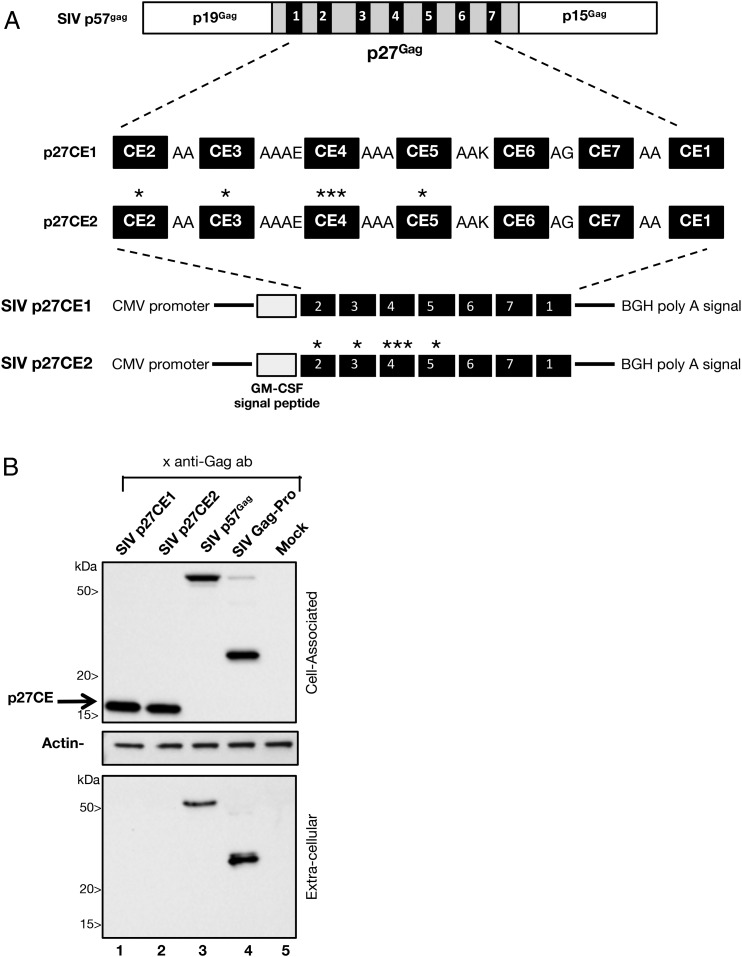
Two p27CE homologs of the HIV p24CE immunogens (Fig. 3A) were generated spanning 12–24 aa and covering 124 aa or 54% of SIV p27gag protein described in Fig. 1. The p27CE1 and p27CE2 molecules differ by the indicated toggle amino acids in CE2, CE3, CE4, and CE5. The CE were separated by linker sequences and were collinearly arranged by analogy to the HIV p24CE DNA, placing CE1 last to avoid a strongly hydrophobic N terminus (20). The RNA/codon optimized p27CE sequences were cloned into the pCMVkan expression vector. The signal peptide from the human GM-CSF was added at the N terminus of SIV p27CE protein to increase protein stability and trafficking.
FIGURE 3.
SIV p27CE expression vectors. (A) The full-length p57Gag precursor protein contains the p19Gag matrix protein, the p27Gag capsid protein, and the C terminal p15Gag processing intermediate. The SIV p27CE1 and p27CE2 proteins are composed of seven conserved elements (CE) derived from the p27Gag sequence, spanning 12–24 aa and collinearly arranged and separated via 2–4 aa linkers in the order shown in the cartoon. The toggle amino acids in p27CE2 is indicated by an asterisk. The proteins contain the GM-CSF signal peptide at the N terminus. Sequences were inserted into the mammalian expression vector pCMVkan providing the CMV promoter and the BGH poly A signal. (B) HEK293T cells were transfected with SIV pDNAs expressing p27CE1 (lane 1), p27CE2 (lane 2), p57Gag (lane 3), Gag-Pro (lane 4). Lane 5 contains a sample from mock-transfected cells. Proteins from the cell-associated (top panel: 1/100 of the sample) and extracellular (bottom panel: 1/250 of the sample) were analyzed. Western immunoblots were probed using the mouse anti-p27Gag Ab KK64, which recognizes an epitope between aa 151–180 and overlapping CE1 and CE2. Equal loading of the blots was controlled by probing the membrane with an anti-actin Ab (middle panel).
Expression of both p27CE DNA plasmids was confirmed upon transient transfection of HEK293 cells and Western immunoblot analysis (Fig. 3B). The SIV p27CE1 and p27CE2 proteins migrated at ∼17 kDa and were only found in the cell-associated fractions (lanes 1 and 2). Expression of SIV p57Gag (lane 3) and SIVGag-Pro (lane 4) proteins served as positive controls and showed the expected expression profiles with unprocessed p57Gag (lane 3) and the processed p27Gag (lane 4) found in both the cell-associated and extracellular fractions.
p27CE pDNA vaccine induces T cell responses with increased CE breadth and cytotoxicity in macaques
Rhesus macaques were vaccinated with a mixture of SIV p27CE1 and p27CE2 plasmids (referred to p27CE pDNA) using i.m. injection followed by in vivo electroporation (Fig. 4). All 14 macaques developed CE-specific (IFN-γ+) cellular responses ranging from 0.03 to 0.8% of total T lymphocytes (Fig. 4A). The responses were mediated both by CD4+ and CD8+ T cells, with 8 of the 14 animals showing a skewing toward CD8+ T cell responses.
Analysis of the T cell breadth in these 14 animals, using peptide subpools specific for the individual CE, showed that all seven CE were immunogenic (Table II). The responses targeted one to four CE per animal (median two CE) and displayed a significant increase in breadth against CE (p < 0.0001) compared with the gag pDNA vaccinated animals (median one) (Fig. 4B). Comparison of the responses to individual CE showed that both regimens favored responses to CE5 > CE3 and CE6 (Fig. 4C), but the p27CE pDNA vaccine showed increased breadth of responses (Fig. 4B), targeting all CE.
Table II. CE-specific T cell response breadth induced by different SIV CE pDNA vaccine regimens.
| p27CE pDNA Prime | pDNA Booster Vaccine | Responses to Individual CE | No. of Positive CE per Animal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CE1 | CE2 | CE3 | CE4 | CE5 | CE6 | CE7 | |||
| T129 | none | + | 1 | ||||||
| T136 | none | + | + | + | 3 | ||||
| T137 | none | + | + | 2 | |||||
| T143 | none | + | + | 2 | |||||
| T144 | none | + | + | + | + | 4 | |||
| T148 | none | + | 1 | ||||||
| T150 | none | + | + | 2 | |||||
| T152 | none | + | + | + | 3 | ||||
| L986a | none | + | + | 2 | |||||
| R108a | none | + | + | 2 | |||||
| R677a | none | + | 1 | ||||||
| R682a | none | + | + | + | 3 | ||||
| R683a | none | + | + | 2 | |||||
| R684a | none | + | + | 2 | |||||
| L986b | gag | + | + | 2 | |||||
| R108b | gag | + | + | 2 | |||||
| R677b | gag | + | 1 | ||||||
| R682b | gag | + | + | + | 3 | ||||
| R683b | gag | + | + | 2 | |||||
| R684b | gag | + | + | + | + | 4 | |||
| RH01A | CE+gag | + | + | + | + | + | + | 6 | |
| RHDE8D | CE+gag | + | + | + | + | 4 | |||
| RHDFHW | CE+gag | + | + | + | + | + | + | + | 7 |
| RHDN8W | CE+gag | + | + | + | + | + | + | 6 | |
| RHFLP | CE+gag | + | + | + | + | + | + | 6 | |
| RHOCM | CE+gag | + | + | + | + | + | + | + | 7 |
Analysis of the same animals after CE pDNA prime booster vaccination.
Analysis of the same animals after the gag pDNA booster vaccination.
A large fraction of the CE-specific IFN-γ+ T cells elicited by p27CE pDNA vaccination was cytotoxic (granzyme B+) with a significant population, especially in the CD8+ T cell compartment, able to degranulate (CD107a+) upon TCR engagement by the peptides (Figs. 4D, 4E). The CE-specific cytotoxic T cell responses (granzyme B+) were compared with those induced by the subgroup of gag pDNA vaccinated animals, which showed positive CE responses (Fig. 2A). We found a significant increase (p = 0.018) in the frequency of CE-specific granzyme B+ T cells induced by the p27CE pDNA vaccine (Fig. 4F). We further noted that the gag pDNA vaccine induced a wider range of cytotoxic CE-specific T cells than the p27CE vaccine. Interestingly, the frequency of cytotoxic CE-specific responses correlated (p = 0.002; Supplemental Fig. 2) with the level of the CE-specific CD8+ T cell responses in gag pDNA vaccinated macaques, supporting the notion of an association of CE responses and cytotoxicity. This finding, together with the potent induction of cytotoxic T cell responses in all the p27CE pDNA vaccinated macaques, supports the conclusion that vaccination with p27CE pDNA induces robust CTL responses recognizing subdominant epitopes and elicits T cell responses of higher functionality than a full-length gag pDNA vaccine.
Optimized CE pDNA prime-boost vaccine regimens increase CE immunogenicity
In an effort to increase the potency of CE recognition, two different vaccine regimens were compared using the SIV p27CE pDNA as a prime (Fig. 5): 1) booster vaccination with gag pDNA [by analogy to HIV CE pDNA prime-gag pDNA boost study (21)], and 2) booster vaccination with codelivery of a mixture of CE+gag pDNA.
To test the first concept, six SIV p27CE pDNA primed animals received a booster vaccination with gag pDNA after a 2 mo rest (Fig. 5A), and were analyzed on the day of vaccination, and 2 wk later (Fig. 5B). The gag pDNA booster vaccination increased the magnitude of the CE-specific responses significantly (p = 0.031, paired t test), reaching up to 1.5% IFN-γ+ T cells, maintaining the distribution among the CD4 and CD8 T cell responses induced by CE priming (Fig. 5C). In addition, the magnitude of the CE-specific responses 2 wk after the last prime and after the gag pDNA boost was significantly higher (p = 0.048; paired Wilcoxon t test). These data recapitulate the augmentation of HIV p24CE primed T cell responses upon a boost with HIV gag pDNA expressing the full-length protein, and reinforce that the immunodominance hierarchy could be reproducibly altered by CE priming. Overall, SIV CE prime-gag pDNA booster vaccination data are similar to our observations from the HIV p24CE prime-gag pDNA boost studies (21).
To test the second vaccine concept, a group of six macaques received a single SIV p27CE pDNA prime followed by three booster vaccinations using codelivery of a mixture of p27CE+gag pDNA (Fig. 5D). Each CE+gag pDNA booster vaccination elicited strong CE-specific responses with median values of 0.2, 0.9, and 0.8% IFN-γ+ T cells, respectively (Fig. 5E). A significant increase in the magnitude of CE-specific T cell responses was found upon the second booster vaccination but no further increase was observed upon the third boost, demonstrating that the inclusion of two CE+gag pDNA booster vaccinations was sufficient to maximize the magnitude of the primed CE-specific responses using this vaccine regimen. The robust responses included CE-specific CD4+ and CD8+ T cells, reaching up to ∼1.8% IFN-γ+ T cells (Fig. 5F). A similar magnitude of the total CE responses was induced by the CE+gag pDNA boost (Fig. 5F) and the gag pDNA boost (Fig. 5C). The high frequency of CE-specific cytotoxic (granzyme B+) T cells measured upon priming with p27CE pDNA was maintained by both booster vaccine regimens.
Together, these data demonstrate that both booster vaccinations (gag pDNA or codelivery of CE+gag pDNA) significantly increased CE-specific T cell responses, and both vaccine regimens effectively induced immune responses to subdominant Gag epitopes.
CE+gag pDNA codelivery as booster vaccination induced T cell responses with greater breadth and cytotoxicity
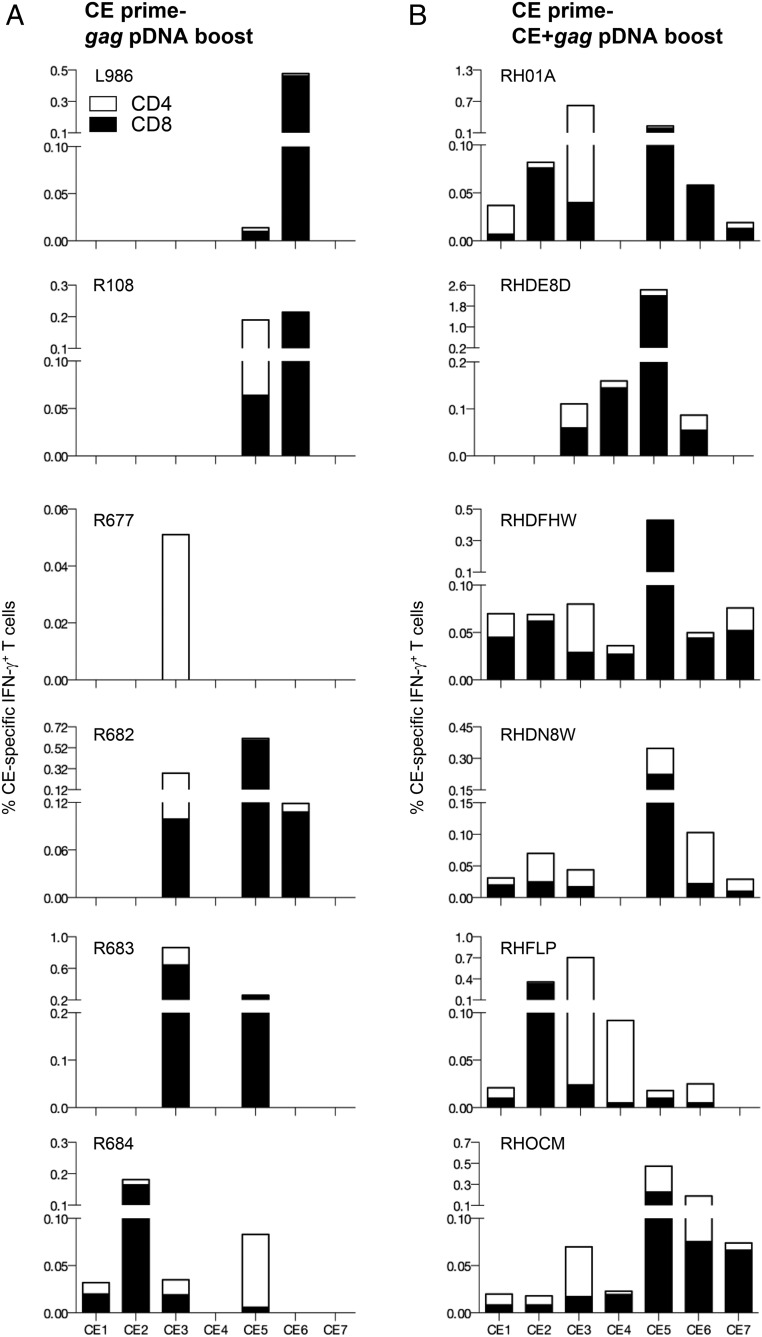
The breadth of CE-specific immunity induced by the two vaccine regimens was analyzed (Fig. 6, Table II) upon stimulation of PBMC with peptide subpools specific for each of the seven individual CE and intracellular cytokine staining followed by flow cytometry. The six animals that received the gag pDNA booster vaccination (Fig. 6A) showed a response breadth similar to animals only receiving a p27CE pDNA prime, with a range of one to four CE per animal (Table II). Thus, a single booster vaccination with gag pDNA did not further increase the breadth of the responses induced by the CE priming (Fig. 7A). An additional gag pDNA booster vaccination in two of the animals produced similar results (data not shown).
FIGURE 6.
Mapping of responses to individual CE in macaques vaccinated with two different prime-boost vaccine regimens. Comparison of the responses to individual CE in macaques that received CE prime followed by either gag pDNA or p27CE+gag pDNA booster vaccination. PBMC were stimulated using peptide pools covering each individual CE (CE1–CE7). The percentages of IFN-γ+ CD4+ (open bars) and CD8+ (filled bars) T cells specific each individual CE 2 wk after the last vaccination are shown. (A) Mapping of the animals boosted with gag pDNA shows a response range of one to four CE (median two CE per animal; Table II). (B) Mapping of the animals boosted with the codelivery of p27CE+gag pDNA shows a response range of four to seven CE (median six CE per animal; Table II).
FIGURE 7.
Comparison of CE response breadth induced by the different SIV p27CE pDNA vaccine regimens. (A) The plot shows the number of CE recognized by each macaque immunized with the different p27CE pDNA vaccine regimens: p27CE pDNA only, or prime-boost regimens including gag pDNA and p27CE+gag pDNA booster vaccinations. The median number of recognized CE, the range of CE responses, and the number of analyzed animals are indicated. The p values are from ANOVA (Dunnett’s test). (B) Comparison of the breadth of the responses induced by the CE prime/gag pDNA prime-boost (blue) and CE prime/CE+gag pDNA prime-boost (orange) vaccine regimen. The bars show the percentage of the vaccinated animals recognizing each specific CE.
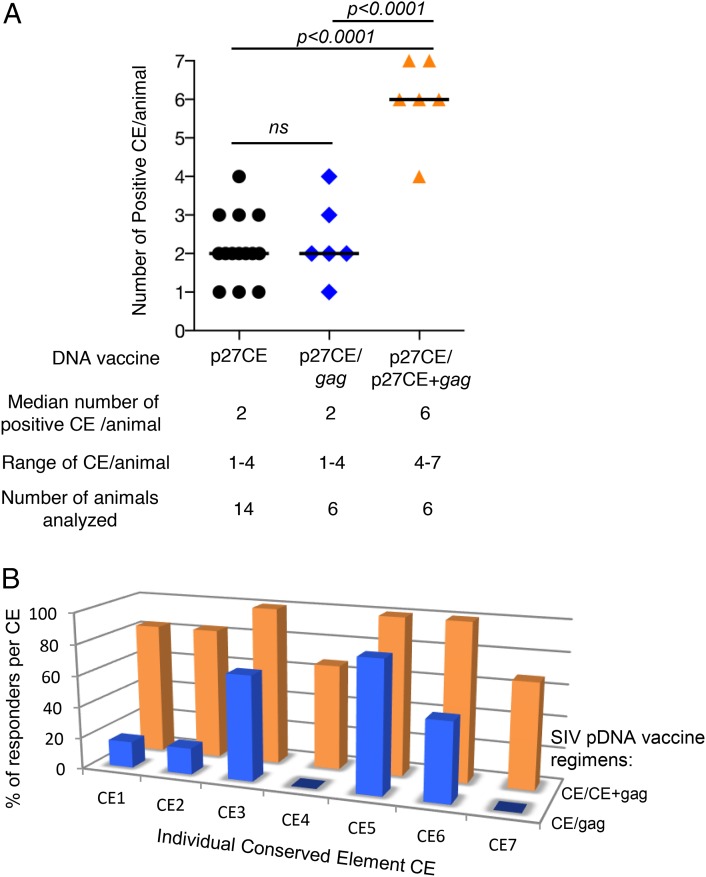
The breadth of the CE cellular responses was also examined in the six animals that received a codelivery of CE+gag pDNA as booster vaccination (Fig. 6B). Interestingly, this regimen significantly expanded CE-specific cellular responses from one to four CE (gag pDNA boost) to four to seven CE (p27CE+gag pDNA) per animal (Fig. 7A, Table II). Although the magnitude of the CE-specific responses in both booster vaccine regimens was similar, analysis of the responses to individual CE revealed a significant difference in the breadth induced by these two vaccine regimens (Fig. 7). Analysis of the responders per CE (Fig. 7B) showed that the CE+gag pDNA boost regimen induced greater responses recognizing all CE for >60% of the animals tested. Thus, the CE pDNA priming vaccine is critical to efficiently induce potent CTL responses to otherwise subdominant epitopes, and codelivery of CE+gag pDNA as booster vaccination is the most effective protocol to induce the broadest cellular immunity targeting the SIV Gag CE, with all seven CE being recognized (Fig. 7B).
Ag-specific T cells elicited by the CE+gag pDNA codelivery as booster vaccination are functional and inhibit SIV infection in vitro
To analyze the functional properties of the T cells targeting the conserved epitopes encoded by the CE pDNA vaccine, PBMC samples from the six macaques boosted with the CE+gag DNA plasmid mixture were monitored using flow cytometry for granzyme B content and their capability of degranulating upon specific TCR stimulation with a pool of CE-specific peptides. Fig. 8A shows two representative animals. We found that the CE-specific T cells from all immunized animals had high levels (range and median for both markers) of granzyme B and actively degranulated (CD107a+), which suggest that the CE-specific T cells induced by the vaccination regimen are actively cytotoxic.
PBMC were available only from two macaques and were used to perform in vitro virus inhibition assays. Autologous CD8-depleted PBMC were used as targets for infection using a stock of SIVmac239. Purified CD8+ cells were used as effectors at different effector to target (E:T) ratios, and p27Gag accumulation in culture supernatant was monitored by ELISA at 7 d postinfection. We found a ∼60% reduction of viral infection at the optimal E:T ratio for both animals compared with the control samples cultured with a similar ratio of CD8+ cells from prevaccination samples (Fig. 8B). The inhibition mediated by the CE-specific CD8+ cells was further confirmed by the detection of SIV-infected cells by intracellular staining with an anti-p27Gag Ab. We found a ∼90% dose-dependent reduction in the number of infected cells (Fig. 8C) as well as a decrease in the mean fluorescent intensity in the signal provided by the anti-p27Gag Ab.
We further compared the cytotoxicity upon CE pDNA prime and the gag pDNA boost in one animal (macaque R682) for which samples were available (Fig. 8D). We found a ∼50% inhibition after the prime compared with ∼70% after the gag pDNA boost (E:T 0.5:1 and 1:1). Thus, the gag pDNA boost increased the virus inhibition capability in agreement with the increased cytotoxic T cell response. Together, these data show that the CE-specific CD8+ cells can both reduce viral replication (p27Gag in supernatant) and prevent accumulation of infected cells (intracellular staining), which, as a result of the antiviral effects mediated by the vaccine induced CD8+ T cells, release lower amounts of virions. Collectively, these data demonstrate that the CE pDNA vaccine elicits functionally relevant Ag-specific T cells that efficiently contribute to reduce SIV infection.
HIV CE+gag pDNA codelivery as booster vaccination increases the breadth of CE-specific T cell responses
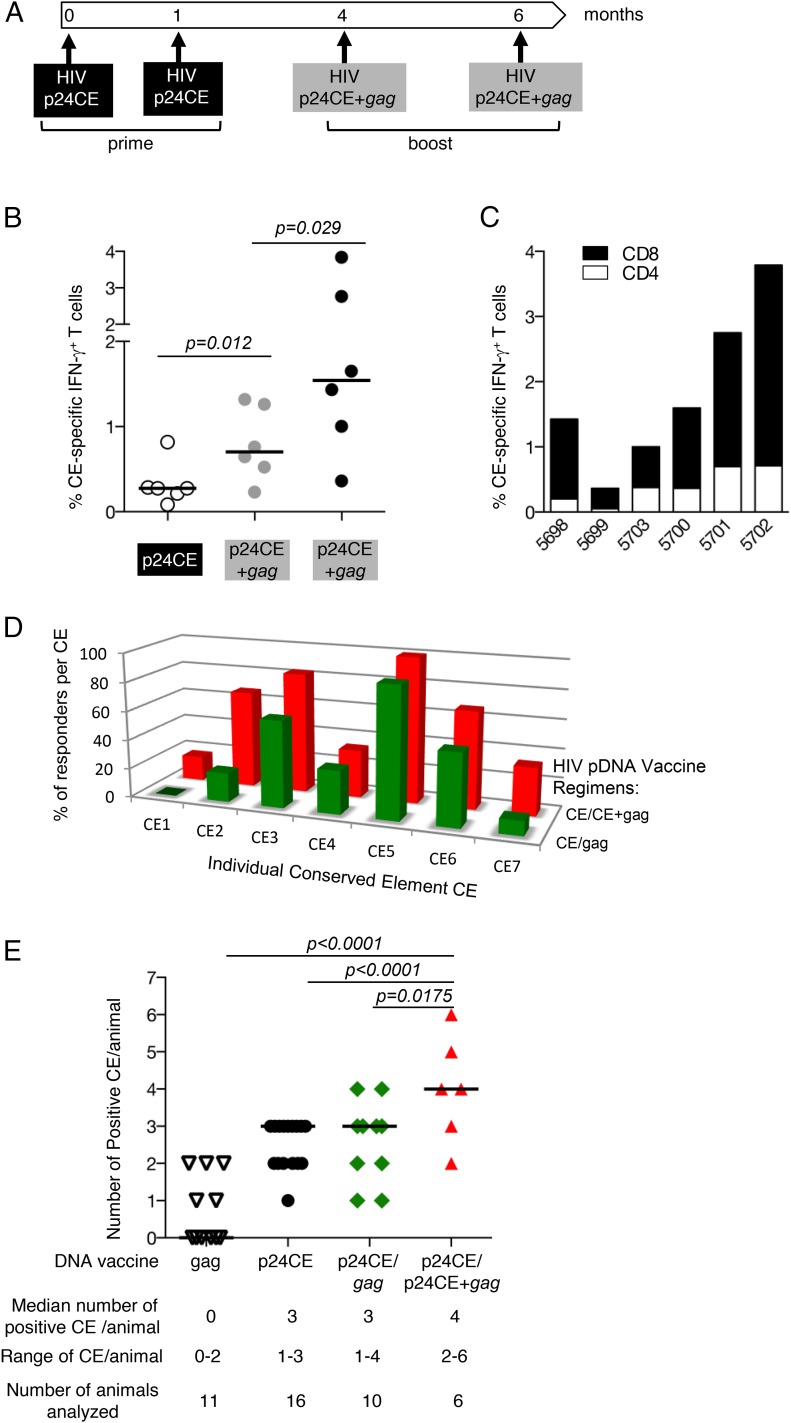
A follow-up study using the analogous molecules from HIV was modeled after SIV CE prime and CE+gag pDNA booster vaccine regimen. A group of six macaques received two priming vaccinations (at 0 and 1 mo) with the dual expression vector p24CE1/2, expressing the HIV CE immunogens p24CE1 and p24CE2, followed by two booster vaccinations (at months 4 and 6) with codelivery of p24CE1/2+p55gag pDNA (Fig. 9A). Priming with p24CE1/2 induced robust CE-specific responses (median 0.3% IFN-γ+ T cells), which were significantly increased by the two HIV CE+p55gag pDNA booster vaccinations (median 0.7 and 1.5% IFN-γ+ T cells, respectively), reaching up to ∼4% IFN-γ+ CE-specific T cells (Fig. 9B). The CE-specific responses were mediated both by CD4+ and CD8+ T cells with a skewing toward CD8+ T cell responses (Fig. 9C). This vaccination regimen also induced highly cytotoxic CE-specific T cells with a frequency of >89% being granzyme B+. Furthermore, the CE+gag pDNA boost induced broader responses compared with the gag pDNA boost resulting in the recognition of all individual CE (Fig. 9D, Table III). The breadth of the responses, measured by the number of CE recognized per animal, was compared among groups that received different vaccines (21), including full-length gag only, CE pDNA only, CE/gag pDNA prime-boost vaccine, and CE/CE+gag pDNA prime-boost vaccine (Fig. 9E). The HIV CE+gag pDNA booster vaccination induced responses with significantly increased breadth (two to six CE per animal) compared with CE only or CE/gag pDNA vaccines (one to three or one to four CE per animal, with no difference among these groups). These data parallel those described above for SIV (Fig. 7). All CE-based vaccine regimens induced broader responses than the HIV p55Gag pDNA only vaccine that induced zero to two CE per animal (Fig. 9E).
FIGURE 9.
Increased breadth of HIV CE using codelivery of CE+gag pDNA booster vaccination regimen. (A) The cartoon depicts the HIV CE pDNA prime/CE+gag pDNA booster vaccination regimen. Cellular immune responses were measured 2 wk after each vaccination. (B) The frequency of the HIV CE-specific IFN-γ+ T cell responses was measured after CE pDNA prime and after each CE+gag pDNA boost. The p values are from ANOVA. (C) The frequency of HIV CE-specific IFN-γ+ T cell responses is shown for CD4+ (open bars) and CD8+ (filled bars) T cells after the second CE+gag pDNA booster vaccination. (D) Comparison of the response breadth induced by the CE prime/gag pDNA prime-boost [data reported in Kulkarni et al. (21)] (green) and CE prime/CE+gag pDNA prime-boost (red). The bars show the percentage of the vaccinated animals recognizing each specific CE. (E) Plot shows the number of CE recognized by each macaque immunized with HIV CE pDNA prime/CE+gag pDNA booster vaccination. The median number CE recognized, range of CE responses, and number of animals analyzed are indicated. The data for animals that received HIV CE pDNA only, HIV CE prime/gag pDNA booster vaccination or HIV gag pDNA were reported previously (21). The p values are from ANOVA (Dunnett’s test).
Table III. Comparison of HIV CE-specific T Cell response breadth induced by gag pDNA and CE+gag pDNA booster vaccination regimens.
| p24CE pDNA Prime | pDNA Booster Vaccine | Responses to Individual CE | No. of Positive CE per Animal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CE1 | CE2 | CE3 | CE4 | CE5 | CE6 | CE7 | |||
| L862a | gag | + | + | + | + | 4 | |||
| M166a | gag | + | + | + | 3 | ||||
| M695a | gag | + | + | 2 | |||||
| R279a | gag | + | + | + | + | 4 | |||
| P314a | gag | + | + | + | 3 | ||||
| P302a | gag | + | + | + | 3 | ||||
| P307a | gag | + | + | + | 3 | ||||
| P308a | gag | + | 1 | ||||||
| R315a | gag | + | + | 2 | |||||
| M437a | gag | + | 1 | ||||||
| 5698 | CE+gag | + | + | + | 3 | ||||
| 5699 | CE+gag | + | + | 2 | |||||
| 5700 | CE+gag | + | + | + | + | 4 | |||
| 5701 | CE+gag | + | + | + | + | + | + | 6 | |
| 5702 | CE+gag | + | + | + | + | + | 5 | ||
| 5703 | CE+gag | + | + | + | + | 4 | |||
Analysis of macaques vaccinated with p24CE prime/p55gag pDNA boost from Kulkarni et al. (21).
Discussion
The current study shows that priming with CE pDNA is critical to induce immune responses to subdominant epitopes, and inclusion of the CE pDNA together with a plasmid expressing the full-length immunogen is the most effective protocol to induce desirable responses including greatest breadth, magnitude and cytotoxic capability. In SIV (as shown in this report) and HIV (21) gag pDNA vaccinated macaques, only ∼50% of the animals developed T cell responses recognizing the highly conserved CE in the p27Gag/p24Gag core Ag, suggestive of immune interference by the more variable epitopes. Interestingly, macaques that developed CE-specific T cell immunity showed more potent CTL responses, suggesting a link between CE epitope recognition and cytotoxic function. To overcome this limitation, we generated novel immunogens derived from SIV Gag protein by analogy to our reported HIV conserved element DNA vaccine. Recapitulating our work with HIV (21), we found that all macaques primed with SIV p27CE pDNA developed robust Ag-specific responses with even higher cytotoxicity than those induced in the subset of CE-reactive gag pDNA vaccinated animals. We previously hypothesized that processing and presentation of Gag peptides representing conserved sequences is not impaired, but their ability to induce de novo responses is negatively affected in the presence of Gag peptides from variable regions (immunodominant decoys). This model is supported by the findings that HIV CE-specific responses could be increased by a gag pDNA booster vaccination and is recapitulated here using SIV homologs. Thus, data from both HIV and SIV vaccine regimens strongly support our hypothesis of impaired immunogenicity of CE-containing peptides when produced from the intact molecules, apparently due to a negative interference by peptides outside of the CE that affect the development of CE-specific T cell responses. In stark contrast, priming with pDNA expressing only CE immunogens allows robust induction of these otherwise subdominant responses, and this is critical for achieving the broadest CE responses.
CE responses can be significantly increased by a gag pDNA booster vaccination. This demonstrates that CE-containing peptides are presented but are unable to efficiently induce de novo responses recognizing these subdominant regions, yet they can potently augment pre-existing, otherwise subdominant responses. Hence, immunity to subdominant epitopes enables a shift in immunodominance hierarchy, resulting in improved development and broadening of subdominant responses.
The finding that the codelivery of CE+gag pDNA as booster vaccination not only increased the magnitude but also the breadth of the CE-primed responses was unexpected. We previously reported that the combination of HIV CE+gag DNA as prime and boost did not increase the breadth of the CE response (21). Thus, increased breadth is only found when CE+gag DNA is used as booster vaccine in CE-primed animals. Although the underlying mechanism is still unclear, we can exclude that this is due to additional vaccinations with CE pDNA, because we found maximal responses using both HIV and SIV pDNA vaccines was typically reached with two vaccinations. We hypothesize that the numerical increase of CE peptides (produced by processing of CE and Gag in the vaccine) on APC may contribute to this. Others reported increased epitope breadth in humans using different vaccine regimens (46, 47). A different vaccine design that also included prime/boost regimens combining DNA and viral vectors, expanded T cell coverage to more conserved epitopes of the HIV proteome and these CTL also efficiently reduced viral infection in vitro (46). Others reported that recombinant adenovirus vectors expressing HIV env immunogens encoding heterologous env sequences elicited T cell responses with broader epitope coverage than using homologous sequences (47). In our study, we found that the vaccine–induced CE-specific T cells are able to reduce viral infection in an autologous in vitro viral inhibition assay, an important feature that has been shown to correlate with in vivo viral control in infected animals (17). Importantly, the successful induction of T cell responses with superior breadth was achieved in our studies by both SIV and HIV CE derived gag pDNA vaccines, however, priming with CE pDNA appeared to be critical for the improved breadth. This regimen is now being further developed in a clinical trial.
An important finding of this work is that CE pDNA induced T cell responses are also highly cytotoxic. Indeed, analysis of the T cell responses induced by vaccination with pDNA expressing intact SIV Gag showed that the subgroup of animals that developed CE-specific responses shows a significantly higher frequency of Gag-specific cytotoxic T cells than the subgroup that developed responses targeting Gag epitopes distinct from the CE. Thus, CE DNA provides a critical contribution to the development of potent vaccine-induced cellular immune responses with efficient induction of CTL responses. In this context, a recent report analyzing respiratory syncytial virus-mediated control of infection alluded to the fact that subdominant CD8 T cell responses were more potent in controlling viremia (48). This work highlighted the importance of designing a vaccine to induce highly efficient and functional T cells, which may be triggered by subdominant T cell epitopes. Our report provides a method for the efficient induction of cytotoxic T cell responses targeting conserved, subdominant epitopes within HIV/SIV Gag with possible application as a preventive and therapeutic vaccine. The efficacy of this improved SIV vaccine regimen to reduce viremia is being tested using a nonhuman primate challenge model.
The vaccine described in this work uses pDNA, a promising platform due to its simplicity, scalability, and possibility for repeated applications due to the lack of immunity against the vector. It can be used as a stand-alone platform or it can be combined with other vaccine modalities (viral vectors, protein), transfer of bNab, and molecular adjuvants (for reviews see 49–53). We have demonstrated that HIV/SIV pDNA vaccines induce responses, including CE-induced (X. Hu, A. Valentin, and B.K. Felber, unpublished observations) responses, which efficiently disseminate to mucosal tissues (39, 54). In addition, the recent demonstration of therapeutic efficacy of a human papilloma virus vaccine delivered by DNA or electroporation in a Phase IIB randomized study in humans (55–57) demonstrates the efficacy of a pDNA platform in inducing mucosal T cell responses in humans. This offers the possibility of using a pDNA vaccination targeting the CE in a therapeutic setting against HIV infection, because this vaccine regimen induces potent cytotoxic T cell responses targeting the presumed weakness of the virus. Therapeutic vaccination by this method could induce novel responses unlikely to be overcome by viral mutation, and has the potential to provide an additional critical step toward reduction of the long-term viral reservoir. We previously reported that therapeutic SIV pDNA vaccination induces potent immune responses (as high as ∼10% of T cells in blood) able to reduce viremia by 1 log following interruption of ART in SIVmac251-infected Indian rhesus macaques (58, 59). Importantly, some animals maintained reduced viral loads for more than 3 y, indicating there is a long-lasting effect of the vaccine-induced T cell responses, another hallmark of the pDNA platform (32, 54). Together, these data provide a proof-of-concept in support of adding pDNA vaccination, and in particular CE followed by CE+gag pDNA booster vaccination to cART regimens to augment potent virus-specific immune responses.
In conclusion, our data demonstrate that priming vaccination with CE pDNA is critical to induce responses to subdominant epitopes with superior cytotoxic potential, and that codelivery of CE+gag DNA as booster vaccination result in maximal breadth of CE-specific responses. This work describes an effective vaccine regimen that focuses the priming response to subdominant epitopes and includes a combination of subdominant and redirected dominant epitopes in the booster vaccination to achieve maximal magnitude and breadth. Such vaccine regimens may be of general application, not limited to HIV.
Supplementary Material
Acknowledgments
We thank D. Weiss, J. Treece, H. Anderson, and the staff at Bioqual, as well as J. Mendoza and L. Humeau at Inovio for excellent support and discussions; N. Miller and staff at the Division of AIDS, National Institutes of Health, for macaques; C. Bergamaschi for critical reading of the manuscript; and T. Jones for editorial assistance. The following reagents were obtained through the AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health: anti-SIVmac251 p27 mAb (KK64) from Dr. Karen Kent and Caroline Powell and SIVmac p27 mAb (55-2F12) from Dr. Niels Pedersen.
This work was supported by the Intramural Research Program of the National Cancer Institute (to G.N.P. and B.K.F.), National Institute of Allergy and Infectious Disease, National Institutes of Health (NIAID/NIH), Grant P01 AI057005 (to M.A.M.), and the University of Washington Centers for AIDS Research Molecular Profiling Computational Biology Core (NIH Grant P30 AI27757; to J.I.M.). This work also was supported in part by the NIAID/NIH Division of AIDS under HIV Vaccine Design and Development Teams Contract HHSN272200800063C (to N.Y.S.).
The online version of this article contains supplemental material.
- BGH
- bovine growth hormone
- CE
- conserved element
- pDNA
- plasmid DNA.
Disclosures
G.N.P. and B.K.F. are the inventors on U.S. government–owned patents related to DNA vaccines and gene expression optimization. G.N.P., B.K.F., A.V., and J.I.M are the inventors on U.S. government– and Washington University–co-owned patent applications on Conserved Element technology. K.E.B. and N.Y.S. are full-time employees of Inovio Pharmaceuticals and, as such, receive compensation in the form of salary and stock options. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The other authors have no financial conflicts of interest.
References
- 1.Nickle D. C., Rolland M., Jensen M. A., Pond S. L., Deng W., Seligman M., Heckerman D., Mullins J. I., Jojic N. 2007. Coping with viral diversity in HIV vaccine design. PLoS Comput. Biol. 3: e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nickle D. C., Jojic N., Heckerman D., Jojic V., Kirovski D., Rolland M., Kosakovsky Pond S., Mullins J. I. 2008. Comparison of immunogen designs that optimize peptide coverage: reply to Fischer et al. [Published erratum appears in 2008 PLoS Comput. Biol. 4.] PLoS Comput. Biol. 4: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch D. H., O’Brien K. L., Simmons N. L., King S. L., Abbink P., Maxfield L. F., Sun Y. H., La Porte A., Riggs A. M., Lynch D. M., et al. 2010. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat. Med. 16: 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santra S., Liao H. X., Zhang R., Muldoon M., Watson S., Fischer W., Theiler J., Szinger J., Balachandran H., Buzby A., et al. 2010. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat. Med. 16: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer W., Perkins S., Theiler J., Bhattacharya T., Yusim K., Funkhouser R., Kuiken C., Haynes B., Letvin N. L., Walker B. D., et al. 2007. Polyvalent vaccines for optimal coverage of potential T cell epitopes in global HIV-1 variants. Nat. Med. 13: 100–106. [DOI] [PubMed] [Google Scholar]
- 6.Fischer, W., H. X. Liao, B. F. Haynes, N. L. Letvin, and B. Korber. 2008. Coping with viral diversity in HIV vaccine design: a response to Nickle et al. PLoS Comp. Biol. 4: e15; author reply e25. [DOI] [PMC free article] [PubMed]
- 7.Doria-Rose N. A., Learn G. H., Rodrigo A. G., Nickle D. C., Li F., Mahalanabis M., Hensel M. T., McLaughlin S., Edmonson P. F., Montefiori D., et al. 2005. Human immunodeficiency virus type 1 subtype B ancestral envelope protein is functional and elicits neutralizing antibodies in rabbits similar to those elicited by a circulating subtype B envelope. J. Virol. 79: 11214–11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullins J. I., Nickle D. C., Heath L., Rodrigo A. G., Learn G. H. 2004. Immunogen sequence: the fourth tier of AIDS vaccine design. Expert Rev. Vaccines 3(Suppl.): S151–S159. [DOI] [PubMed] [Google Scholar]
- 9.Nickle D. C., Jensen M. A., Gottlieb G. S., Shriner D., Learn G. H., Rodrigo A. G., Mullins J. I. 2003. Consensus and ancestral state HIV vaccines. Science 299: 1515–1518, author reply 1515–1518. [DOI] [PubMed] [Google Scholar]
- 10.Dahirel V., Shekhar K., Pereyra F., Miura T., Artyomov M., Talsania S., Allen T. M., Altfeld M., Carrington M., Irvine D. J., et al. 2011. Coordinate linkage of HIV evolution reveals regions of immunological vulnerability. Proc. Natl. Acad. Sci. USA 108: 11530–11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Létourneau S., Im E. J., Mashishi T., Brereton C., Bridgeman A., Yang H., Dorrell L., Dong T., Korber B., McMichael A. J., Hanke T. 2007. Design and pre-clinical evaluation of a universal HIV-1 vaccine. [Published erratum appears in 2011 PLoS One 6.] PLoS One 2: e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosario M., Bridgeman A., Quakkelaar E. D., Quigley M. F., Hill B. J., Knudsen M. L., Ammendola V., Ljungberg K., Borthwick N., Im E. J., et al. 2010. Long peptides induce polyfunctional T cells against conserved regions of HIV-1 with superior breadth to single-gene vaccines in macaques. Eur. J. Immunol. 40: 1973–1984. [DOI] [PubMed] [Google Scholar]
- 13.De Groot A. S., Rivera D. S., McMurry J. A., Buus S., Martin W. 2008. Identification of immunogenic HLA-B7 “Achilles’ heel” epitopes within highly conserved regions of HIV. Vaccine 26: 3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson C. C., McKinney D., Anders M., MaWhinney S., Forster J., Crimi C., Southwood S., Sette A., Chesnut R., Newman M. J., Livingston B. D. 2003. Development of a DNA vaccine designed to induce cytotoxic T lymphocyte responses to multiple conserved epitopes in HIV-1. J. Immunol. 171: 5611–5623. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman D. R., Li F., Cruz A. N., Self S. G., Barouch D. H. 2012. Focus and breadth of cellular immune responses elicited by a heterologous insert prime-boost vaccine regimen in rhesus monkeys. Vaccine 30: 506–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida R. R., Rosa D. S., Ribeiro S. P., Santana V. C., Kallás E. G., Sidney J., Sette A., Kalil J., Cunha-Neto E. 2012. Broad and cross-clade CD4+ T cell responses elicited by a DNA vaccine encoding highly conserved and promiscuous HIV-1 M-group consensus peptides. PLoS One 7: e45267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson K. E., SanMiguel A., Simmons N. L., Smith K., Lewis M. G., Szinger J. J., Korber B., Barouch D. H. 2012. Full-length HIV-1 immunogens induce greater magnitude and comparable breadth of T lymphocyte responses to conserved HIV-1 regions compared with conserved-region-only HIV-1 immunogens in rhesus monkeys. J. Virol. 86: 11434–11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ondondo B., Murakoshi H., Clutton G., Abdul-Jawad S., Wee E. G., Gatanaga H., Oka S., McMichael A. J., Takiguchi M., Korber B., Hanke T. 2016. Novel conserved-region T cell mosaic vaccine with high global HIV-1 coverage is recognized by protective responses in untreated infection. Mol. Ther. 24: 832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mothe B., Llano A., Ibarrondo J., Zamarreño J., Schiaulini M., Miranda C., Ruiz-Riol M., Berger C. T., Herrero M. J., Palou E., et al. 2012. CTL responses of high functional avidity and broad variant cross-reactivity are associated with HIV control. PLoS One 7: e29717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni V., Rosati M., Valentin A., Ganneru B., Singh A. K., Yan J., Rolland M., Alicea C., Beach R. K., Zhang G. M., et al. 2013. HIV-1 p24(gag) derived conserved element DNA vaccine increases the breadth of immune response in mice. PLoS One 8: e60245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni V., Valentin A., Rosati M., Alicea C., Singh A. K., Jalah R., Broderick K. E., Sardesai N. Y., Le Gall S., Mothe B., et al. 2014. Altered response hierarchy and increased T cell breadth upon HIV-1 conserved element DNA vaccination in macaques. [Published erratum appears in 2014 PLoS One 9:e103198.] PLoS One 9: e86254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni V., Valentin A., Rosati M., Rolland M., Mullins J. I., Pavlakis G. N., Felber B. K. 2014. HIV-1 conserved elements p24CE DNA vaccine induces humoral immune responses with broad epitope recognition in macaques. PLoS One 9: e111085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolland M., Heckerman D., Deng W., Rousseau C. M., Coovadia H., Bishop K., Goulder P. J., Walker B. D., Brander C., Mullins J. I. 2008. Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads. PLoS One 3: e1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolland M., Manocheewa S., Swain J. V., Lanxon-Cookson E. C., Kim M., Westfall D. H., Larsen B. B., Gilbert P. B., Mullins J. I. 2013. HIV-1 conserved-element vaccines: relationship between sequence conservation and replicative capacity. J. Virol. 87: 5461–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolland M., Nickle D. C., Mullins J. I. 2007. HIV-1 group M conserved elements vaccine. PLoS Pathog. 3: e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuñiga R., Lucchetti A., Galvan P., Sanchez S., Sanchez C., Hernandez A., Sanchez H., Frahm N., Linde C. H., Hewitt H. S., et al. 2006. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J. Virol. 80: 3122–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masemola A., Mashishi T., Khoury G., Mohube P., Mokgotho P., Vardas E., Colvin M., Zijenah L., Katzenstein D., Musonda R., et al. HIVNET 028 Study Team 2004. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J. Virol. 78: 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiepiela P., Ngumbela K., Thobakgale C., Ramduth D., Honeyborne I., Moodley E., Reddy S., de Pierres C., Mncube Z., Mkhwanazi N., et al. 2007. CD8+ T cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13: 46–53. [DOI] [PubMed] [Google Scholar]
- 29.Honeyborne I., Prendergast A., Pereyra F., Leslie A., Crawford H., Payne R., Reddy S., Bishop K., Moodley E., Nair K., et al. 2007. Control of human immunodeficiency virus type 1 is associated with HLA-B*13 and targeting of multiple gag-specific CD8+ T cell epitopes. J. Virol. 81: 3667–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneidewind A., Brockman M. A., Sidney J., Wang Y. E., Chen H., Suscovich T. J., Li B., Adam R. I., Allgaier R. L., Mothé B. R., et al. 2008. Structural and functional constraints limit options for cytotoxic T lymphocyte escape in the immunodominant HLA-B27-restricted epitope in human immunodeficiency virus type 1 capsid. J. Virol. 82: 5594–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndhlovu Z. M., Stampouloglou E., Cesa K., Mavrothalassitis O., Alvino D. M., Li J. Z., Wilton S., Karel D., Piechocka-Trocha A., Chen H., et al. 2015. The breadth of expandable memory CD8+ T cells inversely correlates with residual viral loads in HIV elite controllers. J. Virol. 89: 10735–10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel V., Jalah R., Kulkarni V., Valentin A., Rosati M., Alicea C., von Gegerfelt A., Huang W., Guan Y., Keele B., et al. 2013. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous SIV challenge. Proc. Natl. Acad. Sci. USA 110: 2975–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schell J. B., Bahl K., Folta-Stogniew E., Rose N., Buonocore L., Marx P. A., Gambhira R., Rose J. K. 2015. Antigenic requirement for Gag in a vaccine that protects against high-dose mucosal challenge with simian immunodeficiency virus. Virology 476: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schell J. B., Rose N. F., Bahl K., Diller K., Buonocore L., Hunter M., Marx P. A., Gambhira R., Tang H., Montefiori D. C., et al. 2011. Significant protection against high-dose simian immunodeficiency virus challenge conferred by a new prime-boost vaccine regimen. J. Virol. 85: 5764–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen S. G., Piatak M., Jr., Ventura A. B., Hughes C. M., Gilbride R. M., Ford J. C., Oswald K., Shoemaker R., Li Y., Lewis M. S., et al. 2013. Immune clearance of highly pathogenic SIV infection. Nature 502: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen S. G., Wu H. L., Burwitz B. J., Hughes C. M., Hammond K. B., Ventura A. B., Reed J. S., Gilbride R. M., Ainslie E., Morrow D. W., et al. 2016. Broadly targeted CD8⁺ T cell responses restricted by major histocompatibility complex E. Science 351: 714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosati M., von Gegerfelt A., Roth P., Alicea C., Valentin A., Robert-Guroff M., Venzon D., Montefiori D. C., Markham P., Felber B. K., Pavlakis G. N. 2005. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J. Virol. 79: 8480–8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kent K. A., Gritz L., Stallard G., Cranage M. P., Collignon C., Corcoran T., Silvera P., Stott E. J. 1991. Production and of monoclonal antibodies to simian immunodeficiency virus envelope glycoproteins. AIDS 5: 829–836. [DOI] [PubMed] [Google Scholar]
- 39.Valentin A., McKinnon K., Li J., Rosati M., Kulkarni V., Pilkington G. R., Bear J., Alicea C., Vargas-Inchaustegui D. A., Jean Patterson L., et al. 2014. Comparative analysis of SIV-specific cellular immune responses induced by different vaccine platforms in rhesus macaques. Clin. Immunol. 155: 91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosati M., Alicea C., Kulkarni V., Virnik K., Hockenbury M., Sardesai N. Y., Pavlakis G. N., Valentin A., Berkower I., Felber B. K. 2015. Recombinant rubella vectors elicit SIV Gag-specific T cell responses with cytotoxic potential in rhesus macaques. Vaccine 33: 2167–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jalah R., Patel V., Kulkarni V., Rosati M., Alicea C., Ganneru B., von Gegerfelt A., Huang W., Guan Y., Broderick K. E., et al. 2012. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum. Vaccin. Immunother. 8: 1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mothe B., Hu X., Llano A., Rosati M., Olvera A., Kulkarni V., Valentin A., Alicea C., Pilkington G. R., Sardesai N. Y., et al. 2015. A human immune data-informed vaccine concept elicits strong and broad T cell specificities associated with HIV-1 control in mice and macaques. J. Transl. Med. 13: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martins M. A., Wilson N. A., Reed J. S., Ahn C. D., Klimentidis Y. C., Allison D. B., Watkins D. I. 2010. T cell correlates of vaccine efficacy after a heterologous simian immunodeficiency virus challenge. J. Virol. 84: 4352–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins J. R., Sutjipto S., Marx P. A., Pedersen N. C. 1992. Shared antigenic epitopes of the major core proteins of human and simian immunodeficiency virus isolates. J. Med. Primatol. 21: 265–269. [PubMed] [Google Scholar]
- 45.Mansfield K. G., Lerch N. W., Gardner M. B., Lackner A. A. 1995. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J. Med. Primatol. 24: 116–122. [DOI] [PubMed] [Google Scholar]
- 46.Borthwick N., Ahmed T., Ondondo B., Hayes P., Rose A., Ebrahimsa U., Hayton E. J., Black A., Bridgeman A., Rosario M., et al. 2014. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol. Ther. 22: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh S. R., Moodie Z., Fiore-Gartland A. J., Morgan C., Wilck M. B., Hammer S. M., Buchbinder S. P., Kalams S. A., Goepfert P. A., Mulligan M. J., et al. HVTN 083 Study Group and the NIAID HVTN 2016. Vaccination with heterologous HIV-1 envelope sequences and heterologous adenovirus vectors increases T cell responses to conserved regions: HVTN 083. J. Infect. Dis. 213: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J., Haddad E. K., Marceau J., Morabito K. M., Rao S. S., Filali-Mouhim A., Sekaly R. P., Graham B. S. 2016. A numerically subdominant CD8 T cell response to matrix protein of respiratory syncytial virus controls infection with limited immunopathology. PLoS Pathog. 12: e1005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salonius K., Simard N., Harland R., Ulmer J. B. 2007. The road to licensure of a DNA vaccine. Curr. Opin. Investig. Drugs 8: 635–641. [PubMed] [Google Scholar]
- 50.Ferraro B., Morrow M. P., Hutnick N. A., Shin T. H., Lucke C. E., Weiner D. B. 2011. Clinical applications of DNA vaccines: current progress. Clin. Infect. Dis. 53: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulmer J. B., Wahren B., Liu M. A. 2006. DNA vaccines for HIV/AIDS. Curr. Opin. HIV AIDS 1: 309–313. [DOI] [PubMed] [Google Scholar]
- 52.Felber B. K., Valentin A., Rosati M., Bergamaschi C., Pavlakis G. N. 2014. HIV DNA vaccine: stepwise improvements make a difference. Vaccines (Basel) 2: 354–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flingai S., Czerwonko M., Goodman J., Kudchodkar S. B., Muthumani K., Weiner D. B. 2013. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front. Immunol. 4: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel V., Valentin A., Kulkarni V., Rosati M., Bergamaschi C., Jalah R., Alicea C., Minang J. T., Trivett M. T., Ohlen C., et al. 2010. Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine 28: 4827–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bagarazzi M. L., Yan J., Morrow M. P., Shen X., Parker R. L., Lee J. C., Giffear M., Pankhong P., Khan A. S., Broderick K. E., et al. 2012. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 4: 155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trimble C. L., Morrow M. P., Kraynyak K. A., Shen X., Dallas M., Yan J., Edwards L., Parker R. L., Denny L., Giffear M., et al. 2015. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386: 2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim T. J., Jin H.-T., Hur S.-Y., Yang H. G., Seo Y. B., Hong S. R., Lee C. W., Kim S., Woo J. W., Park K. S., et al. 2014. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 5: 5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Gegerfelt A. S., Rosati M., Alicea C., Valentin A., Roth P., Bear J., Franchini G., Albert P. S., Bischofberger N., Boyer J. D., et al. 2007. Long-lasting decrease in viremia in macaques chronically infected with simian immunodeficiency virus SIVmac251 after therapeutic DNA immunization. J. Virol. 81: 1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valentin A., von Gegerfelt A., Rosati M., Miteloudis G., Alicea C., Bergamaschi C., Jalah R., Patel V., Khan A. S., Draghia-Akli R., et al. 2010. Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit. Vaccine 28: 1962–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.